Chronic pelvic pain (CPP) is defined as noncyclic pain of at least 6 months duration, severe enough to cause disability or seeking of medical attention, and occurring in locations such as the pelvis, anterior abdominal wall at or below the umbilicus, lower back, or buttocks [1]. The pathophysiology of CPP is complex. The source of pain may include the viscera (urologic, gynaecologic, and anorectal) and neuromuscular system (e.g. pudendal neuralgia, piriformis syndrome). Furthermore, the clinical presentation is often influenced by psychological factors [2]. Therefore, a multidisciplinary approach to management is recommended [2]. As part of this management plan, neural block and injection of muscles within the pelvis play both diagnostic and therapeutic roles [2]. The methods of neural block in the past were either landmark-based (blind) or equipment-guided techniques. The latter are indirect methods providing surrogate markers (such as bony landmarks for the nerve in fluoroscopy) or electrophysiological changes (such as nerve stimulation or electromyography). Both of these have intrinsic limitations in precisely locating a soft tissue structure. In either method, the nerve or muscle of interest is not reliably visualized. The emergence of ultrasound guidance to assist in needle placement and injection has provided the pain clinician with many benefits over previous modalities. Among the advantages of ultrasound are improved visualization of the nerve and surrounding vascular, bony, muscular, and visceral structures, more precise deposit of medication in the vicinity of the nerve of interest, and real-time guidance for needle advancement, thereby improving targeting and reducing inadvertent damage to surrounding neurovascular structures, as well as making it possible to better identify intravascular and intraneuronal injection [3]. Furthermore, the relatively easy access to ultrasonography and its portability and lack of radiation exposure make it an attractive imaging modality for the interventional pain physician [4–8] This chapter concentrates on anatomy, sonoanatomy, and ultrasound-assisted techniques for needle placement in three procedures associated with CPP: (1) ilioinguinal, iliohypogastric, and genitofemoral nerve block, (2) piriformis muscle injection, and (3) pudendal nerve block.
1. ILIOINGUINAL, ILIOHYPOGASTRIC, AND GENITOFEMORAL NEURALGIA
The ilioinguinal (II), iliohypogastric (IH), and genitofemoral (GF) nerves are known as “border nerves”, providing sensory innervation to the skin lying between the thigh and abdomen [9]. Because of their location and variable course, these nerves are susceptible to injury in surgical procedures involving the lower abdomen. Injury to the II and IH nerves is a known risk in open appendectomy incisions, inguinal herniorrhaphy, low transverse incisions (e.g. Pfannenstiel incision), and during trocar insertion for laparoscopic surgery of the abdomen and pelvis [10–14]. These nerves can be injured by several mechanisms, including direct nerve trauma with or without neuroma formation, compression of the nerve with scar tissue or haematoma, and suturing of the nerve into fascial closure or mesh incorporation [15, 16].
Patients presenting with pain secondary to irritation of these nerves usually complain of groyne pain, which may radiate to the scrotum or testicle in males, the labia majora in women, and the medial aspect of the thigh [5]. One review has identified chronic pain after inguinal repair to be as high as 54%, and one third of these patients report the pain to be moderate to unbearable [17]. block of the II and IH nerves is often performed to provide intraoperative and postoperative analgesia for hernia repair [18]. In addition, block of these nerves serves a diagnostic and therapeutic purpose in patients complaining of chronic pain in this nerve distribution [5, 6, 8, 19, 20].
2. ANATOMY
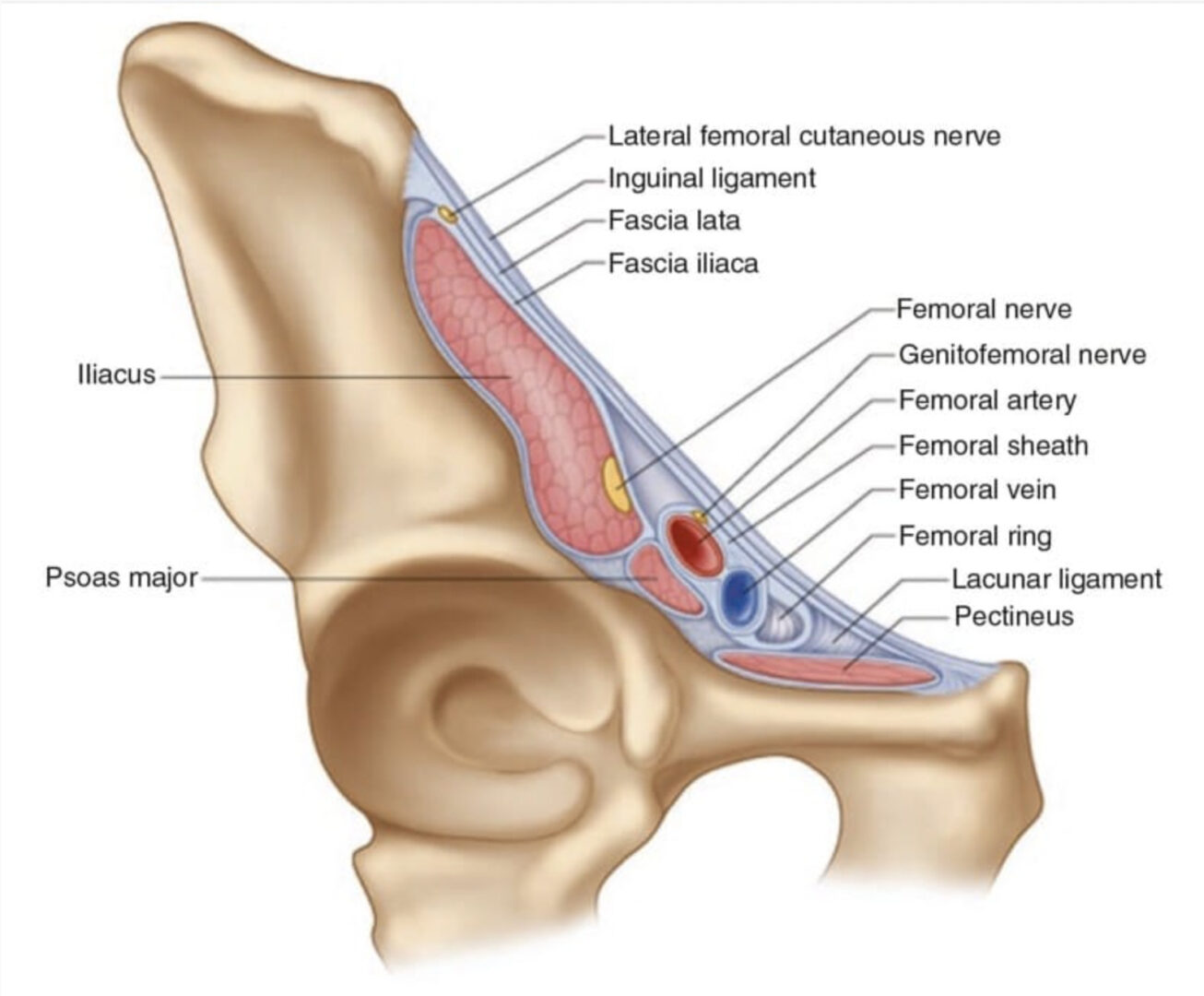
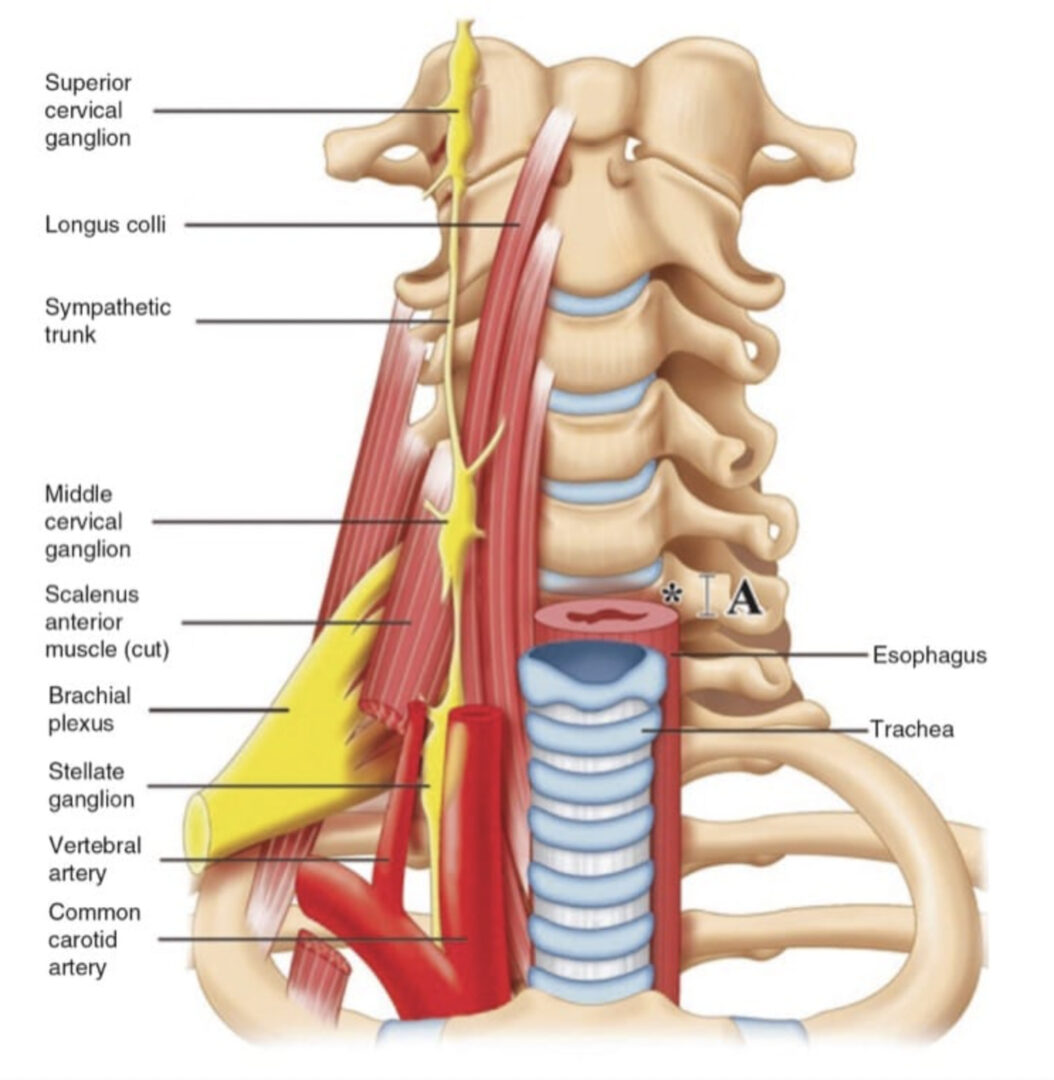
