Manoj K. Karmakar, Roy A. Greengrass, Malikah Latmore, and Matthew Levin
THORACIC PARAVERTEBRAL BLOCK
Thoracic paravertebral block (TPVB) is the technique of injecting local anesthetic alongside the thoracic vertebra close to where the spinal nerves emerge from the intervertebral foramen. This produces unilateral, segmental, somatic, and sympathetic nerve block, which is effective for anesthesia and in treating acute and chronic pain of unilateral origin from the chest and abdomen. Hugo Sellheim of Leipzig (1871–1936) is believed to have pioneered TPVB in 1905. Kappis, in 1919, developed the technique of paravertebral injection, which is comparable to the one in present day use.
Although paravertebral block (PVB) was fairly popular in the early 1900s, it seemed to have fallen into disfavor during the later part of the century; the reason for which is not known. In 1979, Eason and Wyatt re popularized the technique after describing paravertebral catheter placement. Our understanding of the safety and efficacy of TPVB has improved significantly in the last 25 years, with renewal of interest in this technique. Currently, it is used not only for analgesia but also for surgical anesthesia, and its application has been extended to children. Introduction of ultrasound to the practice of regional anesthesia led to the renewed efforts to increase safety and consistency of PVBs.
Anatomy
The thoracic paravertebral space (TPVS) is a wedge-shaped space located on either side of the vertebral column (Figure 1). The parietal pleura forms the anterolateral boundary. The base is formed by the vertebral body, intervertebral disc, and the intervertebral foramen with its contents.
The transverse process and the superior costotransverse ligament form the posterior boundary. Lying in between the parietal pleura anteriorly and the superior costotransverse ligament posteriorly is a fibroelastic structure, the endothoracic fascia, which is the deep fascia of the thorax (Figures 1 through 3). Medially, the endothoracic fascia is attached to the periosteum of the vertebral body. A layer of loose areolar connective tissue, the subserous fascia, lies between the parietal pleura and the endothoracic fascia.
Therefore, there are two potential fascial compartments in the TPVS: the anterior extrapleural paravertebral compartment and the posterior subendothoracic paravertebral compartment (see Figures 1 and 2). The TPVS contains adipose tissue within which lie the intercostal (spinal) nerve, the dorsal ramus, intercostal vessels, and rami communicantes and anteriorly the sympathetic chain. The spinal nerves are segmented into small bundles and lie freely in the adipose tissue of the TPVS, which make them accessible to local anesthetic solutions injected in the TPVS. The TPVS communicates with the epidural space medially and with the intercostal space laterally.
The TPVSs on either side of the thoracic vertebra also communicate with each other through the epidural and prevertebral space. The cranial extension of the TPVS is challenging to define and may significantly vary; however, there is direct paravertebral spread of radiopaque contrast medium from the thoracic to the cervical paravertebral space, indicating anatomic continuity. The TPVS also communicates caudally through the medial and lateral arcuate ligaments with the retroperitoneal space behind the fascia transversalis, where the lumbar spinal nerves are located.
Learn more about Neuraxial Anatomy.
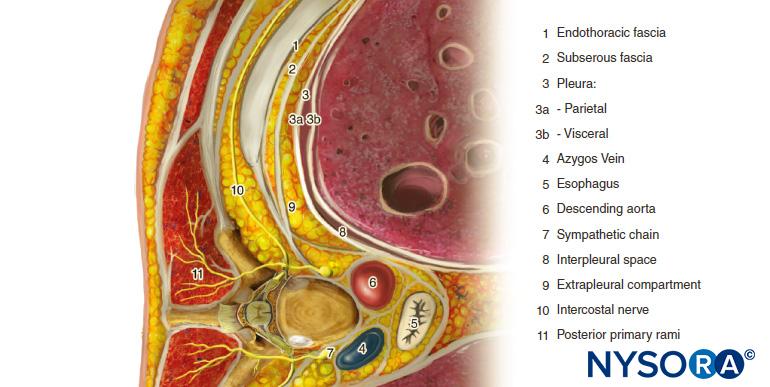
FIGURE 1. Anatomy of the thoracic paravertebral space, chest cavity and intercostal nerves.
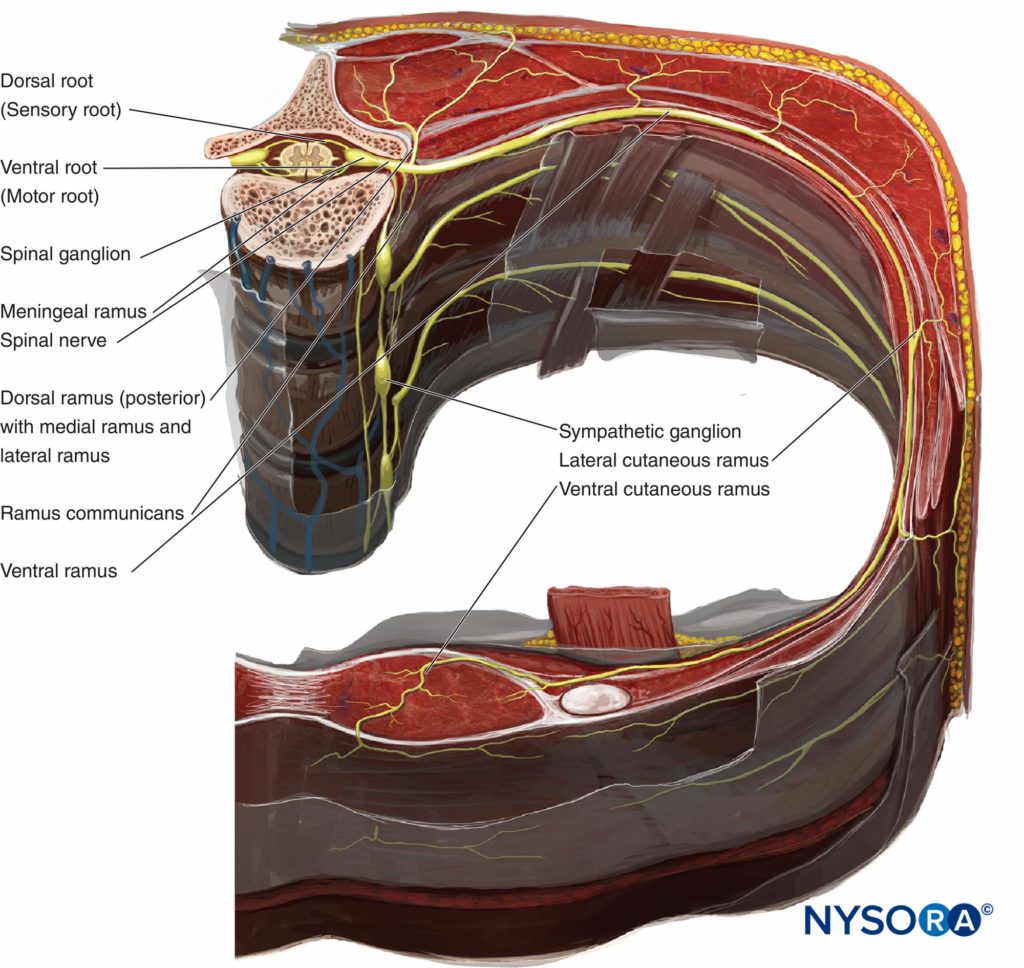
FIGURE 2. Crossectional anatomy of the vertebra and chest wall demonstrating relationship of paraveretbral space, sympathetic ganglia, spinal and intercostal nerves.
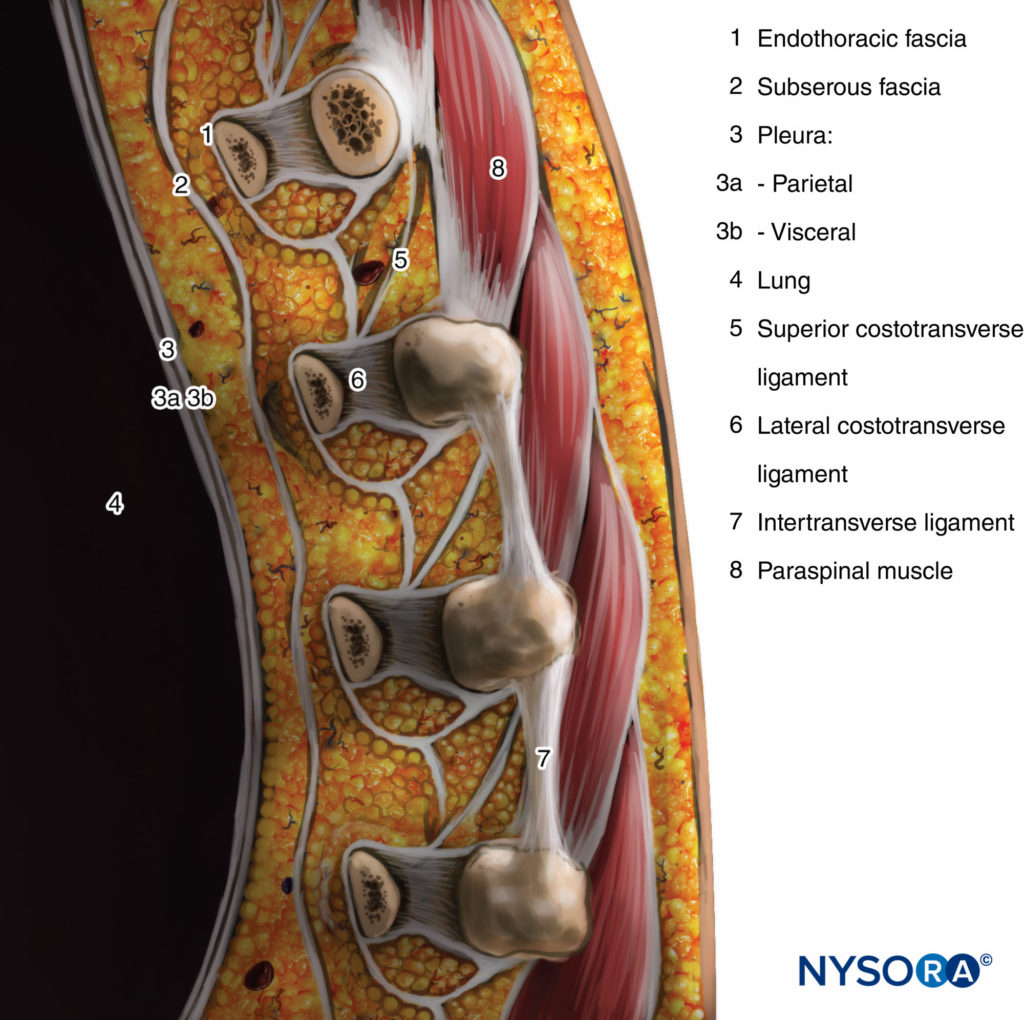
FIGURE 3. Sagittal section through the thoracic paravertebral space.
