Peripheral nerve stimulation (PNS) is currently a topic of increased interest after decades of apparent decline. Some of this increased popularity can be attributed to the advent of new imaging techniques, including ultrasound. Two recent feasibility studies in fresh cadavers suggested that ultrasound (US) could be used to place electrodes without apparent nerve injury next to peripheral nerves, similar to nerve catheter placement [1, 2]. These reports were followed by a small case series of patients receiving permanent implants, with generally good outcomes. US-guided placement allowed a percutaneous trial, preventing incision in nonresponders, and in many cases produced durable analgesia beyond 1 year. Percutaneous leads designed for spinal cord stimulation placed via US allowed the intraoperative testing of multiple different stimulation parameters. US visualization also allowed electrode placement superior or inferior to the nerve or even two parallel leads placed abreast of the nerve [3]. Historical uses of PNS came about after publishing of the gate control theory [4]. Wall and Sweet’s initial experiments with PNS essentially sought to put “gate control” to the test [5]. Early studies by multiple authors were promising, yet technical difficulties and patient selection problems were common [6–9]. Due to declining interest, lead design/technical improvement for peripheral nerve leads has lagged behind the comparative technical advances for spinal cord stimulation leads over the last two decades. Early versions of cuff electrodes and button electrodes have been largely replaced by the current commercial leads (flat lead with four circular contacts). Neurosurgical open procedures will likely continue to be the predominant method of placement of these devices. Whether the US-guided technique will serve as a method of trial only, will allow permanent placement in some anatomical areas, or will help develop the evidence basis for PNS remains to be answered.
1. CURRENT EVIDENCE
There are no major prospective studies to date, which has been chronicled recently by Bittar and Teddy [10]. Davis lamented this lack of evidence in an editorial on the subject of peripheral neuromodulation [11]. Questions regarding the role of neurolysis on the analgesia seen after PNS, placebo effects, physical therapy effects, analgesic drug changes, or merely increased attention to the patients’ needs were all raised as possible confounding factors. The largest clinical series in print are those from Eisenberg et al. [12] and the Cleveland Clinic [9]. In Eisenberg’s series, 46 patients with isolated painful neuropathies received PNS. They reported good results in 78% of patients and poor in 22%. Visual analog pain scores decreased from 69 ± 12 prior to surgery to 24 ± 28 postoperatively [12]. Four major etiologies were identified: nerve lesions following operation around the hip or knee, entrapment neuropathy, pain following nerve graft, or painful neuropathy after traumatic nerve injection [12]. In the Cleveland Clinic series, the most notable result was the high requirement for surgical revision, a mean of 1.6 operations per patient [9]. In some cases, a neuroma may be the cause of the neuropathic pain (Fig. 1).
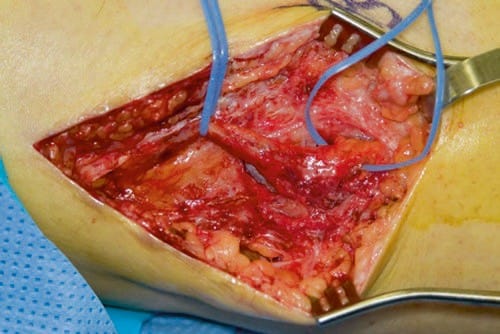
Fig.1 A peroneal nerve is depicted with large neuroma. (Photo courtesy of Spinner, Robert J., M.D. Mayo Clinic)
2. PATIENT SELECTION AND ROLE OF NEUROLYSIS
Patient selection for peripheral nerve procedures is of paramount importance. It is important to properly diagnose the condition, as many disorders are categorized as complex regional pain or “neuropathic pain” due to imprecise terminology. Sympathetically maintained syndromes may respond well to PNS implants, particularly if the pain is predominately in one nerve distribution [8, 9]. Pain that is resistant to previous surgical procedure such as transposition of the nerve and a neuroma in continuity with good functional preservation are other possible candidates. Pain that persists despite previous external or internal neurolysis may also be good candidates. Patients should- have previously failed good pharmacologic therapy with standard neuromodulatory drugs. External neurolysis refers to the removal of scar tissue around the nerve in circumferential fashion. If entrapment of the nerve is seen, it is mobilized and freed. External neurolysis poses little risk of fascicular injury. Nerve action potentials can be utilized to better assess nerve function than clinical or standard EMG/nerve conduction studies. Internal neurolysis can be used for pain syndromes, especially if incomplete loss of nerve function distally is present. The risk of fascicular injury or disruption is higher with internal neurolysis [13].