The II and IH nerves originate from the ventral rami of L1 with contributing filaments from T12 [9, 21]. The IH nerve emerges along the upper lateral border of the psoas major (Fig. 1). The nerve then crosses quadratus lumborum inferolaterally, travelling to the iliac crest [9]. At a point midway between the iliac crest and the twelfth rib, the nerve pierces the transversus abdominis (TA) muscle superior to the anterior superior iliac spine (ASIS) [21]. The IH nerve then runs inferomedially, piercing the internal oblique (IO) muscle above the anterior superior iliac spine [21]. From this point, the nerve runs between the internal and external oblique (EO) muscles, piercing the external oblique aponeurosis approximately 1 in. above the superficial inguinal ring [9]. As the nerve courses between the abdominal oblique muscles, it divides into lateral and anterior cutaneous branches [12]. The lateral cutaneous branch provides sensory innervations to the skin of the gluteal region [21]. The anterior cutaneous branch supplies the skin over the hypogastric region, including the skin over the lower region of the rectus abdominis muscle [21]. The II nerve emerges along the lateral border of psoas major, inferior to the IH nerve (Fig. 1) [21]. The II runs parallel and below the IH nerve. In contrast to the IH nerve, the II nerve pierces the internal oblique at its lower border and then passes between the crura of the superficial inguinal ring, anterior to the spermatic cord [9, 21]. The nerve provides sensory fibres to the skin over the root of the penis and scrotum (or the mons pubis and labium majus) and superomedial thigh region [21].
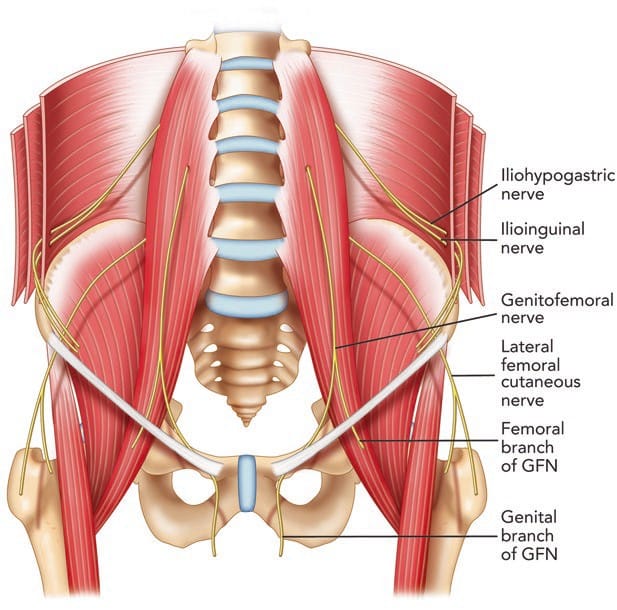
Fig. 1 The pathways of the ilioinguinal (II) nerve, iliohypogastric (IH) nerve, and genitofemoral nerve (GFN). (Reproduced with permission from Philip Peng Educational Series)
Observation of the course of the nerves in imaging and cadaver studies has shown that the area where both II and IH nerves are found is most consistently (90%) at the point midway between the iliac crest and the twelfth rib, where the nerves are located between the TA and IO muscles [21, 22].
The GF nerve arises from the L1 and L2 nerve roots [9]. The nerve travels anteriorly, passing through the psoas muscle at the level of the third and fourth lumbar vertebrae [9]. It then runs on the ventral surface of the muscle, under the peritoneum, and behind the ureter [23]. The nerve divides into the genital and femoral branches above the level of the inguinal ligament (Fig. 1) [23]. This point of division is variable. The genital branch passes through the deep inguinal ring, providing motor innervations to the cremaster muscle and sensory fibres to the scrotum [9, 23]. The course of this nerve in relation to the spermatic cord in the inguinal canal is varied, with ventral, dorsal, or inferior locations [9, 24], or it may be found as part of the cremaster muscle [23]. In females, the genital branch runs with the round ligament supplying the mons pubis and labium majus [9]. The femoral branch follows the external iliac artery, passing through fascia lata and providing sensory innervations to the skin of the femoral triangle [9].
Success, consistency, and reliability in block of the border nerves with blind techniques have been poor [25, 26]. These results are likely to be due to the high degree of anatomic variability not only in the course of the nerves but also in their branching patterns, areas of penetration of the fascial layers, and dominance patterns [8]. The above description of the II and IH nerve anatomy may be consistent in only 41.8% of patients [27]. Furthermore, the sites at which the II and IH nerves pierce the abdominal wall muscle layers vary significantly [14]. By far the most consistent location of the II and IH nerves is lateral and superior to the ASIS, where the nerves are found between the TA and IO muscular layers [5, 6, 8, 21].
3. LITERATURE REVIEW ON INJECTION TECHNIQUES FOR ILIOINGUINAL, ILIOHYPOGASTRIC, AND GENITOFEMORAL NERVE BLOCK
A number of injection techniques for II and IH nerves have been described, virtually all of which are landmark-based [28–30]. Unfortunately, all these techniques suggest a needle entry anterior to the ASIS (Fig. 2), where the anatomy of these nerves is highly variable. Thus, the failure rates with those techniques range between 10% and 45% [18, 25, 26, 31]. Furthermore, the misguided needle may result in femoral nerve block [32], bowel perforation [33, 34], and pelvic haematoma [35].