Mechanism of Block & Distribution of Anesthesia
TPVB produces ipsilateral somatic and sympathetic nerve block (Figure 4) due to a direct effect of the local anesthetic on the somatic and sympathetic nerves in the TPVS, extension into the intercostal space laterally, and the epidural space medially. The overall contribution of epidural spread to the dermatomal distribution of anesthesia following a TPVB is not well defined. However, some degree of ipsilateral spread of local anesthetic toward the epidural space probably occurs in the majority of the patients, resulting in a greater distribution of anesthesia than occurs with paravertebral spread alone. The dermatomal distribution of anesthesia following a single injection of a large volume varies and is often unpredictable, but the injected solutions routinely spread both cephalad and caudad to the site of injection to some extent (Figure 5). Nevertheless, the multiple injection technique, where small volumes (3–4 mL) of local anesthetic are injected at several contiguous thoracic levels, is preferable over single, large-volume injection. This is particularly important when reliable anesthesia over several ipsilateral thoracic dermatomes is desired, such as when TPVB is used for anesthesia during breast surgery. Segmental contralateral anesthesia, adjacent to the site of injection, occurs in approximately 10% of patients after single-injection TPVB and may be due to epidural or prevertebral spread.

FIGURE 4. Segmental thoracic anesthesia achieved with paravertebral blocks.
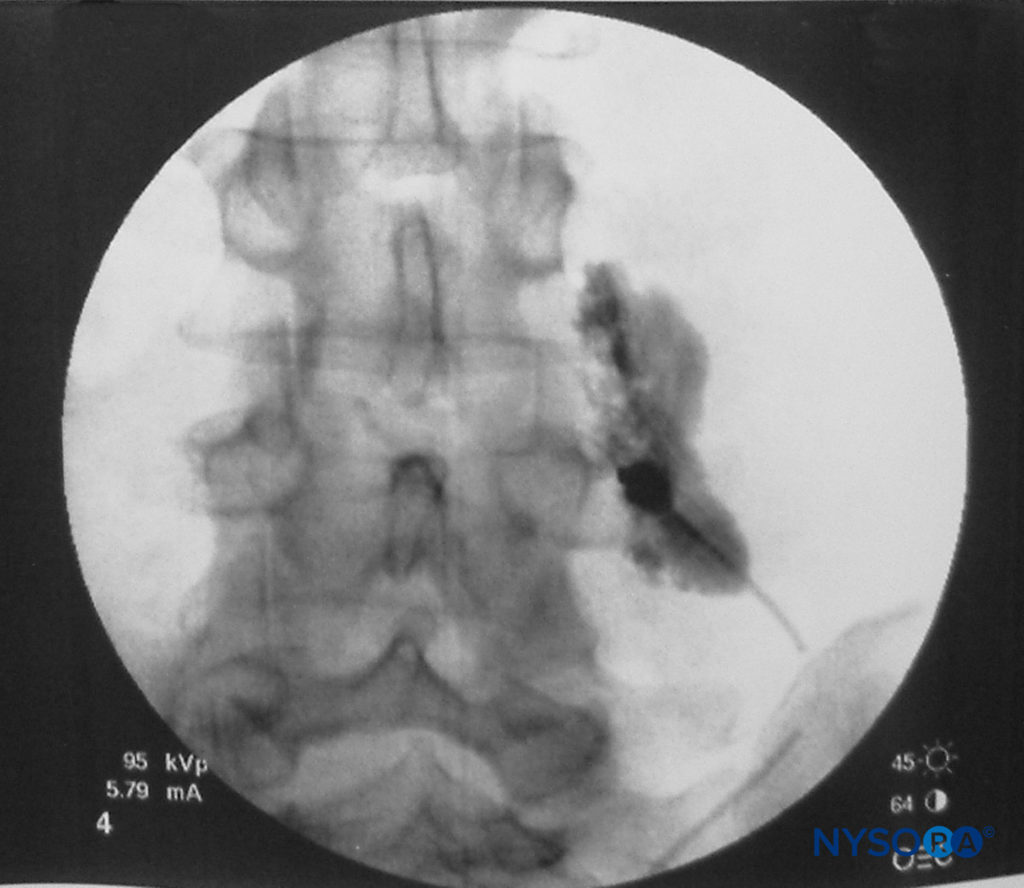
FIGURE 5. Spread of 3 mL of local anesthetic solution after a paravertebral block (lumbar spine).
Bilateral symmetric anesthesia due to extensive epidural spread or unintentional intrathecal injection into a dural sleeve may occur, particularly when the needle is directed medially or when a larger volume of local anesthetic (>25 mL) is used. For this reason, patients should be monitored using the same vigilance and methods as those employed for injection using the large-volume, single-injection epidural technique. The ipsilateral ilioinguinal and iliohypogastric nerves may also occasionally be involved after lower thoracic paravertebral injections. This is either due to epidural spread or extended subendothoracic fascial spread to the retroperitoneal space where the lumbar spinal nerves are located. The effect of gravity on the dermatomal spread of anesthesia after TPVB is unknown, but there may be a tendency for preferential pooling of injected solution toward the dependent levels.
- Technique
It is preferable to perform TPVB with the patient in the sitting position because the surface anatomy is better visualized and patients are often more comfortable. However, when this is not possible or practical, TPVB can also be performed with the patient in the lateral, or prone position. The number and levels of injections are selected according to the desired spread of local anesthesia. In this example, description of the TPVB for breast surgery is described. Surface landmarks are identified and marked with a skin marker before block placement (Figure 6). Skin markings are also made 2.5 cm lateral to the midline at the thoracic levels that are to be blocked.
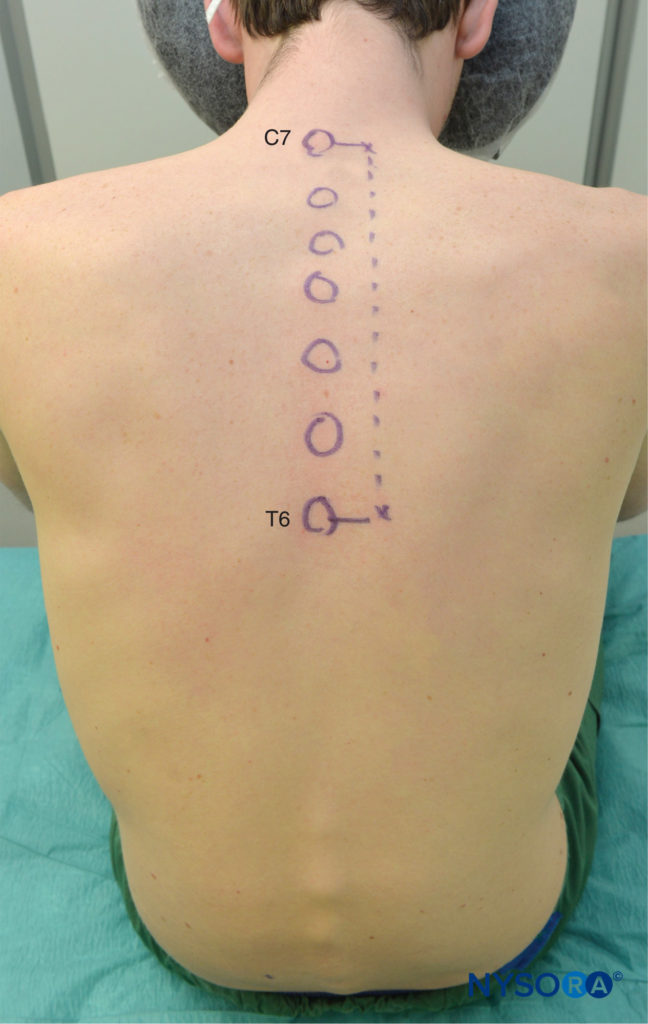
FIGURE 6. Surface landmarks for thoracic paravertebral blocks.