3. ANATOMICAL CONSIDERATIONS
One issue that complicates any peripheral nerve electrode placement in the four extremities is that nerves must freely glide within fascial/muscle planes along with their vascular supply as the extremity moves. Nerves can be entrapped by scar tissue, and the rough edges of an external electrode could, over time, cause constriction and scarring. Mixed peripheral nerves are also characterized by a complex internal fascicular arrangement. Briefly, nerve trunks may have sensory, motor, and mixed axons at various locations within the peripheral nerve. This complex cross-sectional anatomical configuration means that optimal stimulation of the desired sensory fascicle might, for example, be at the medial aspect of the ulnar nerve in a supracondylar placement but change location within a matter of a few millimeters to a posterior location. If the amplitude of stimulation is too high above sensory threshold, motor fascicles deeper within the trunk may easily be activated causing muscle cramping and/ or pain. A recent study looked at these issues more closely, specifically the effects of the fascicle perineural thickness, diameter, and position within the nerve trunk on axonal excitation thresholds and neural recruitment. A model of human femoral nerve within a nerve circumferential cuff electrode was studied. The study showed that stimulation of target fascicles is strongly dependent upon the cross-sectional anatomy of the nerve being stimulated. The mean thickness of perineurium was 3.0 ± 1.0% of the fascicle diameter. Increased thickness of the human perineurium or larger fascicle diameter increases the threshold for electrical activation. If a large neighbor fascicle was present, it could also affect stimulation activation of the target fascicle by as much as 80 ± 11% [14].
4. RADIAL NERVE STIMULATION
The radial nerve is very close to the lateral surface of the humerus at a point 10–14 cm proximal to the lateral epicondyle. The nerve is scaphoid shaped and superficial enough to be seen reasonably well under US. Ultrasound scanning usually begins at the elbow and, with the probe in a transverse orientation to the arm, continues proximally until the desired approach is identified. The needle can be advanced in-plane with the transducer to lie between the nerve and humerus. The lateral head of the triceps muscle is overlying the nerve here, and although one would desire to avoid transgression of large amounts of muscular tissue, there is no more optimal approach to the nerve in a superficial location above the humerus. Vascular structures including the profunda brachii artery and recurrent radial artery branch may be in anatomic proximity and should be scanned, as one would desire to avoid injuries to these structures [14]. The electrode(s) may be anchored in the superficial fascia of the triceps muscle. A tension loop at the site where the electrode exits the muscle is also desirable. Generator placement should be as close as possible to the leads to eliminate traction and lead migration. The fascicular arrangement of the radial nerve may not be favorable for the stimulation of more distal pain syndromes, e.g., the distal radial nerve sensory branch in locations above the elbow. In one patient in the first case series of US-guided stimulator placement, for example [3], the patient’s threshold between sensory and motor activation was too narrow to be therapeutic. A de Quervain’s tenosynovectomy, for example, may have caused injury to the superficial distal radial branch nerve. Thus, a better approach to stimulate this distal radial branch was in the mid-forearm, immediately deep to the brachioradialis muscle. Ultimately, the patient above [3] required open placement of a flat electrode at the distal superficial radial branch to improve her analgesia. Open operative findings included perineural scarring and neuroma. This branch could have been visualized with ultrasound near the radial artery where imaging may be improved by using color flow Doppler.