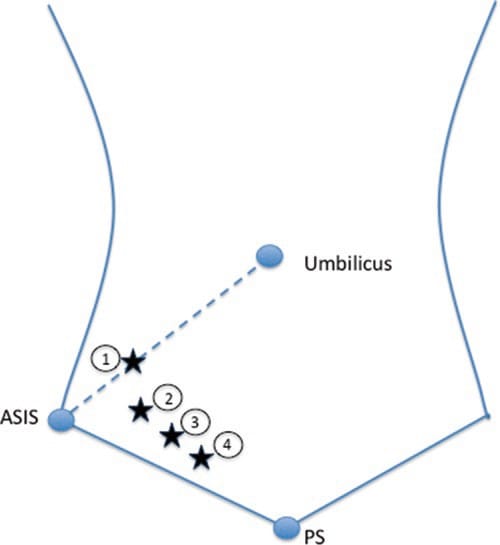
Fig. 2 Three methods (four landmarks) described for ilioinguinal and iliohypogastric nerves injection [28–30]. ASIS anterior superior iliac spine, PS pubic symphysis. (Reproduced with permission from Philip Peng Educational Series)
Two key elements contribute to an improvement in the success rate. One is to perform the injection cephalad and posterior to the ASIS, where both the II and IH nerves consistently (>90%) can be found between the TA and IO muscles [21]. The other is the use of ultrasound for the guidance of injection. Techniques utilizing ultrasound to inject the II and IH nerves have been published [5, 8, 36, 37]. The accuracy of ultrasound guidance has been validated in a cadaver study with the injection site superior to the ASIS, and the block success rate was 95% [36]. The success of using ultrasound to guide II and IH nerve block has been replicated in the clinical setting. Based on visualization of the abdominal muscles, the fascial planes, and the deep circumflex iliac artery, the authors were able to demonstrate a clinically successful block in all their cases, based on sensory loss corresponding to the II and IH nerves following injection [37, 38]. The ease and importance of identifying the abdominal muscle planes before attempting to visualize the nerves have been supported by a study assessing the training of anaesthesiologists with little experience in using ultrasound to assist in needle placement [39].
Neural block of the GF nerve is not commonly performed. Review of the literature shows that techniques described in the past were blind and rely on the pubic tubercle, inguinal ligament, inguinal crease, and femoral artery as landmarks [40, 41]. One of the blind methods involves infiltration of 10 mL local anaesthetic immediately lateral to the pubic tubercle, caudad to the inguinal ligament [42]. In another method, a needle is inserted into the inguinal canal to block the genital branch, a method that can be reliably performed only during surgery [41]. The blind techniques described are essentially infiltration techniques and rely on high volumes of local anaesthetic for consistent results [42].
Ultrasound-guided block of the genital branch of the GF nerve has been described in several review articles [5, 6, 8]. The genital nerve is difficult to visualize, and block is achieved by identification of the inguinal canal [5, 6, 8]. In males, the GF nerve may travel within or outside the spermatic cord. Thus, the local anaesthetic and steroid are deposited both outside and within the spermatic cord [5, 6, 8]. In addition to simple neural block with analgesic medication, ultrasound has also been utilized to achieve successful cryoablation of the genitofemoral nerve for chronic inguinal pain, but these authors directed their therapy only to the femoral branch of the GF nerve [43].
4. ULTRASOUND-GUIDED TECHNIQUE OF ILIOINGUINAL, ILIOHYPOGASTRIC, AND GENITOFEMORAL NERVE block
Ilioinguinal and Iliohypogastric Nerves
When performing II and IH nerve block under ultrasound guidance, it is important to clearly identify the abdominal wall muscle layers: EO, IO, and TA (Fig. 1). The patient is placed in the supine position. Both nerves are relatively superficial, so a high-frequency (6–13 MHz) linear probe will provide optimal visualization. The recommended area for initial scanning is posterior and superior to the ASIS. The probe should be placed perpendicular to the direction of the II and IH nerves (which is usually parallel to the inguinal ligament), with the lateral edge on top of the iliac crest (Fig. 3). At this position, the iliac crest will appear as a hyperechoic structure, adjacent to which will appear the three muscular layers of the abdominal wall (Fig. 4). Below the TA, peristaltic movements of the bowel may be detected. The probe may need to be tilted either caudad or cephalad to optimize the image. Once the muscular layers are identified, the II and IH nerves will be found in the split fascial plane between the IO and TA muscle layers. Both nerves should be within 1.5 cm of the iliac crest at this site, with the II nerve closer to the iliac crest [36]. The nerves are usually in close proximity to each other [27] and located on the “upsloping” split fascia close to the iliac crest. In some cases, the nerves may run approximately 1 cm apart [8]. The deep circumflex iliac artery, which is close to the two nerves in the same fascial layer, can be revealed with the use of colour Doppler (Fig. 4). A neural structure within the fascial split may also be seen medial and on the flat part of the IO and TA muscle junction. This is the subcostal nerve; if it is mistaken for the II or IH nerve, the nerve block will result in an aberrant distribution of anesthesia.