These markings indicate the needle insertion sites and should lie over the transverse process of the vertebra (Figure 7). A standard regional anesthesia tray is prepared, and strict asepsis should be maintained during block placement. A 22-gauge Tuohy needle is recommended for TPVB (Figure 8). Ideally, the needle should have depth markings on its shaft. Alternatively, a depth guard (see Figure 8) is recommended. An epidural set is used if insertion of a catheter into the TPVS is planned. TPVB requires proper premedication to ensure patient acceptance and comfort during block placement.
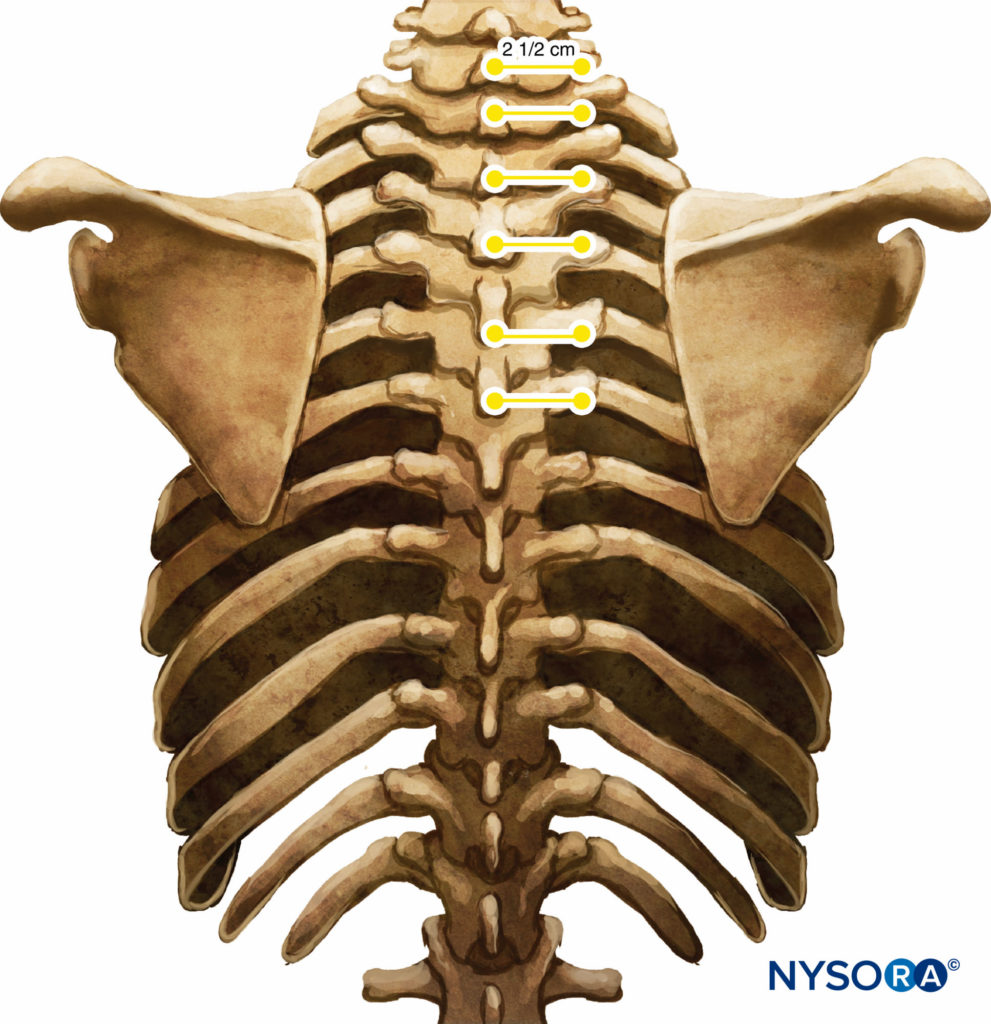
FIGURE 7. Relationship between spinous and transverse processes.
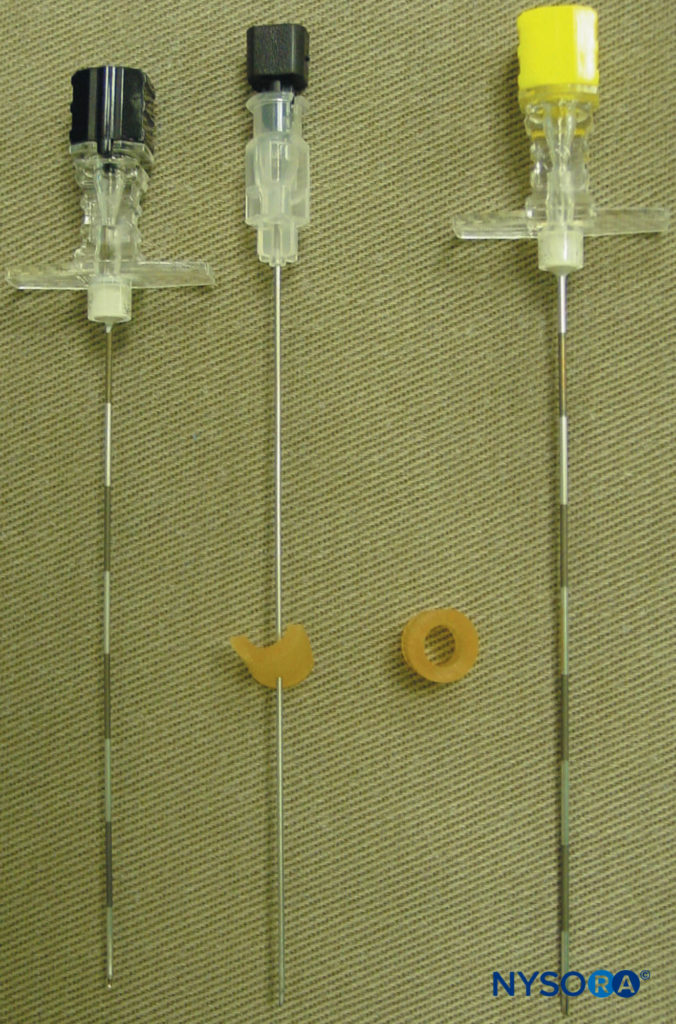
FIGURE 8. Needles commonly used for a single- or multiple-injection thoracic paravertebral block. Note the depth guard that is attached to the needle for assessment of the depth.
Loss-of-Resistance Technique
There are several different techniques of TPVB. The classic technique involves eliciting loss of resistance. The skin and underlying tissue is infiltrated with lidocaine 1%, and the block needle is inserted perpendicular to the skin in all planes to contact the transverse process of the vertebra. Note that due to the acute angulation of the thoracic spines in the midthoracic region, the transverse process that is contacted is the one from the lower vertebra (Figures 9 and 10).
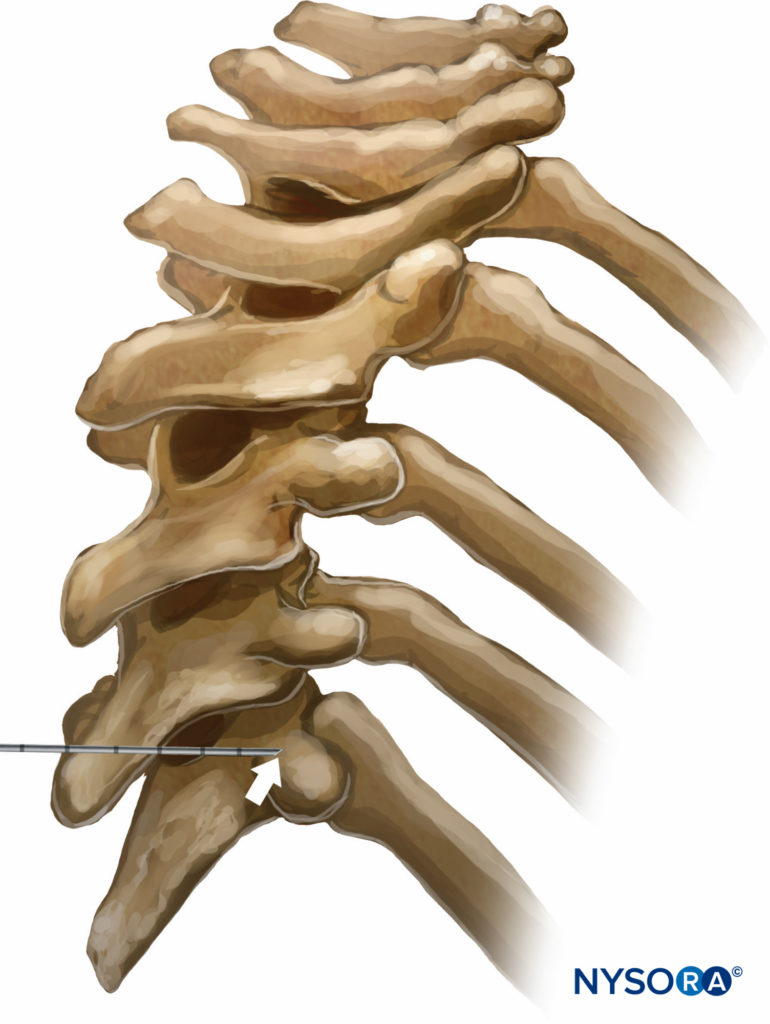
FIGURE 9. Relationship between the spinous and transverse processes at the thoracic level. Due to the steep downward angulation of the spinous processes at the thoracic levels, the needle inserted at the level of the spinous process contacts the transverse process that belongs to the vertebra below it.