5. ULNAR NERVE STIMULATION
The ulnar nerve is very near the surface of the skin, superficial to the medial head of the triceps muscle. In the recent anatomical feasibility studies [1, 2], the nerve was identified at a point 9–13 cm proximal to the medial epicondyle in the medial/posterior arm, a location in which it was usually easily identifiable and also in close proximity to the humerus. Ultrasound scanning can commence at the elbow and, with the probe in a transverse orientation to the arm, continue to scan more proximally until the nerve fascicular arrangements can be well identified. The needle may be advanced from posterior to anterior on the medial aspect of the arm to lie between nerve and humerus, staying superficial to the medial head of the triceps. Often, patients with ulnar nerve pain syndromes such as cubital tunnel syndrome status-post failed transposition surgery may be good candidates. In these cases, the nerve may have already been surgically transposed, making it more easily identifiable. US may allow large neuromas to be visualized. The nerve passes into the cubital tunnel after passing into the ulnar groove behind the medial epicondyle. The cubital tunnel is formed by the aponeurotic arch of the flexor carpi ulnaris as its ceiling where the aponeurosis attaches to the medial epicondyle and olecranon, with the floor formed by the medial ligaments of the elbow and the flexor digitorum profundus muscle [14]. This area is a potential area of compression of the nerve.
6. MEDIAN NERVE STIMULATION
The median nerve enters the antecubital fossa medial to the biceps muscle and its tendon and next to the brachial artery. The artery serves as a good landmark to scan the neurovascular bundle, identify the median nerve, and continue to scan distally. In the upper forearm at a point approximately 4–6 cm distal to the antecubital crease, the nerve passes between the two heads of the pronator teres muscle and then passes under the sublimis bridge of the two heads of the flexor digitorum superficialis (Fig. 2). There are numerous potential neural fascicular communications between the median and ulnar nerves which are often in the forearm. The most important one is the Martin-Gruber anastomosis. Most of these Martin-Gruber anastomoses involve fibers from the median nerve passing to the ulnar nerve, with the reverse much less common. Other anomalous connections may exist as well. Interestingly, the very first series of PNS5 likely involved some type of abnormal connection, with both median and ulnar sensory distributions being stimulated by the application of stimulation to the ulnar nerve.
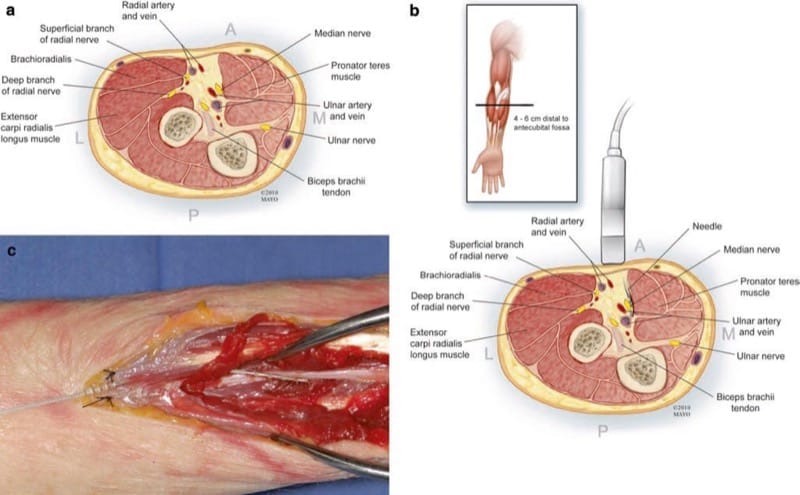
Fig.2 (a) Cross-sectional anatomy of the median nerve approxi-mately 4–6 cm distal to the antecubital fossa in the upper forearm. (b) A long axis in-plane US approach to the median nerve is depicted, keeping the needle and electrode closer to the muscle and avoiding the ulnar artery. (c) Fresh cadaver dissection after US-guided electrode placement. Anatomical entry site approximately 4–6 cm distal to the antecubital fossa (anchor sutured to superficial fascia) showing a lead placed longitudinally and lying anterior to the median nerve
Median nerve stimulation may be accomplished either superior to the elbow or inferior. Stimulation below the elbow might encounter one of these aberrant anastomoses or stimulate the nerve between the pronator heads where compression may be more likely.