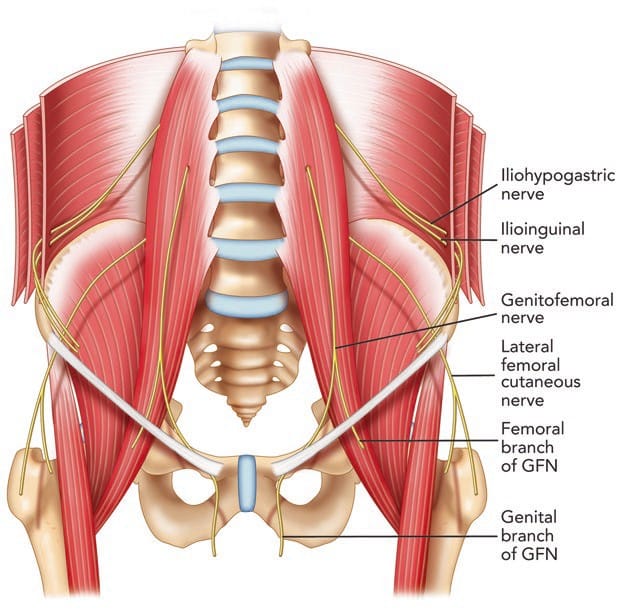
Fig. 1 The pathways of the ilioinguinal (II) nerve, iliohypogastric (IH) nerve, and genitofemoral nerve (GFN). (Reproduced with permission from Philip Peng Educational Series)
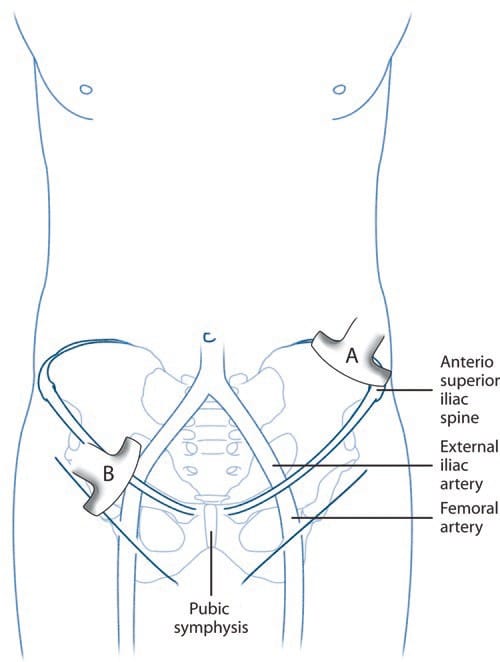
Fig. 3 Ultrasound guidance for II and IH nerve block. The position of the ultrasound probe is shown. The probe A is placed above and posterior to the ASIS and is in the short axis of the course of the II nerve. The probe B is placed in the inguinal line in the long axis of the femoral and external iliac arteries. (Reproduced with permission from Philip Peng Educational Series)
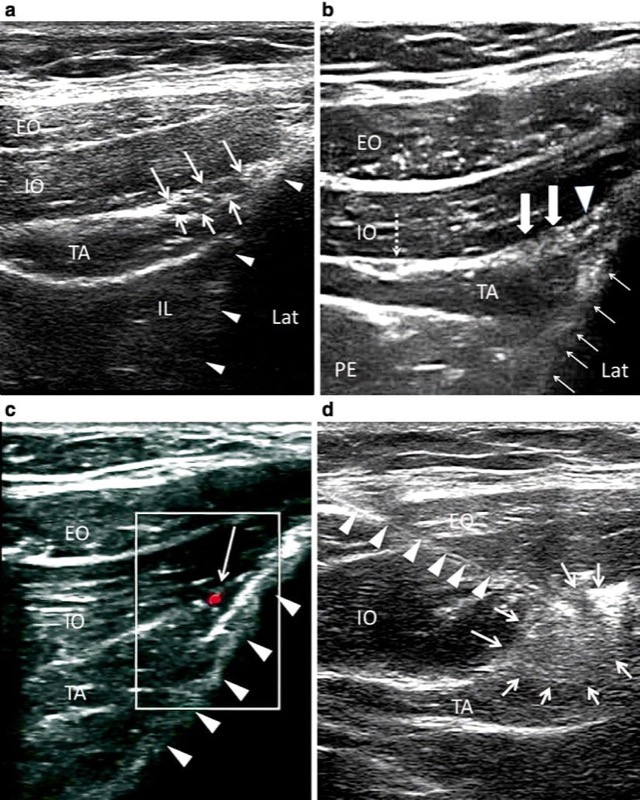
Fig. 4 Ultrasound guidance for II and IH nerve block. (a) The three layers of muscles and the fascia split with the II and IH nerves inside. Solid triangles outline the iliac crest. (b) In a similar view to A, solid arrows show the II nerve (lateral) and IH nerve (medial). Solid triangle shows the deep circumflex iliac artery. Dashed arrows point to the fascia split with subcostal nerve (T12). Usually the fascia split for the II and IH nerves appears adjacent to the iliac crest. When it appears far away from the iliac crest (as in this figure), one should suspect subcostal nerve. Solid arrows outline the iliac crest. (c) Similar view as B, with color Doppler showing the deep circumflex iliac artery (red). Line arrows outline the iliac crest. (d) The needle (outlined by solid triangle) is inserted with in-plane technique; line arrows outline the spread of the local anesthetic and steroid solution. EO external oblique muscle, IL iliacus, IO internal oblique muscle, LAT lateral, PE peritoneum, TA transverse abdominis muscle. (Reproduced with permission from Philip Peng Educational Series)
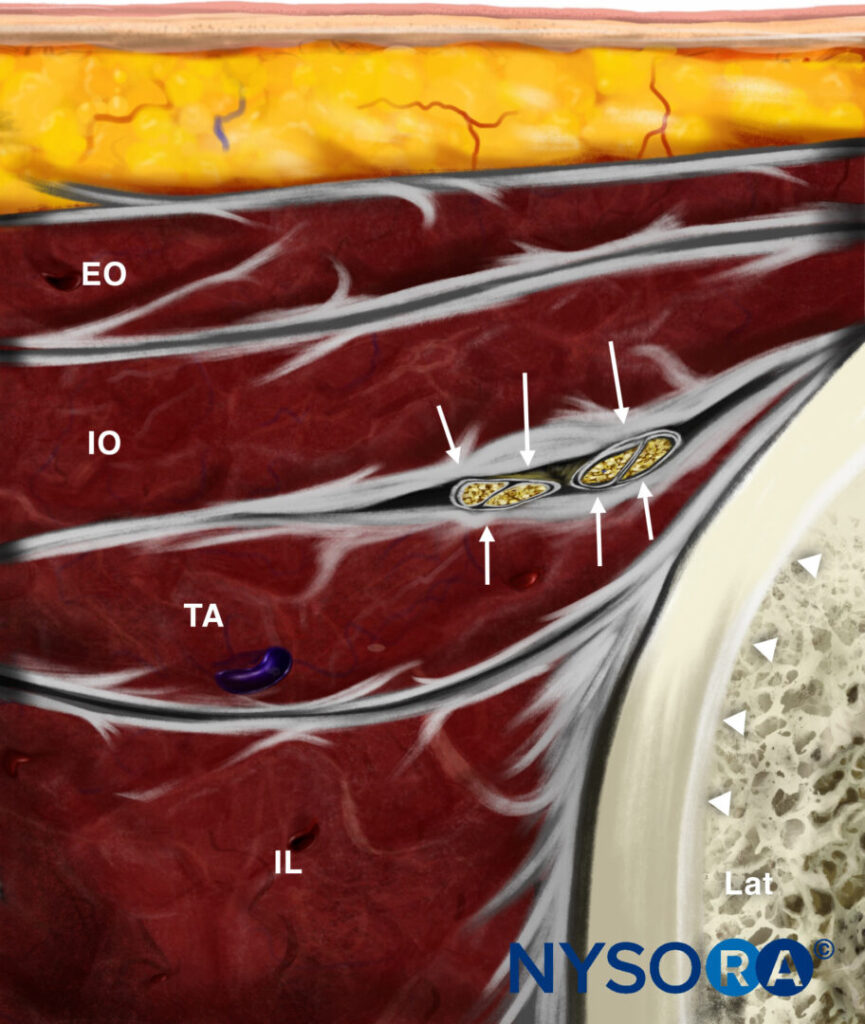
Reverse Ultrasound Anatomy illustration of figure 4a. EO, external oblique; IO, internal oblique; TA, transversus abdominis; IL, iliacus; Lat, lateral.
Once satisfied with visualization of the nerves, a 22-gauge spinal needle is advanced to the nerves under real-time guidance. We favor an out-of-plane technique. The needle is advanced so that the tip lies in the split fascial plane between the IO and TA muscles and adjacent to the II and IH nerves (Fig.4). At this point, hydrodissection with normal saline can confirm adequate position of the needle tip and spread within the fascial plane. In some cases, the nerves may be difficult to visualize. In this situation, injectate may be deposited in the fascial plane between the TA and IO muscles, ensuring satisfactory medial and lateral spread [38]. The injectate usually consists of 6–8 mL of local anesthetic (bupivacaine 0.5%) and steroid (Depo-Medrol 40 mg). The desired result is observation of spread of the solution in the split fascial plane to surround both nerves.
5. GENITAL BRANCH OF GENITOFEMORAL NERVE
The genital branch of the GF nerve cannot be visualized directly. The major structure sought on scanning is the inguinal canal and its content (the spermatic cord in males or the round ligament in females).
The patient is positioned supine, and a linear ultrasound probe with high frequency (6–13 MHz) is used. Initially, the probe is placed in the transverse plane below the inguinal ligament. In this plane, the femoral artery is identified and positioned in the middle of the screen. The probe is then rotated so the artery lies in the long axis (Fig. 3). The ultrasound probe is then moved cranially to trace the femoral artery until it dives deep into the abdomen to become the external iliac artery (Fig. 5). At this point, an oval or circular structure may be seen superficial to the femoral artery. This structure is the inguinal canal, which contains the spermatic cord in men and the round ligament in women. The probe may be moved slightly medial to trace the spermatic cord or round ligament. In males, arterial pulsations may be visible within the spermatic cord. These pulsations represent the testicular artery and the artery to the vas deferens and may be confirmed by the use of colour Doppler. The blood vessels may be made more prominent by asking the patient to perform a Valsalva manoeuvre, which increases blood flow through the pampiniform plexus. In addition to the arteries, a thin tubular structure within the spermatic cord may also be visible; this is the vas deferens. In females, the round ligament can be difficult to visualize, and the target is the inguinal canal.