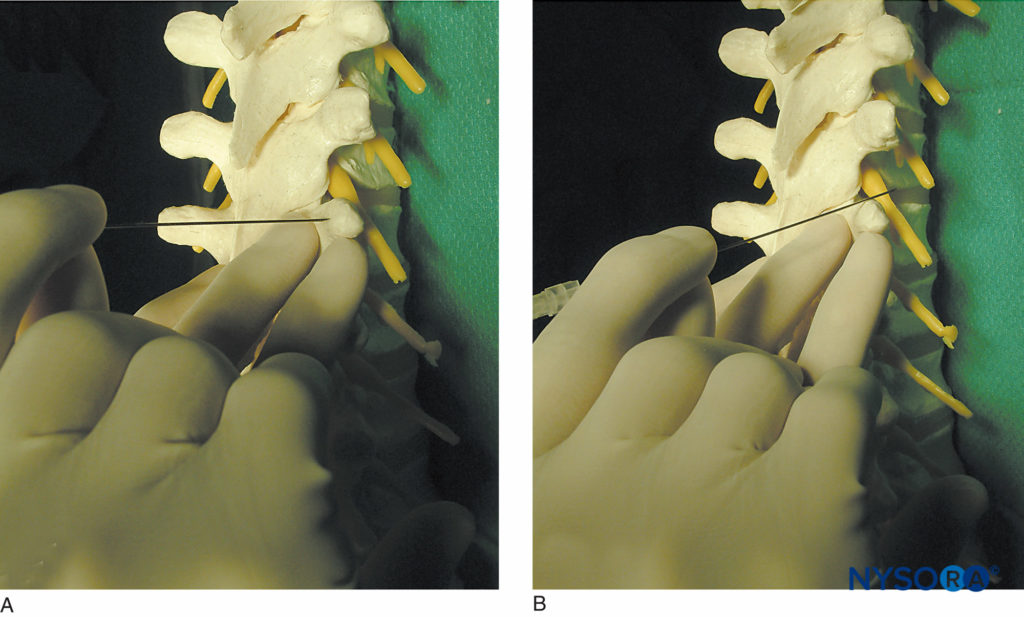
FIGURE 10. Technique of “walking off” the transverse process. A: The needle is shown contacting the transverse process. B: The needle is shown walking off the superior aspect of the transverse process. Walking off the inferiorly may be safer at thoracic level.
The depth at which the transverse process is contacted varies (3–4 cm) and depends on the build of the individual and the level at which the needle is inserted. The depth is deeper at the cervical and lumbar spine level and shallower at the thoracic levels.
During needle insertion, it is possible to miss the transverse process and inadvertently puncture the pleura. Therefore, it is imperative to search and make contact with the transverse process before advancing the needle too deep and risking pleural puncture. To minimize this complication, the block needle should initially be inserted only to a maximum depth of 4 cm at thoracic and 5 cm at cervical and lumbar levels. If the bone is not contacted, it should be assumed that the needle is in between two adjacent transverse processes. The needle should be withdrawn to the subcutaneous tissue and reinserted with a cephalad or caudad direction to the same depth (4 cm) until the bone is contacted.
If bone is still not encountered, the needle is advanced a further centimeter and the above procedure repeated until the transverse process is identified. The needle is then walked above or bellow (safer) the transverse process and gradually advanced until a loss of resistance is elicited as the needle traverses the superior costotransverse ligament into the TPVS (Figure 11; see Figure 3).
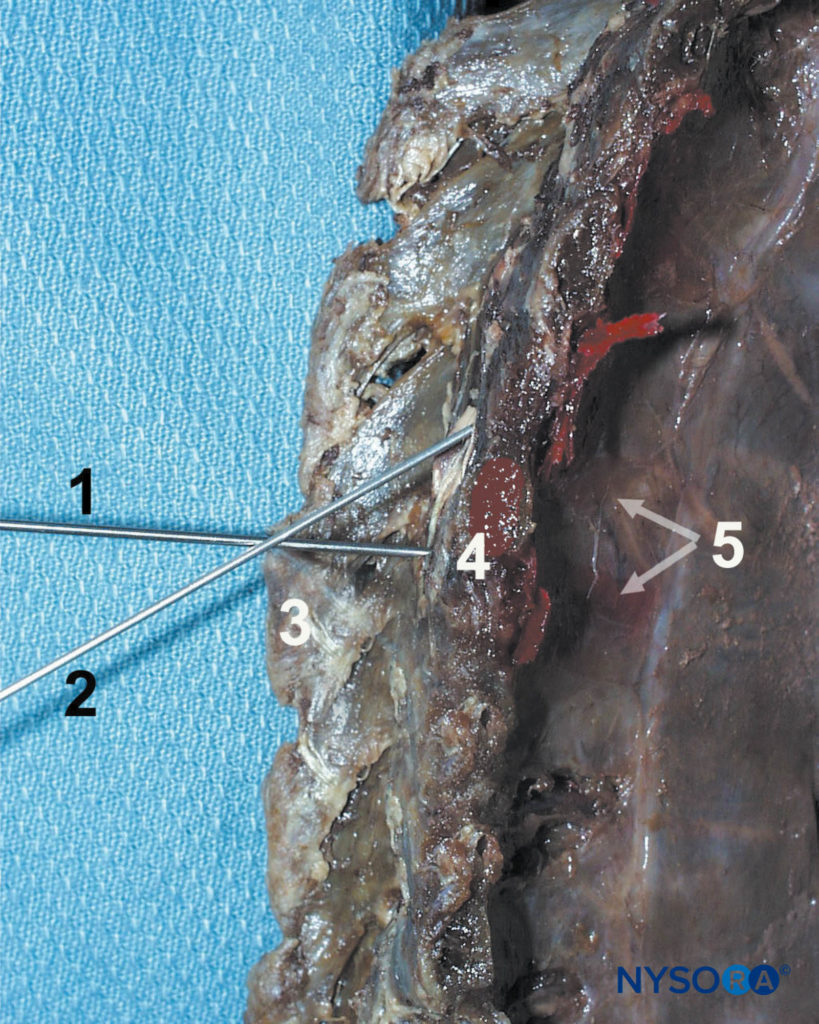
FIGURE 11. Paravertebral block technique. The needle (1) is first advanced to contact the transverse process (4), then redirected cephalad (2) or caudad to walk off the transverse process and enter the paravertebral space. Other structures shown are spinous process (3) and the dispersion of the dye in the paravertebral space and intercostal sulcus.
NYSORA Tips
- “Walking” off the lower aspect of the transverse process is recommended in case the needle has contacted the rib rather than transverse process. When this happens, walking off the rib cephalad may result in pneumothorax.
- This usually occurs within 1.0–1.5 cm from the superior edge of the transverse process (see Figure 3). Although a subtle “pop” or “give” may be appreciated as the needle traverses the superior costotransverse process, this should not be entirely relied on. Instead, the depth of the needle placement should be guided by the initial bone contact (skin-transverse process + 1.0–1.5 cm).
Predetermined Distance Technique
TPVB can also be performed by advancing the needle by a fixed predetermined distance (1 cm) once the needle is walked off the transverse process, without eliciting loss of resistance (Figure 12A and B). Proponents of this technique have used it very successfully with low risk of pneumothorax. The use of a depth marker is recommended to avoid inadvertent pleural or pulmonary puncture.
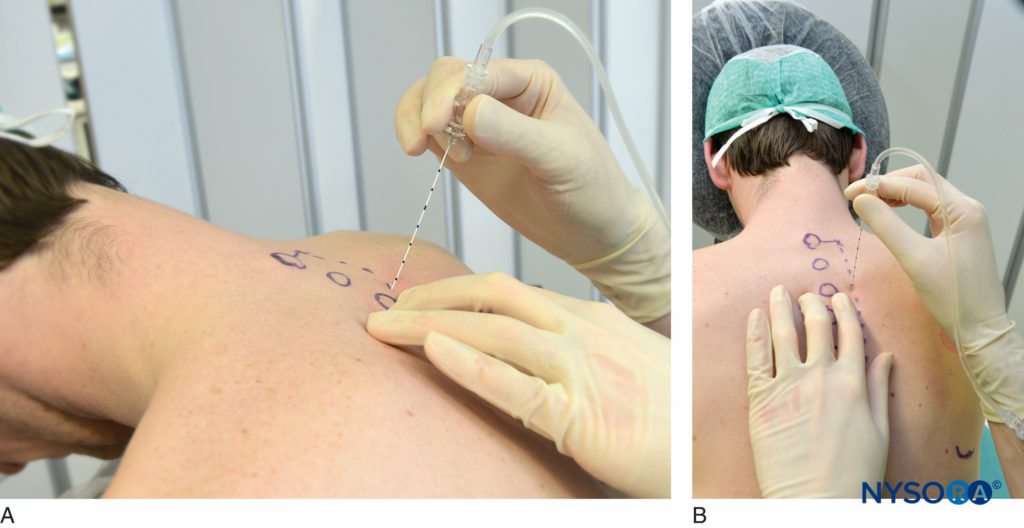
FIGURE 12. Needle angle to contact the transverse process (A) and to walk off the transverse process inferiorly (B). Once the transverse process is contacted, the needle is walked off and inserted 1.5 cm deeper while paying attention to the depth marks or using a rubber stopper (Figure 8).
NYSORA Tips
- Perform TPVB with the patient in the sitting position.
- Surface landmarks should always be identified and marked with a skin marker.
- Use needles with depth markings to facilitate estimation of the depth of insertion.
- It is imperative to search and make contact with the transverse process before advancing the needle any farther.