7. SCIATIC NERVE AT POPLITEAL BIFURCATION
The common peroneal nerve may be identified at its branch point from the sciatic nerve, a point 6–12 cm proximal to the popliteal crease. Ultrasound scanning usually commences at the popliteal crease and, with the probe in a transverse orientation to the leg, continued proximally until the desired nerve is identified. Either transverse or longitudinal placement can be utilized, with transverse placement being more forgiving of movement, but a greater number of possible electrodes contacting the nerves with longitudinal placement. Location of the popliteal artery is noted to avoid vascular puncture during electrode placement. The needle may be advanced from posterolateral to anteromedial in a slightly oblique plane, attempting to avoid passing through the biceps femoris muscle (Fig. 3). The area distal to the bifurcation of the sciatic nerve, a short distance beyond the tibial branch is reasonably easily seen with ultrasound. The electrode can be anchored on the fascia of the biceps femoris muscle. During anatomical feasibility studies, the area near the fibular head was also evaluated for potential US-guided placements, but there is very little room to maneuver anatomically, and current leads are not well designed for this area. Supramalleolar areas may be attractive sites to target the superficial peroneal nerve but have not yet been attempted.
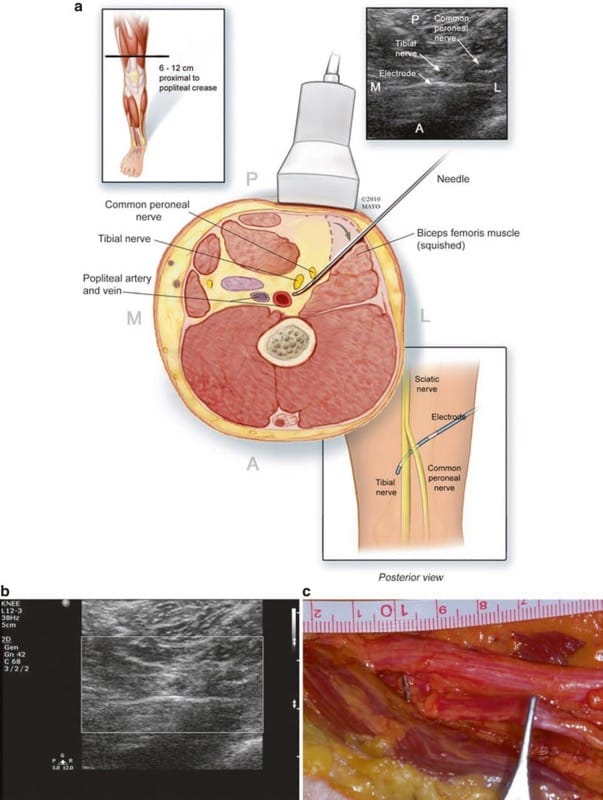
Fig.3 (a) Cross-sectional anatomy and technique of short-axis US visualization, with perpendicular electrode placement to cover both the tibial and common peroneal nerves. (b) An enlarged view of US view in (a). (c) Anatomical dissection of electrode placement just distal to sciatic bifurcation similar to (a) and (b) but passing between the tibial and common peroneal (CP) nerves. Note that two electrical contacts can be seen under the tibial and common peroneal nerve branches. The forceps are on the CP more distally.
8. POSTERIOR TIBIAL NERVE
The posterior tibial nerve can also be approached more distally in the leg. Approximately 8–14 cm proximal to the medial malleolus, the nerve is in close proximity to the tibialis posterior muscle, the digitorum profundus, one or two large veins, and the flexor hallucis longus. Ultrasound scanning usually begins at the ankle near the medial malleolus, with the probe in a transverse orientation to the leg, and is then continued proximally until the desired approach is identified. Location of the posterior tibial artery is noted to avoid vascular puncture during electrode placement. The needle may be advanced from anterior to posterior along the medial aspect of the ankle to lie just superficial (or deep) to the nerve. Care should be taken to minimize trauma to surrounding tissues and avoid transgression of these muscular structures. The pulse generator may be placed superficial to the fascia of the medial gastrocnemius muscle.
9. CONCLUSION
PNS may be accomplished using minimally invasive guidance. In general, performing permanent implantations should continue to be done in open fashion until both significant clinical experience is accomplished and long-term outcomes are clearer. Future prospective double-blinded studies and development of new electrodes may be helpful in furthering this minimally invasive technique.