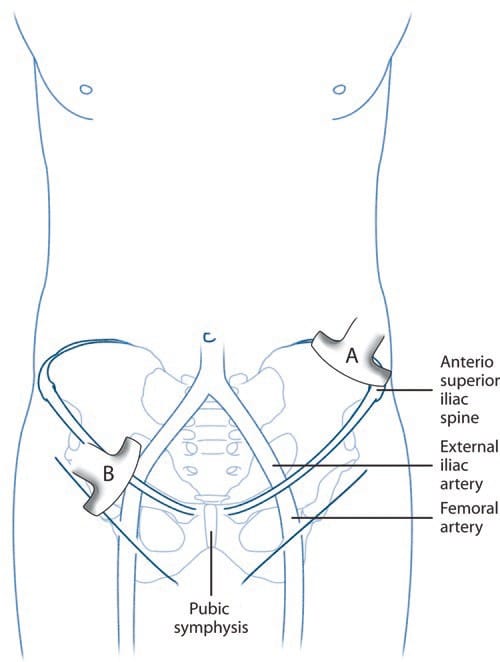
Fig. 3 Ultrasound guidance for II and IH nerve block. The position of the ultrasound probe is shown. The probe A is placed above and posterior to the ASIS and is in the short axis of the course of the II nerve. The probe B is placed in the inguinal line in the long axis of the femoral and external iliac arteries. (Reproduced with permission from Philip Peng Educational Series)
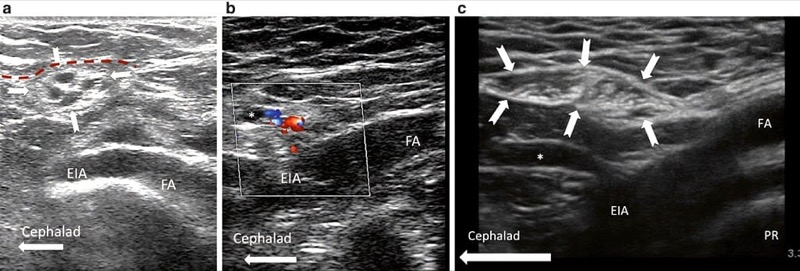
Fig. 5 Ultrasound-guided GFN block. (a) Long-axis view of the femoral artery (FA) and external iliac artery (EIA) showing the crosssection of spermatic cord (solid arrows) in a male patient. The deep abdominal fascia is outlined (red dashed line). (b) Similar view as A, with colour Doppler showing the vessels inside the spermatic cord. (c) Similar view as A but in a female patient. The inguinal canal is outlined (bold arrows). PR, pubic ramus. (Reproduced with permission from Philip Peng Educational Series)
An out-of-plane technique is used to guide needle placement. The needle is inserted on the lateral aspect of the probe and is directed to pierce the deep abdominal fascia and enter the inguinal canal (Fig. 5). Once the needle has pierced the fascia, hydrodissection with normal saline confirms spread within the inguinal canal. A volume of 4 mL of anaesthetic solution is deposited within the inguinal canal but outside the spermatic cord, with another 4 mL deposited inside the spermatic cord. The injection is divided because of the anatomic variability of the genital branch. The local anaesthetic solution should not contain epinephrine, as there is a risk of vasoconstriction of the testicular artery. In addition to local anaesthetic, steroids may be added for cases with chronic pain. In females, 8 mL of solution will be deposited into the inguinal canal.
6. PIRIFORMIS SYNDROME
Piriformis syndrome is a potential cause of pain occurring in the back, buttock, or hip [44–47]. One clinical study reported a prevalence of 17.2% of piriformis syndrome in patients who complained of low back pain [48]. The characteristic symptoms of piriformis syndrome are buttock pain with radiation into the ipsilateral thigh and lower leg, which may resemble sciatica [45, 49]. The pain is exacerbated by walking, stooping, or lifting [50]. On physical examination, there may be gluteal atrophy and tenderness on palpation, pain on stretching of the piriformis muscle, and a positive Lasegue sign [48, 50, 51]. Often, it is a diagnosis of exclusion with clinical assessment and investigations necessary to rule out pathology of the lumbar spine, hips, and sacroiliac joint [50–52].
Often, piriformis syndrome will improve with a conservative regimen of physical therapy and simple analgesic pharmacotherapy. For those patients not responding, more interventional therapy may be required in the form of muscle injections or surgery [53]. The piriformis muscle may be injected with local anaesthetic and steroid [54], which will also aid in diagnosis if therapeutically successful. Furthermore, botulinum toxin has been injected into the piriformis muscle with evidence of longer periods of analgesia [55, 56]. If there is failure to improve after three injections, surgical release of the piriformis muscle may be considered [44].
7. ANATOMY
The origin of the piriformis muscle is via fleshy digitations on the ventral surface of the S2 to S4 vertebrae (Fig. 6) [47]. Running laterally anterior to the sacroiliac joint, the piriformis muscle exits the pelvis through the greater sciatic foramen [51]. At this point, the muscle becomes tendinous, inserting into the upper border of the greater trochanter as a round tendon [52]. The piriformis functions as an external rotator of the lower limb in the erect position, an abductor when supine, and a weak hip flexor when walking [52].
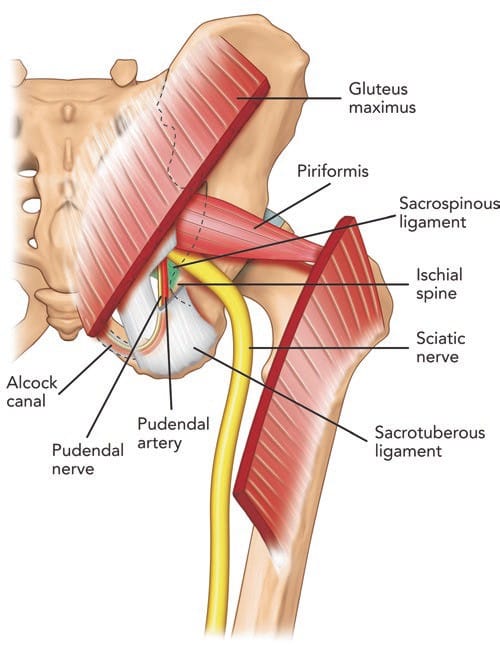
Fig.6 Posterior view of pelvis showing the pudendal neurovascular bundle and piriformis muscle. The gluteus maximus muscle was cut to show the deeper structures. Note that the pudendal nerve and artery run in the interligamentous plane between the sacrospinous and sacro-tuberous ligament and subsequently into Alcock’s canal. (Reproduced with permission from Philip Peng Educational Series)
All neurovascular structures exiting the pelvis to the buttock pass through the greater sciatic foramen [52]. The superior gluteal nerve and artery pass superior to the piriformis [52]. Inferior to the piriformis lie the inferior gluteal artery and nerve, the internal pudendal artery, the pudendal nerve, the nerve to the obturator internus, the posterior femoral cutaneous nerve, the nerve to the quadratus lumborum, and the sciatic nerve [52]. The anatomical relationship between the piriformis muscle and sciatic nerve is variable. Most commonly (78–84%), the sciatic nerve passes below the piriformis muscle [57, 58]. Less frequently (12–21%), the nerve is divided, passing through and below the muscle [58]. Occasionally, the divided nerve may pass through and above the piriformis muscle or both above and below the muscle, or the undivided nerve may pass above the piriformis muscle or through the muscle [57, 58]. The close relationship of the piriformis muscle to the sciatic nerve explains why patients experiencing piriformis syndrome may also experience symptoms of sciatic nerve irritation [46].
8. LITERATURE REVIEW ON PIRIFORMIS MUSCLE INJECTIONS
Reported techniques used to inject the piriformis muscle have included fluoroscopy [54], CT [59], and MRI [60] to assist with accurate needle placement within the muscle. Electrophysiologic guidance also has been used alone and in conjunction with the above modalities [56, 61, 62]. Regardless of whether EMG guidance is used, fluoroscopically guided piriformis muscle injections depend on the presence of a characteristic intrapiriformis contrast pattern to confirm needle placement within the piriformis muscle (Fig. 7) [54], which has been shown to be unreliable [63]. A validation study with cadavers suggested that the fluoroscopically guided contrast-controlled injection was accurate in guiding an intrapiriformis injection in only 30% of the injections [63]. When the needle was incorrectly placed, the usual final position of the needle was within the gluteus maximus muscle, which overlies the piriformis.
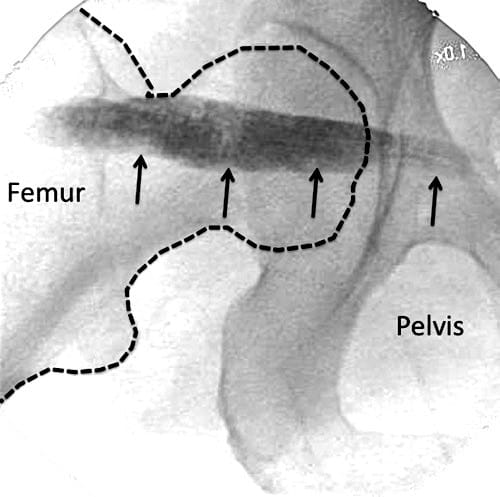
Fig.7 Radiographic contrast (line arrows) outlining the piriformis muscle. (Reproduced with permission from Philip Peng Educational Series)
Ultrasound is seen as an attractive imaging technique, as it provides visualization of the soft tissue and neurovascular structures and allows real-time imaging of needle insertion towards the target [64]. The use of ultrasound for piriformis muscle injection was first reported in 2004 [65]. Since then, multiple reports of ultrasound-guided piriformis muscle injection have been published, with similar techniques described [4, 5, 63, 66]. The accuracy of needle placement with ultrasound was recently validated in a cadaveric study that suggested an accuracy of 95% [63]. In clinical practice, the accuracy of ultrasound-guided needle placement within the piriformis muscle has been confirmed with electromyography [67].