- The depth at which the transverse process is contacted varies in the same patient at different thoracic levels. It is deepest in the cervical, upper and lower thoracic, and shallowest in the midthoracic region.
- The needle should not be advanced more than 1.5 cm beyond the contact with the transverse process.
- Avoid directing the needle medially to prevent inadvertent epidural or intrathecal needle misadventure.
- Placement of Thoracic Paravertebral Catheter
If a continuous TPVB (CTPVB) is planned, a catheter is inserted through a Tuohy needle into the TPVS. Unlike epidural catheterization, certain resistance is commonly encountered during insertion of the paravertebral catheter. This can be facilitated by injecting 5–10 mL of saline to create a space before catheter insertion. An unusually seamless passage of catheter should arouse the suspicion of interpleural placement. Perhaps the safest and simplest method of placing a catheter into the TPVS is to place it under direct vision from within the open chest cavity. Obviously, this requires an open thorax and is, therefore, done exclusively in patients undergoing a thoracotomy.
This technique involves reflecting the parietal pleura from the posterior wound margin onto the vertebral bodies over several thoracic segments, thereby creating an extrapleural paravertebral pocket (Figure 13) into which a percutaneously inserted catheter is placed against the angles of the exposed ribs. The pleura is reapposed to the chest wall, and the thorax is closed. This method can be combined very effectively with a preincisional, single-shot, percutaneous thoracic paravertebral injection to provide perioperative analgesia during thoracic surgery.
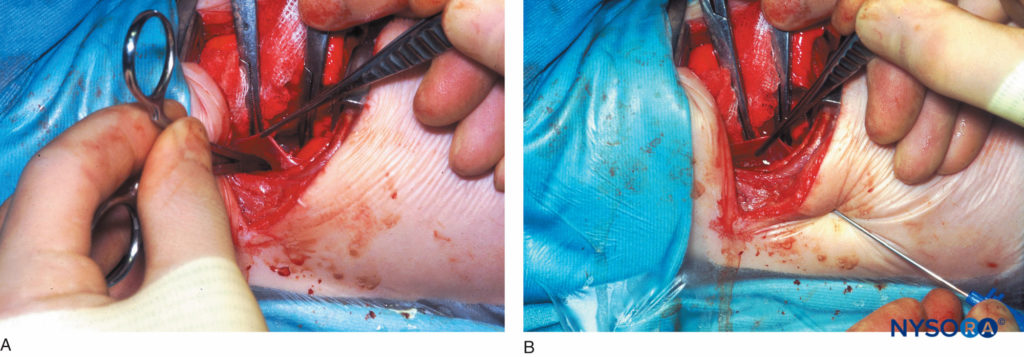
FIGURE 13. A: Extrapleural paravertebral catheter placement under direct vision in an infant. Figure shows a curved artery forceps that has been inserted into the extrapleural paravertebral pocket that was created by reflecting the parietal pleura from the posterior wound margin on to the vertebral bodies over several thoracic dermatomes. B: Extrapleural paravertebral catheter placement under direct vision in an infant. Figure shows a Tuohy needle that has been inserted from the lower intercostal space into the thoracic paravertebral space; .i.e, the extrapleural paravertebral pocket previously created. A catheter is then inserted through the Tuohy needle and secured in place against the angles of the exposed ribs, after which the pleura is reapposed and the chest is closed.
NYSORA Tips
- Injecting saline or the bolus dose of the local anesthetic before catheter insertion makes it easier to insert a catheter.
- Very easy passage of catheter (>6 cm) should raise the suspicion of intrapleural placement.- Catheter should not be inserted >3 cm to prevent their migration toward epidural space.
Indications
TPVB is indicated for anesthesia and analgesia for unilateral surgical procedures in the chest and abdomen. Commonly reported indications are listed in Table 1. The use of bilateral TPVB has also been reported.
TABLE 1. Indications for thoracic paravertebral block.
| Anesthesia |
|---|
| Breast surgery |
| Herniorrhaphy (thoracolumbar anesthesia) |
| Chest wound exploration |
| Postoperative Analgesia (as part of a balanced analgesic regimen) |
|---|
| Thoracotomy |
| Thoracoabdominal esophageal surgery |
| Video-assisted thoracoscopic surgery |
| Cholecystectomy |
| Renal surgery |
| Breast surgery |
| Herniorrhaphy |
| Liver resection |
| Appendicectomy |
| Minimally invasive cardiac surgery |
| Conventional cardiac surgery (bilateral TPVB) |
| Chronic Pain Management |
|---|
| Benign and malignant neuralgia |
| Miscellaneous |
|---|
| Postherpetic neuralgia |
| Relief of pleuritic chest pain |
| Multiple fractured ribs |
| Treatment of hyperhydrosis |
| Liver capsule pain after blunt abdominal trauma |
Contraindications
Contraindications for TPVB include infection at the site of injection, allergy to local anesthetic drug, empyema, and a neoplastic mass occupying the paravertebral space. Coagulopathy, bleeding disorders, or patients receiving anticoagulant drugs are relative contraindication for TPVB. One must exercise caution in patients with kyphoscoliosis or deformed spines and those who have had previous thoracic surgery. The chest deformity in the former may predispose to inadvertent thecal or pleural puncture, and the altered paravertebral anatomy due to fibrotic obliteration of the paravertebral space or adhesions of the lung to the chest wall in the latter may predispose to pulmonary puncture.
Choice of Local Anesthetic
Since TPVB does not result in motor weakness of the extremities, long-lasting analgesia is nearly always desirable with TPVB. Consequently, long-acting local anesthetic drugs are typically used. These include bupivacaine or levobupivacaine 0.5% and ropivacaine 0.5%. For single-injection TPVB, 20–25 mL of local anesthetic is injected in aliquots, whereas for multiple-injection TPVB, 4–5 mL of local anesthetic is injected at each level planned. The maximum dose of local anesthetic must be adjusted in the elderly, poorly nourished, and frail patients.
The TPVS is well vascularized, leading to relatively rapid absorption of local anesthetic into the systemic circulation. Consequently, peak plasma concentration of the local anesthetic agent is attained quickly. Epinephrine (2.5–5.0 mcg/mL) containing local anesthetic solutions may be used during the initial injection because it reduces systemic absorption and thereby reduces the potential for toxicity.
Epinephrine also helps in increasing the maximum allowable dose of local anesthetic. The duration of anesthesia after TPVB ranges from 3–4 h, but analgesia often lasts much longer (8–18 h). If a continuous TPVB (CTPVB) is planned, e.g., for postoperative analgesia after thoracotomy or continuous pain relief for multiple fractured ribs, then an infusion of bupivacaine or levobupivacaine 0.25% or ropivacaine 0.2% at 0.1–0.2 mL/kg/h is started after the initial bolus injection and continued for 3–4 days or as indicated. It is our experience that using a higher concentration of local anesthetic (e.g., bupivacaine 0.5% instead of 0.25%) for the CTPVB does not result in better quality of analgesia, and it may increase the potential for local anesthetic toxicity.
NYSORA Tips
- Consider using lidocaine or chloroprocaine for skin and subcutaneous infiltration to reduce the total dose of the more toxic long- acting local anesthetic.
- Use an epinephrine-containing (e.g., 1:200 000 or 1:400 000) long-acting local anesthetic because it reduces systemic absorption and, therefore, the potential for systemic toxicity.
- Local anesthetic dose should be adjusted in the elderly and those with impairment of hepatic and renal function.
Practical Management of Thoracic Paravertebral Block
Breast Surgery
Thoracic paravertebral injection of local anesthetic at multiple levels (C7 through T6) in conjunction with intravenous sedation is effective for surgical anesthesia during major breast surgery (Figure 14). The C7 spinous process is the most prominent cervical spinous process; the inferior border of the scapula corresponds to T7. Compared with patients who receive only general anesthesia (GA), patients who receive a multiple-injection TPVB for major breast surgery have less postoperative pain, require fewer analgesics, and have less nausea and vomiting after surgery.
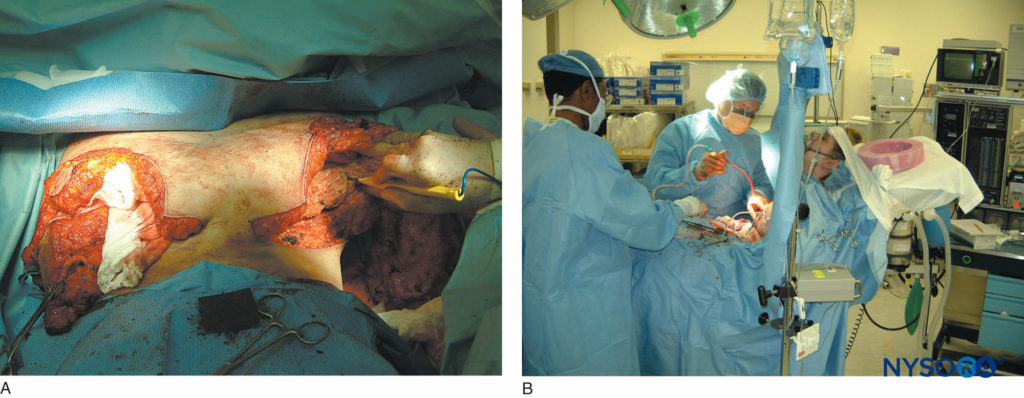
FIGURE 14. A: Extensive breast reconstruction surgery being performed under paravertebral block. B: The patient is sedated using propofol infusion. The images demonstrate how potent paravertebral blocks can be both as anesthetic and analgesic techniques.