9. ULTRASOUND-GUIDED TECHNIQUE FOR PIRIFORMIS MUSCLE INJECTION
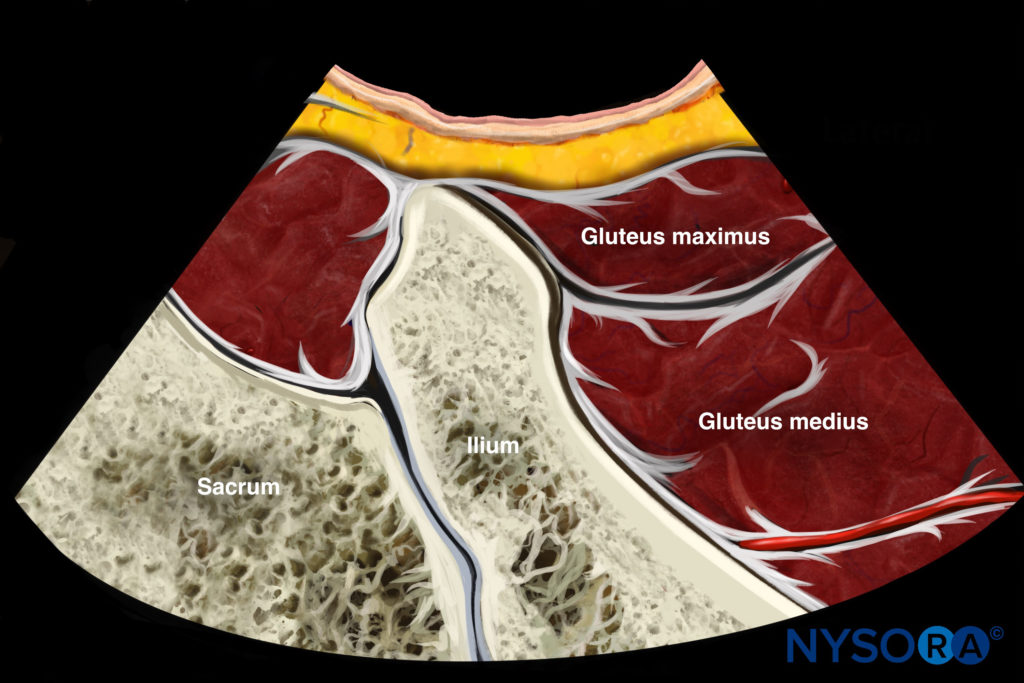
The patient is placed in the prone position. A low-frequency (2–5 Hz) curvilinear probe is held in the transverse plane and initially positioned over the posterior superior iliac spine (PSIS). The transducer is then moved laterally to visualize the ilium, which will be identifiable as a hyperechoic line descending diagonally across the screen from the superomedial to inferolateral corners (Fig. 8a). Once the ilium is visualized, the probe is oriented in the direction of the piriformis muscle and moved in a caudad direction until the sciatic notch is found (Fig. 8b). At the sciatic notch level, the hyperechoic shadow of the bone will disappear from the medial aspect, and two muscle layers will be visible: the gluteus maximus and the piriformis (Fig. 8c). Confirmation of the piriformis muscle can be made by having an assistant rotate the hip externally and internally with the knee flexed. This movement will demonstrate side-to-side gliding of the piriformis muscle on ultrasound. It is important to identify the sciatic notch, as failure to do so may lead the practitioner to mistakenly identify one of the other external hip rotators (e.g. the gemelli muscles) as the piriformis.
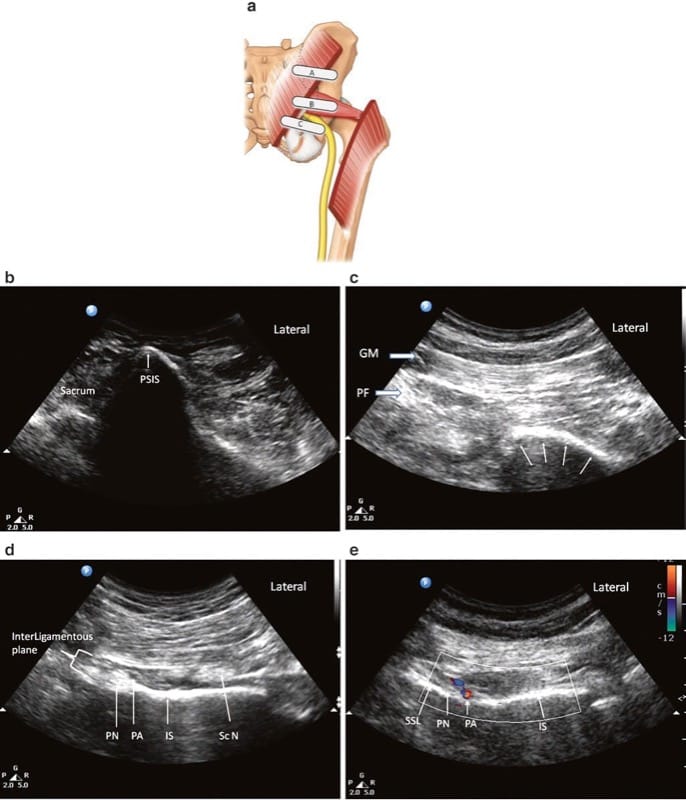
Fig.8 Ultrasonographic scan of the piriformis muscle and the pudendal nerve. (a) Three different positions of the ultrasound probe. (b) Ultrasound image at probe position A. (c) Ultrasound image at probe position B. (d) Ultrasound image at probe position C. (e) Colour Doppler to show the pudendal artery. GM gluteus maximus muscle, Pu A pudendal artery, Pu N pudendal nerve, Sc N sciatic nerve, SSL sacro-spinous ligament. (Reproduced with permission from Philip Peng Educational Series)
Reverse Ultrasound Anatomy illustration of figure 8b. PSIS, posterior superior iliac spine.
Because of the depth of the muscle, a 22-gauge, 120-mm nerve-stimulating needle is used. For the less experienced practitioner, we recommend the concomitant use of a nerve stimulator to avoid unintentional injection of the sciatic nerve, as the passage of the sciatic nerve in this territory varies, as described above. In addition, the use of a nerve stimulator also allows identification of the needle tip within the piriformis muscle by the visualization of piriformis muscle twitches on the monitor.
An in-plane technique is used, with the needle being inserted on the medial aspect of the probe and passing laterally into the muscle belly of the piriformis in the sciatic notch. If intramuscular injection is the objective, the needle should be slowly advanced further until strong contractions of the piriformis muscle are evident on the monitor. A small volume of normal saline (0.5 mL) may be injected to confirm position within the muscle. Once satisfied with the needle position, a small volume (1–2 mL) of medication (either a mixture of 1 mL 0.5% bupivacaine and 40 mg Depo-Medrol or 50 units of botulinum toxin A diluted in 1 mL of normal saline) may be injected into the muscle.
10. PUDENDAL NEURALGIA
The pudendal nerve supplies the anterior and posterior urogenital areas (clitoris, penis, vulva, and perianal area) [68–70]. Pudendal neuralgia refers to CPP in which pain is experienced in the regions innervated by the pudendal nerve [68]. Typically, the pain is exacerbated by sitting and may be reduced by lying on the nonpainful side, standing, or sitting on a toilet seat [71]. On physical examination, there may be evidence of hypoesthesia, hyperalgesia, or allodynia in the perineal area [71]. The pain may be reproduced or exaggerated when pressure is applied against the ischial spine during a vaginal or rectal examination. Pudendal nerve block is an important tool in the diagnosis of this condition [72].
Often the cause of the symptoms in patients suffering from pudendal neuralgia will not be readily identifiable, but recognized risk factors in the development of pudendal neuralgia include bicycle riding [73], vaginal delivery [74, 75], countertraction devices in orthopaedic surgery [76, 77], pelvic trauma [76], and intensive athletic activity [78].
The pudendal nerve is susceptible to entrapment in two anatomical regions along its path: the interligamentous plane, which lies between the sacrotuberous and sacrospinous ligaments at the level of the ischial spine [79], and Alcock’s canal [80] (Fig. 9).
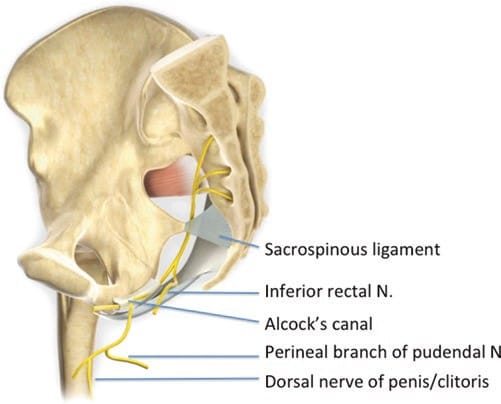
Fig.9 The pudendal nerve is seen arising from S2 to S4 and exiting the pelvis to enter the gluteal region through the greater sciatic foramen. The nerve gives rise to the inferior rectal nerve, the perineal nerve, and the dorsal nerve of the penis or clitoris. The inferior rectal nerve branches from the pudendal nerve prior to Alcock’s canal. N, nerve. (Reproduced with permission from Philip Peng Educational Series)
11. ANATOMY
The pudendal nerve contains both motor and sensory fibres [81]. Relative to the major nerves of the extremities, the pudendal nerve is thin (0.6–6.8 mm) and is situated deep within the body, surrounded by fatty tissue [82]. It arises from the anterior rami of the second, third, and fourth sacral nerves (S2, S3, and S4) [81] and passes through the greater sciatic notch [82]. Once out of the pelvis, the pudendal nerve travels ventrally in the interligamentous plane between the sacrospinous and sacrotuberous ligament at the level of the ischial spine (Fig. 6) [68, 83]. At this level, 30–40% of pudendal nerves will have two or three trunks [68, 84, 85]. Within the interligamentous plane, the pudendal artery is located lateral to the pudendal nerve in the vast majority of cases (90%) [82]. This region is of clinical importance, as the nerve may be compressed between the sacrospinous and sacrotuberous ligaments [79]. Furthermore, elongation of the ischial spine due to repetitive muscular forces represents a potential source of microtrauma affecting the pudendal nerve [78].
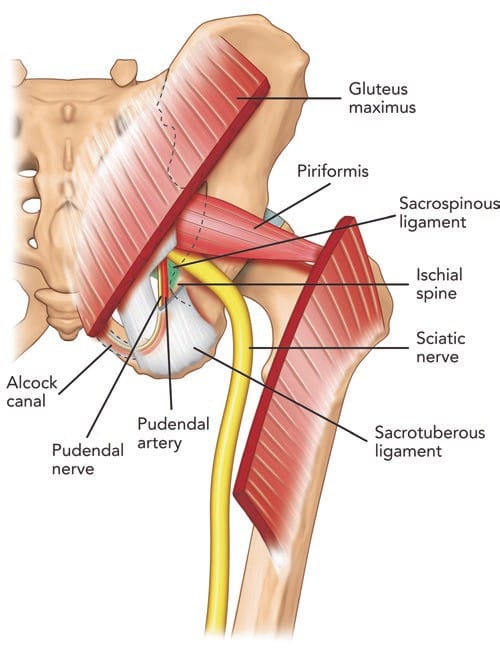
Fig.6 Posterior view of pelvis showing the pudendal neurovascular bundle and piriformis muscle. The gluteus maximus muscle was cut to show the deeper structures. Note that the pudendal nerve and artery run in the interligamentous plane between the sacrospinous and sacro-tuberous ligament and subsequently into Alcock’s canal. (Reproduced with permission from Philip Peng Educational Series)
Following its passage between the two ligaments, the pudendal nerve swings anteriorly to enter the pelvis through Alcock’s canal of the lateral ischiorectal fossa [84–86]. Alcock’s canal is a fascial sheath formed by the duplication of the obturator internus muscle, underlying the plane of the levator ani [84]. At this site, the pudendal nerve is also susceptible to entrapment by either the fascia of the obturator internus or the falciform process of the sacrotuberous ligament [80].
As the pudendal nerve travels through the ischiorectal fossa, it gives off three terminal branches: the dorsal nerve of the penis, the inferior rectal nerve, and the perineal nerve. The dorsal nerve of the penis runs lateral to the dorsal artery and deep dorsal vein of the penis, terminating in the glans penis [83, 87, 88]. The course of the nerve under the subpubic arch makes it susceptible to compression by the saddle nose of a bicycle [89]. The inferior rectal nerve supplies the external anal sphincter [83, 87, 88]. The remaining portion of the pudendal nerve trunk becomes the perineal nerve, which continues to supply sensation of the skin of the penis or clitoris, the perianal area, and the posterior surface of the scrotum or labia majora [88]. The perineal nerve also provides motor supply to the deep muscles of the urogenital triangle [87, 88].
12. LITERATURE REVIEW ON PUDENDAL NERVE INJECTIONS
block of the pudendal nerve may be performed in two anatomical regions: the interligamentous plane [79] and Alcock’s canal [80]. The pudendal nerve has been blocked by various routes in the literature. These include the transvaginal [90], transperineal [91, 92], and transgluteal approaches [93]. The transgluteal approach is popular, allowing block at the ischial spine and Alcock’s canal. Traditionally, fluoroscopy has been used to guide needle placement, using the ischial spine as a surrogate landmark [68]. The needle is placed medial to the ischial spine, which corresponds to the course of the pudendal nerve at this level [93, 94]. The major limitation of fluoroscopy is that it cannot accurately demonstrate the interligamentous plane [5, 8]. At the level of the ischial spine, the pudendal nerve lies medial to the pudendal artery in the majority of cases (76–100%) [7, 82]. Therefore, injectate may not spread to the pudendal nerve using this landmark. In addition, the potential proximity of the sciatic nerve at this level makes it susceptible to the anaesthetic if spread of the injectate is not visualized in real time. Furthermore, the depth for needle insertion cannot be assessed with fluoroscopy.
Both ultrasound and CT scan are ideal for visualizing the interligamentous plane, as they identify all the important landmarks: ischial spine, sacrotuberous ligament, sacrospinous ligament, pudendal artery, and pudendal nerve (8). They also allow visualization of the sciatic nerve and other vascular structures, so more selective needle placement and block can occur. Ultrasound has the advantage of not exposing the patient to radiation, and it is more accessible to clinicians. Early reports only described ultrasound visualization of the pudendal nerve [82, 95], but the actual technique of block was later reported in greater detail [5, 7, 8]. A consistent feature of published techniques on ultrasoundguided pudendal nerve block is identification of the ischial spine and its medial aspect, which contains the sacrotuberous and sacrospinous ligaments, internal pudendal artery, and pudendal nerve [5–8]. In a more recent study, the pudendal nerve itself could only be identified accurately in 57% of cases, but sonographic identification of the ischial spine (96%), sacrotuberous ligament (100%), sacrospinous ligament (96%), and pudendal artery (100%) was highly accurate [96].
This study also compared pudendal nerve block performed under ultrasound guidance versus fluoroscopic guidance [96]. The investigators found that there was no difference in the efficacy of pudendal nerve block (as assessed to pin prick and cold sensation) between the ultrasound-guided and fluoroscopy-guided techniques [96]. In addition, there was no difference in the rate of adverse effects between the two modes of neural block [96].
At the level of Alcock’s canal, ultrasound cannot accurately identify or guide needle placement. CT is the only form of imaging that can accurately guide the needle into the canal [97].
13. ULTRASOUND-GUIDED TECHNIQUE FOR PUDENDAL NERVE INJECTION
Pudendal nerve block at the level of the ischial spine with ultrasound guidance is performed via the transgluteal approach, with the patient in the prone position. The aim of scanning is to identify the ischial spine and therefore reliably identify the interligamentous plane, which will appear on its medial aspect. A curvilinear probe (2–5 Hz) is recommended for scanning because of the depth of the nerve. Scanning begins with the probe held in the transverse plane over the PSIS, a technique similar to the technique for scanning the piriformis muscle (Fig. 8c). The probe is then moved caudad until the piriformis muscle is identified, as described above for the piriformis muscle injection. At this level, the ischium can be identified as a curved, hyperechoic line. The probe is then moved further caudad to identify the ischial spine. Four features will help to identify the level of the ischial spine (Fig. 8d):
1. The ischial spine will appear as a straight, hyperechoic line, as opposed to the ischium, which is a curved, hyperechoic line.