However, to effectively use the multiple-injection TPVB technique for anesthesia during breast surgery, one must understand the complex innervation of the breast. The anterior and lateral chest wall receives sensory innervation from the anterior and lateral cutaneous branches of the intercostal nerves (T2 through T6), the axilla (T1–T2), the infraclavicular region from the supraclavicular nerves (C4–C5), and the pectoral muscles from the lateral (C5–C6) and medial (C7–C8) pectoral nerves.
There also may be overlapping sensory innervation from the contralateral side of the chest. This complex innervation of the breast from the C4–T6 spinal segments explains why TPVB may not provide complete anesthesia for dissection over the pectoral muscle or the infraclavicular region. However, this can be overcome with proper sedation during surgery as well as by injections of local anesthetic by the surgeon intraoperatively into the sensitive areas. Injection of a local anesthetic subcutaneously along the inferior border of the clavicle or to perform ipsilateral superficial cervical plexus block in order to anaesthetize the supraclavicular nerves (C4–C5) will minimize discomfort and sedative and analgesic requirements during surgery.
A combination of midazolam, or propofol infusion, or IV opioid can be used to provide comfort to the patients intraoperatively. Dexmedetomidine, a highly selective α2-adrenoceptor agonist, with its sedative, analgesic, and minimal or no respiratory depression properties, is a useful alternative for sedation during breast surgery under TPVB.
When combined with general anesthesia, a single injection TPVB with ropivacaine (2 mg/kg diluted to 20 mL with 0.9% saline) with 1:200 000 epinephrine, performed prior to the induction of GA can be used. This provides excellent postoperative analgesia, reduces postoperative analgesic requirement, reduces postoperative vomiting, facilitates the earlier resumption of oral fluid intake, reduces the postoperative decline in respiratory function, and augments the recovery of postoperative respiratory mechanics.
Postthoracotomy Pain Relief
CTPVB is an effective method of providing analgesia after thoracotomy (Figure 15). Ideally, TPVB should be established before the thoracotomy incision, via a catheter that is inserted percutaneously, and continued for 4–5 days after surgery. However, if an extrapleural paravertebral catheter is being placed under direct vision from within the chest during surgery, then a single-injection TPVB can be performed at the level of the thoracotomy incision before the surgical incision, and a continuous infusion of local anesthetic is commenced after the catheter placement. Analgesia achieved by CTPVB is comparable to epidural analgesia but with less hypotension, urinary retention, and the side effects commonly seen with epidural opioid administration. The opioid requirement with such an approach is significantly reduced by the CTPVB, and analgesia is superior to IVPCA alone.
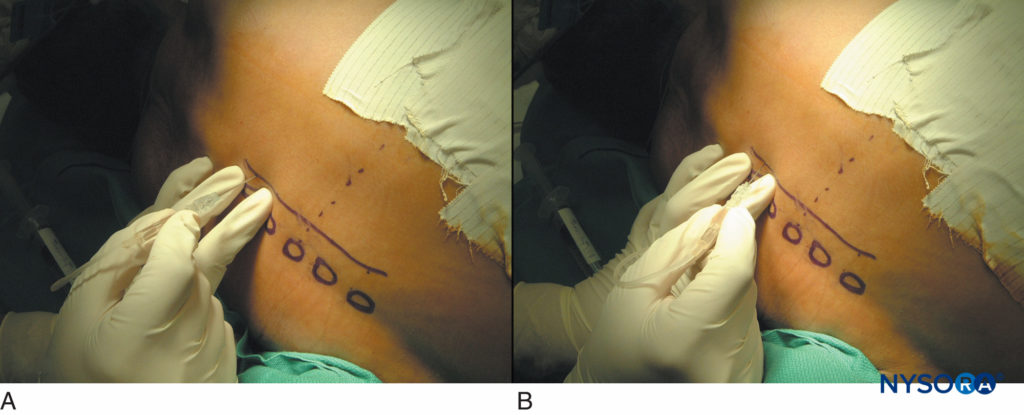
FIGURE 15. Thoracic paravertebral blocks in patients after thoracotomy. A typical sequence of touching the transverse process (A) and walking off 1 cm deeper to the transverse process superiorly or inferiorly (B).
Multiple Fractured Ribs
TPVB is an effective method of providing pain relief in patients with unilateral multiple fractured ribs. A single thoracic paravertebral injection of 25 mL of bupivacaine 0.5% produces pain relief for a mean duration of 10 h and improves respiratory function and arterial blood gases. To avoid recurrence of pain and deterioration in respiratory function, a thoracic paravertebral catheter can be inserted midway between the highest and the lowest fractured rib, and a CTPVB can be commenced after administration of the initial bolus injection.
CTPVB in combination with an NSAID provides continuous pain relief and produces a sustained improvement in respiratory parameters and arterial oxygenation. Since TPVB does not cause urinary retention or affect lower limb motor function, it is useful in patients with multiple fractured ribs who also have concomitant lumbar spinal trauma since it also allows continuous neurologic assessment for signs of spinal cord compression.
Pharmacokinetic Considerations
Relatively large doses of local anesthetics are commonly used during CTPVB. Therefore, there is the potential for local anesthetic toxicity, and patients should be closely monitored during CTPVB and the infusion stopped if signs develop. During a prolonged thoracic paravertebral infusion there is progressive accumulation of local anesthetic in the plasma, and the plasma concentration of the drug may exceed the threshold for central nervous system toxicity (e.g, 2.0–4.5 mcg/mL for bupivacaine). Despite the systemic accumulation, local anesthetic toxicity is rare. This may be the case because, although the total plasma concentration of the local anesthetic increases postoperatively, the free fraction of the drug remains unchanged and may be due to the postoperative increase in α1-acid glycoprotein concentration, the protein that binds to local anesthetic drugs. There is also a greater increase in the S-bupivacaine enantiomer, which is associated with lower toxicity, than the R-enantiomer. Due to concerns of systemic accumulation and local anesthetic toxicity with prolonged paravertebral infusion, it is preferable to use a local anesthetic with lower potential for toxicity, such as ropivacaine. One must also exercise caution in the elderly and frail patients as well as in patients with impaired hepatic and renal function.
Complications and How to Avoid Them
Based on published data the incidence of complications after TPVB is relatively low and varies from 2.6%–5%. These include vascular puncture (3.8%), hypotension (4.6%), pleural puncture (1.1%), and pneumothorax (0.5%). Unlike with thoracic epidural anesthesia, hypotension is rare in normovolemic patients after TPVB because the sympathetic block is unilateral. However, TPVB may unmask hypovolemia and result in hypotension. Therefore, TPVB should be used with caution in patients who are hypovolemic or hemodynamically labile. Nevertheless, hypotension is rare even after bilateral TPVB, probably owing to the segmental nature of the bilateral sympathetic block.
Pleural puncture and pneumothorax are two complications that often dissuade anesthesiologists from performing a TPVB. Inadvertent pleural puncture is uncommon after TPVB and may not result in a pneumothorax, which is usually minor and can be managed conservatively. Clues that suggest pleural puncture during a TPVB pronounced loss of resistance as the needle enters chest cavity, cough, onset of sharp chest or shoulder pain, or sudden hyperventilation. Contrary to the common belief, air cannot be aspirated through the needle unless the lung is also inadvertently punctured or air that may have entered the pleural cavity during removal of the stylet is aspirated. Such patients should be closely monitored for the possible development of a pneumothorax. It should be kept in mind that pneumothorax may be delayed in onset and a chest radiograph taken too early to exclude a pneumothorax may not be conclusive. Even a radiologic contrast study using a chest radiograph may be difficult to interpret because the intrapleural contrast disperses rapidly, does not define any specific anatomic plane, and tends to spread to the diaphragmatic angles or horizontal fissure. Systemic local anesthetic toxicity can occur due to inadvertent intravascular injection or from using an excessive dose of local anesthetic. The local anesthetic solution must be injected in aliquots, and the dosage must be adjusted in the elderly and frail patient. An epinephrine-containing local anesthetic solution is suggested to enable the recognition of the intravascular injection and reduce the absorption of the local anesthetic in the systemic circulation. Inadvertent epidural, subdural, or intrathecal injection and spinal anesthesia can also occur. Published data suggest that these complications are more frequent when the needle is directed medially but may also occur with a normally positioned needle due to the close proximity of the needle to the dural cuff and intervertebral foramen. Therefore, the needle must never be directed medially, and care must be taken to exclude intrathecal injection by routinely performing an aspiration test before injection. Transient ipsilateral Horner syndrome can occasionally develop after TPVB. This is due to cephalad spread of the local anesthetic to the stellate ganglion or to the preganglionic fibers of the first few segments of the thoracic spinal cord. Bilateral Horner syndrome has also been reported and may be due to epidural spread or prevertebral spread to the contralateral stellate ganglion. Sensory changes in the arm and lower extremity may also occur after a TPVB. The former is due to spread of local anesthetic to the lower components of the ipsilateral brachial plexus (C8 and T1), and the latter is due to extended subendothoracic fascial spread to the ipsilateral retroperitoneal space where the lumbar spinal nerves are located (discussed earlier), but epidural spread as a cause cannot be excluded. Motor block or bilateral symmetric anesthesia involving the lower extremity is rare. It generally suggests significant epidural spread and may be more common if large volumes of local anesthetic (>25–30 mL) are injected at a single level. Therefore, if a wide segmental spread of anesthesia is desired, it is preferable to perform the multiple-injection technique or inject a smaller volume of local anesthetic at several levels a few dermatomes apart.