2. The sacrospinous ligament will be visualized as a hyperechoic line lying medial to and in contact with the ischial spine. Unlike bony structures, however, the sacrospinous ligament does not cast an anechoic shadow deep to its image.
3. The piriformis muscle will disappear. Deep to the gluteus maximus lies the sacrotuberous ligament. Although it is difficult to differentiate between this ligament and the fascial plane of the gluteus maximus, the sacrotuberous ligament can be felt easily as the needle advances through this thick ligament.
4. The internal pudendal artery can be seen, usually situated on the medial portion of the ischial spine. This artery can be confirmed with color Doppler (Fig. 8e).
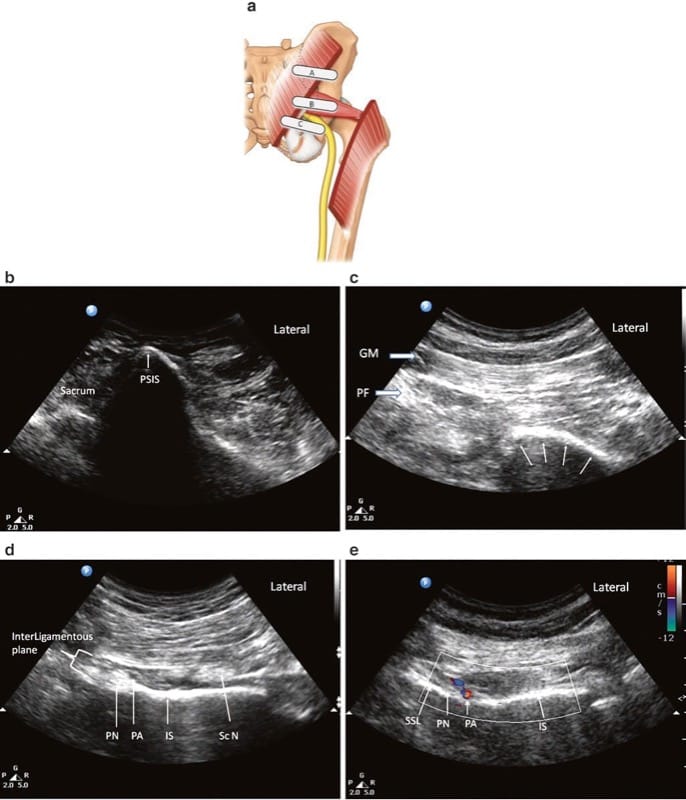
Fig.8 Ultrasonographic scan of the piriformis muscle and the pudendal nerve. (a) Three different positions of the ultrasound probe. (b) Ultrasound image at probe position A. (c) Ultrasound image at probe position B. (d) Ultrasound image at probe position C. (e) Colour Doppler to show the pudendal artery. GM gluteus maximus muscle, Pu A pudendal artery, Pu N pudendal nerve, Sc N sciatic nerve, SSL sacro-spinous ligament. (Reproduced with permission from Philip Peng Educational Series)
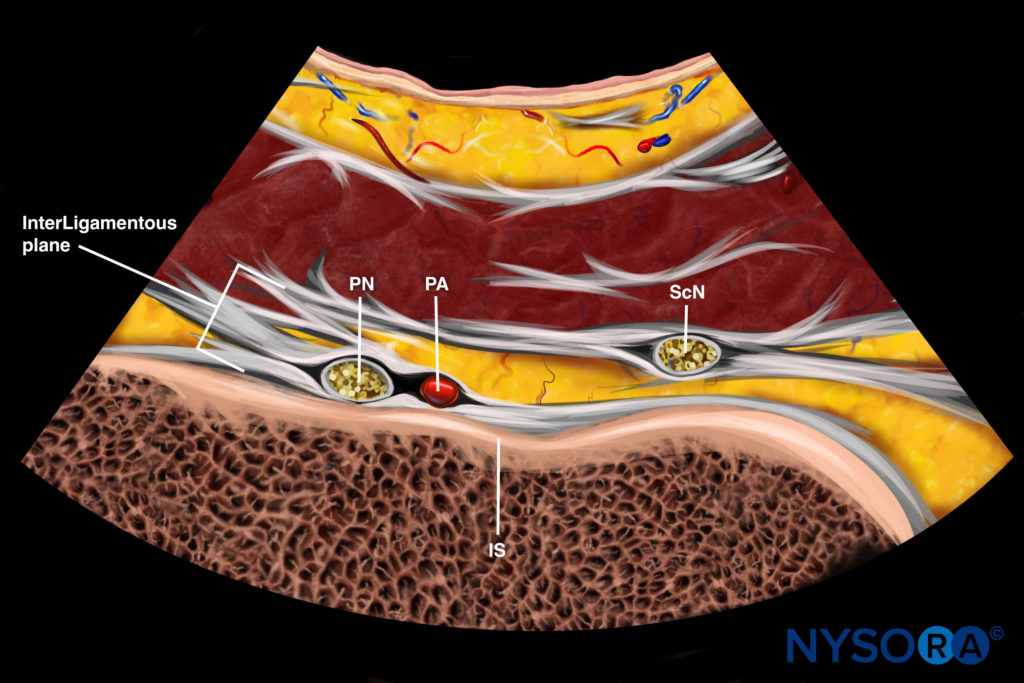
Reverse Ultrasound Anatomy illustration of figure 8d. PN, pudendal nerve; PA, pudendal artery; IS, ischial spine; ScN, sciatic nerve.
The pudendal nerve will lie medial to the pudendal artery at this level, but it may be difficult to visualize because of its depth and its small diameter. On dynamic scan, the sciatic nerve and inferior gluteal artery can be seen lateral to the ischial spine tip. Visualization of these structures is important because if they are mistaken for the internal pudendal artery, sciatic nerve block will result.
Once satisfied with the identification of the ischial spine, pudendal artery, and interligamentous plane, a 22-gauge, 120-mm insulated peripheral nerve-stimulating needle is inserted from the medial aspect of the probe. The target is for the needle tip to be situated between the sacrotuberous ligament and sacrospinous ligament. Because of the depth of the pudendal nerve, it is helpful to insert the needle several centimeters medial to the medial edge of the probe to reduce the steepness of the needle path and therefore assist in visualization of the needle tip as it passes to the target site. The needle is advanced so that it will pass through the sacrotuberous ligament, on the medial side of the pudendal artery. As the needle is passing through the sacrotuberous ligament, increased resistance will be felt. Once the needle is through, the resistance will diminish. A small volume of normal saline is injected to confirm position within the interligamentous plane. The pudendal nerve itself will be difficult to visualize owing to a combination of its depth [7, 82], its small diameter [68, 82, 85], and the possibility of anatomical division into two or three trunks [68, 84, 85].
If hydrodissection confirms adequate spread within the interligamentous plane and no intravascular spread, a mixture of local anesthetic and steroid may be injected. In our experience, a mixture of 4 mL of 0.5% bupivacaine and 40 mg of steroid (Depo-Medrol) is commonly injected, and clinical signs of pudendal nerve block are present shortly after. During injection, the clinician should ensure that there is spread of the injectate medial to the pudendal artery and that the injectate does not pass too far laterally past the artery. Excessive lateral spread may result in inadvertent sciatic nerve block. The patient should be assessed for signs of successful block following the procedure. This may be achieved simply by assessing sensation to pinprick and alcohol swab in the perineal area ipsilateral to the site of block. Successful block will result in reduced sensation to both stimuli in this region.
14. CONCLUSION
Ultrasound is a valuable tool for imaging peripheral structures, guiding needle advancement, and confirming the spread of injectate around the target tissue, all without exposing healthcare providers and patients to the risks of radiation. In patients with chronic pelvic pain, the target structures for interventional procedures can be well visualized with the use of ultrasound. Most of the ultrasound-guided interventional procedures for chronic pelvic pain have been validated and thus can be accurately performed.