LUMBAR PARAVERTEBRAL BLOCK
Lumbar paravertebral block (LPVB) is technically similar to a TPVB but due to differences in anatomy between the thoracic and lumbar paravertebral spaces the two paravertebral techniques are described separately. LPVB is used most commonly in combination with a TPVB, as a thoracolumbar paravertebral block, for surgical anesthesia during inguinal herniorrhaphy.
Anatomy
The lumbar paravertebral space (LPVS) is limited anterolaterally by the psoas major muscle; medially by the vertebral bodies, the intervertebral discs, and the intervertebral foramen with its contents; and posteriorly by the transverse process and the ligaments that are interposed between the adjoining transverse processes. Unlike the TPVS, which contains adipose tissue, the LPVS is occupied primarily by the psoas major muscle. The psoas major muscle is made up of a fleshy anterior part that forms the main bulk of the muscle, and a thin accessory posterior part. The main bulk originates from the anterolateral surface of the vertebral bodies and the accessory part originates from the anterior surface of the transverse process. The two parts fuse to form the psoas major muscle except near the vertebral bodies where the two parts are separated by a thin fascia within which lie the lumbar spinal nerve roots and the ascending lumbar veins. The ventral rami of the lumbar spinal nerve roots extend laterally in this intramuscular plane formed by the two parts of the psoas major muscle and form the lumbar plexus within the substance of the psoas major muscle. The psoas muscle is enveloped by a fibrous sheath, “the psoas sheath,” which continues laterally as the fascia covering the quadratus lumborum muscle. During a LPVB the local anesthetic is injected anterior to the transverse process into a triangular space between the two parts of the psoas major muscle containing the lumbar spinal nerve root. The LPVS communicates medially with the epidural space.
A series of tendinous arches extends across the constricted parts of the lumbar vertebral bodies, which are traversed by the lumbar arteries and veins and sympathetic fibers. These tendinous arches may provide a pathway for the spread of local anesthetic from the LPVS to the anterolateral surface of the vertebral body, the prevertebral space, and the contralateral side and may be the pathway through which the ipsilateral lumbar sympathetic chain may occasionally be involved.
Learn more about Neuraxial Anatomy.
- Mechanism of Block and Distribution of
Anesthesia
A lumbar paravertebral injection produces ipsilateral dermatomal anesthesia (Figure 16) by a direct effect of the local anesthetic on the lumbar spinal nerves and by medial extension into the epidural space via the intervertebral foramen. The contribution of epidural spread to the overall distribution of anesthesia after a LPVB is unknown but probably occurs in the majority of patients and depends on the volume of local anesthetic injected at a given level.
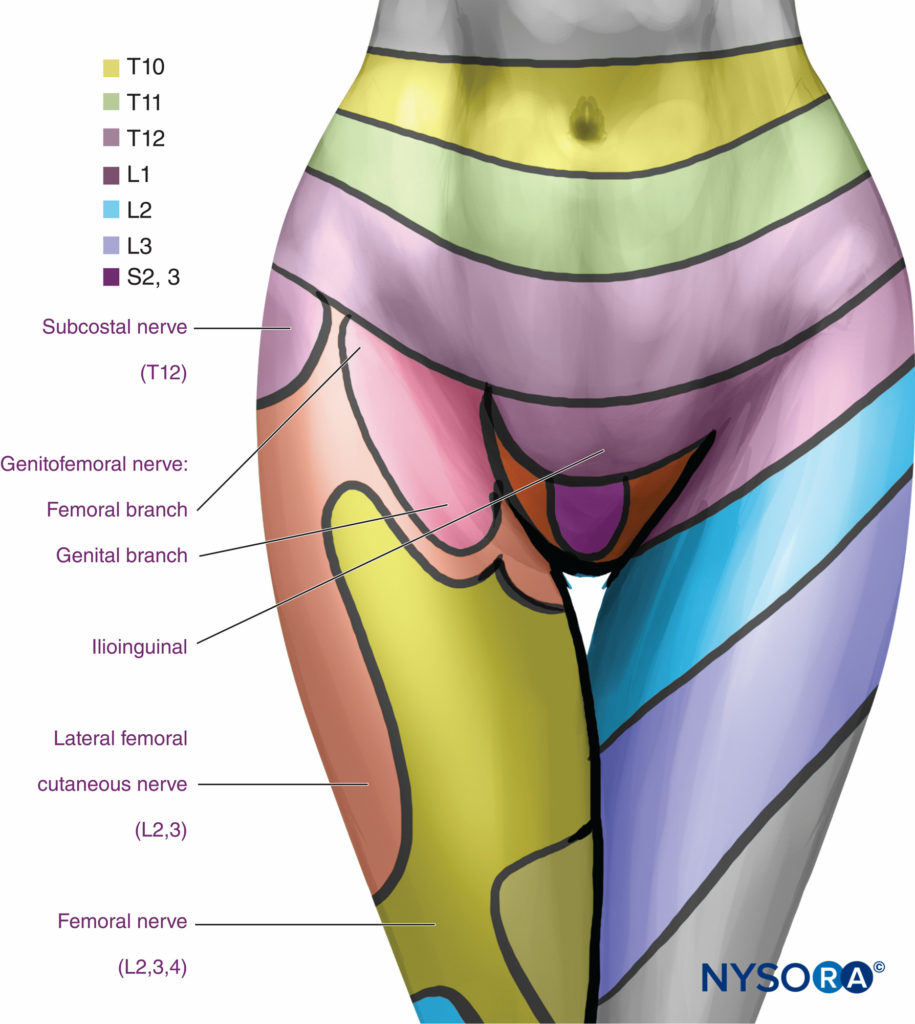
FIGURE 16. Segmental distribution of anesthesia with lumbar paravertebral levels.
Ipsilateral sympathetic block may also occur due to epidural spread or spread of local anesthetic anteriorly via the tendinous arches to the rami communicantes or the lumbar sympathetic chain.
- Technique
Lumbar paravertebral block can be performed with the patient in the sitting, lateral, or prone position. Surface landmarks must be identified and marked with a skin marker before block placement. The spinous process of the vertebra at the levels to be blocked represents the midline, the iliac crest corresponds to the L3-L4 interspace, and the tip of scapula corresponds to the T7 spinous process. Skin markings are also made 2.5 cm lateral to the midline at the levels that are to be blocked (Figure 17A) or one can draw a line 2.5 cm lateral to the midline and perform the injections along this line (Figure 17B and C).
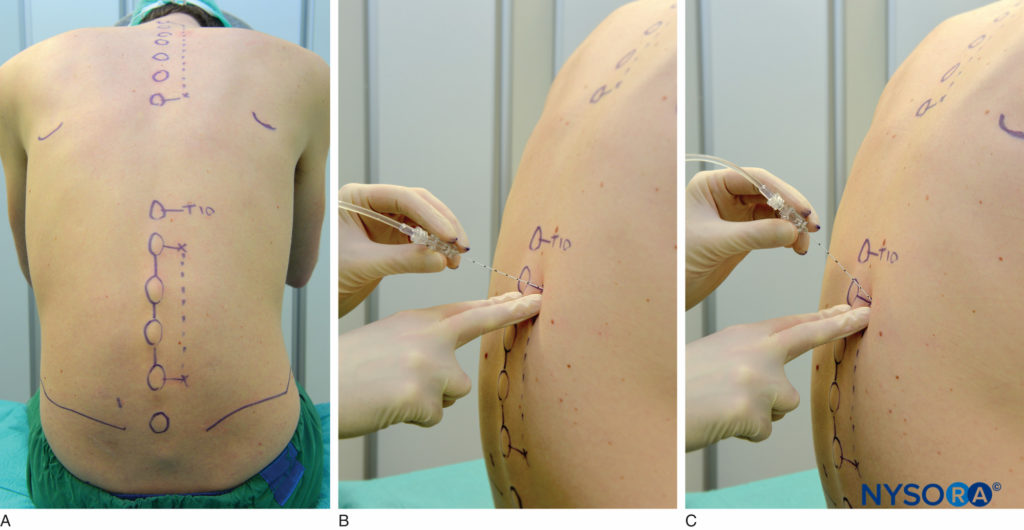
FIGURE 17. A: Surface landmarks and needle insertion sites for lumbar paravertebral block. B and C: Needle insertion.
A standard regional anesthesia tray is prepared; strict asepsis should be maintained during block placement. An 8-cm, 22-gauge, Tuohy tip needle (see Figure 1–8) is used for LPVB. Similarly to the recommendations for TPVB, the use of needles with depth markings on the shaft of the needle or a guard indicating the depth (see Figure 1–8) is recommended. Advancing the needle by a fixed predetermined distance (1.5–2.0 cm) beyond the transverse process, without eliciting paresthesia, is the method most commonly used to perform LPVB. The block needle is inserted perpendicular to the skin until the transverse process is contacted. The depth at which the transverse process is contacted is variable (4–6 cm) and depends on the build of the patient. Once the transverse process is identified, the marking on the needle is noted or the depth marker is adjusted so that it is 1.5–2.0 cm beyond the skin–transverse process depth. The needle is then withdrawn to the subcutaneous tissue and reinserted at a 10- to 15-degree superior or inferior angle so that it slides off the superior or inferior edge of the transverse process, similarly to the technique in thoracic paravertebral block (see Figure 11). The needle is advanced by a further 1.5–2.0 cm beyond the contact with the transverse process or until the depth marker is reached. After negative aspiration for blood or cerebrospinal fluid (CSF), the local anesthetic is injected. Since spread of local anesthetic after a single large-volume lumbar paravertebral injection is unpredictable, the multiple-injection technique in which 4–5 mL of local anesthetic is injected at each level is more commonly used.
Choice of Local Anesthetic
As for TPVB, long-acting local anesthetic agents such as bupivacaine 0.5%, ropivacaine 0.5%, or levobupivacaine 0.5% are commonly used for LPVB. During a multiple injection LPVB, 4–5 mL of the local anesthetic is injected at each level. Anesthesia develops in about 15–30 minutes and lasts for 3–6 h. Analgesia is also long-lasting (12–18 h) and generally outlasts the duration of anesthesia. There are no data on the pharmacokinetics of local anesthetic after LPVB. Nevertheless, the addition of epinephrine (2.5–5.0 mcg/mL) to the local anesthetic may reduce systemic absorption and reduce the potential for toxicity.
Indications and Contraindications
LPVB is commonly used in combination with TPVB (T10 through L2) for surgical anesthesia during inguinal herniorrhaphy. It can be also effective for rescue in patients with severe pain after total hip replacement. It can also be used for diagnostic purpose during evaluation of groin or genital pain, such as that following nerve entrapment syndrome after inguinal herniorrhaphy.
Contraindications for LPVB are similar to TPVB, but caution should be exercised in patients who are anticoagulated or are receiving prophylactic anticoagulants since psoas hematoma with lumbar plexopathy has been reported.
Complications and How to Avoid Them
Published data suggest that complication is rare after LPVB. Nevertheless, it is possible to inadvertently inject local anesthetic into the intravascular, epidural, or intrathecal spaces during LPVB, and this may be more common if the needle is directed medially. Therefore, the direction of the block needle should be maintained perpendicular to the skin during insertion, and medial angulation should be avoided. Intraperitoneal injection or visceral injury (renal) may also occur, although this can occur only as a result of gross technical error. Motor weakness involving the ipsilateral quadriceps muscle may result if the L2 spinal nerve is blocked (femoral nerve L2–L4).
SUMMARY
Proper training is necessary to acquire stereotactic techniques required to ensure a high success rate. Thoracic paravertebral block produces unilateral somatic and sympathetic nerve block that is adequate for surgical anesthesia during breast surgery and for analgesia when pain is of unilateral origin from the chest or abdomen. It has been also described as a rescue analgesic therapy in patients with rib fractures and respiratory compromise. Lumbar paravertebral block is less commonly used in clinical practice. As a thoracolumbar paravertebral block, it is effective for surgical anesthesia during inguinal herniorrhaphy.
Hemodynamic stability is usually maintained after a paravertebral block due to the unilateral nature of sympathetic block. Bladder and lower limb motor function are also preserved, and no additional nursing vigilance is required during the postoperative period. Successful clinical applications of a bilateral paravertebral block have also been reported.
REFERENCES
- Karmakar MK: Thoracic paravertebral block. Anesthesiology 2001;95:771–780.
- Richardson J, Lonnqvist PA: Thoracic paravertebral block. Br J Anaesth 1998;81:230–238.
- Cheema SP, Ilsley D, Richardson J, et al: A thermographic study of paravertebral analgesia. Anesthesia 1995;50:118–121.
- Eason MJ, Wyatt R: Paravertebral thoracic block—a reappraisal. Anesthesia 1979;34:638–642.
- Coveney E, Weltz CR, Greengrass R, et al: Use of paravertebral block anesthesia in the surgical management of breast cancer: experience in 156 cases. Ann Surg 1998;227:496–501.
- Greengrass R, O’Brien F, Lyerly K, et al: Paravertebral block for breast cancer surgery. Can J Anaesth 1996;43:858–861.
- Klein SM, Bergh A, Steele SM, et al: Thoracic paravertebral block for breast surgery. Anesth Analg 2000;90:1402–1405.
- Karmakar MK, Booker PD, Franks R, et al: Continuous extrapleural paravertebral infusion of bupivacaine for post-thoracotomy analgesia in young infants. Br J Anaesth 1996;76:811–815.
- Lonnquist PA, Hesser U: Radiological and clinical distribution of thoracic paravertebral block in infants and children. Paediatr Anaesth 1993;3: 83–87.
- Lonnqvist PA: Continuous paravertebral block in children. Initial experience [see comments]. Anesthesia 1992;47:607–609.
- Dugan DJ, Samson PC: Surgical significance of the endothoracic fascia. The anatomic basis for empyemectomy and other extrapleural technics. Am J Surg 1975;130:151–158.
- Karmakar MK, Kwok WH, Kew J: Thoracic paravertebral block: radiological evidence of contralateral spread anterior to the vertebral bodies. Br J Anaesth 2000;84:263–265.
- Karmakar MK, Chung DC: Variability of a thoracic paravertebral block. Are we ignoring the endothoracic fascia? [letter]. Reg Anesth Pain Med 2000;25:325–327.
- Moore DC, Bush WH, Scurlock JE: Intercostal nerve block: a roentgenographic anatomic study of technique and absorption in humans. Anesth Analg 1980;59:815–825.
- Tenicela R, Pollan SB: Paravertebral-peridural block technique: a unilateral thoracic block. Clin J Pain 1990;6:227–234.
- Nunn JF, Slavin G: Posterior intercostal nerve block for pain relief after cholecystectomy. Anatomical basis and efficacy. Br J Anaesth 1980;52:253–260.
- Conacher ID: Resin injection of thoracic paravertebral spaces. Br J Anaesth 1988;61:657–661.
- Purcell-Jones G, Pither CE, Justins DM: Paravertebral somatic nerve block: a clinical, radiographic, and computed tomographic study in chronic pain patients. Anesth Analg 1989;68:32–39.
- Karmakar MK, Gin T, Ho AM: Ipsilateral thoraco-lumbar anesthesia and paravertebral spread after low thoracic paravertebral injection. Br J Anaesth 2001;87:312–316.
- Saito T, Gallagher ET, Cutler S, et al: Extended unilateral anesthesia. New technique or paravertebral anesthesia? Reg Anesth 1996;21:304–307.
- Saito T, Den S, Tanuma K, et al: Anatomical bases for paravertebral anesthetic block: fluid communication between the thoracic and lumbar paravertebral regions. Surg Radiol Anat 1999;21:359–363.
- Karmakar MK, Critchley LA, Ho AM, et al: Continuous thoracic paravertebral infusion of bupivacaine for pain management in patients with multiple fractured ribs. Chest 2003;123:424–431.
- Gilbert J, Hultman J: Thoracic paravertebral block: a method of pain control. Acta Anaesthesiol Scand 1989;33:142–145.
- Richardson J, Jones J, Atkinson R: The effect of thoracic paravertebral block on intercostal somatosensory evoked potentials. Anesth Analg 1998;87:373–376.
- Sabanathan S, Smith PJ, Pradhan GN, et al: Continuous intercostal nerve block for pain relief after thoracotomy. Ann Thorac Surg 1988;46:425–426.
- Richardson J, Sabanathan S, Jones J, et al: A prospective, randomized comparison of preoperative and continuous balanced epidural or paravertebral bupivacaine on post-thoracotomy pain, pulmonary function and stress responses. Br J Anaesth 1999;83:387–392.
- Weltz CR, Greengrass RA, Lyerly HK: Ambulatory surgical management of breast carcinoma using paravertebral block. Ann Surg 1995;222:19–26.
- Sabanathan S, Mearns AJ, Bickford SP, et al: Efficacy of continuous extrapleural intercostal nerve block on post-thoracotomy pain and pulmonary mechanics. Br J Surg 1990;77:221–225.
- Matthews PJ, Govenden V: Comparison of continuous paravertebral and extradural infusions of bupivacaine for pain relief after thoracotomy. Br J Anaesth 1989;62:204–205.
- Carabine UA, Gilliland H, Johnston JR, et al: Pain relief for thoracotomy. Comparison of morphine requirements using an extrapleural infusion of bupivacaine. Reg Anesth 1995;20:412–417.
- Karmakar MK, Chui PT, Joynt GM, et al: Thoracic paravertebral block for management of pain associated with multiple fractured ribs in patients with concomitant lumbar spinal trauma. Reg Anesth Pain Med 2001;26:169–173.
- Dauphin A, Gupta RN, Young JE, et al: Serum bupivacaine concentrations during continuous extrapleural infusion. Can J Anaesth 1997;44: 367–370.
- Berrisford RG, Sabanathan S, Mearns AJ, et al: Plasma concentrations of bupivacaine and its enantiomers during continuous extrapleural intercostal nerve block. Br J Anaesth 1993;70:201–204.
- Clark BJ, Hamdi A, Berrisford RG, et al: Reversed-phase and chiral highperformance liquid chromatographic assay of bupivacaine and its
enantiomers in clinical samples after continuous extraplural infusion. J Chromatogr 1991;553:383–390. - Lonnqvist PA, MacKenzie J, Soni AK, et al: Paravertebral block. Failure rate and complications. Anesthesia 1995;50:813–815.
- Richardson J, Sabanathan S: Thoracic paravertebral analgesia. Acta Anaesthesiol Scand 1995;39:1005–1015.
- Farny J, Drolet P, Girard M: Anatomy of the posterior approach to the lumbar plexus block. Can J Anaesth 1994;41:480–485.
- Klein SM, Greengrass RA, Weltz C, et al: Paravertebral somatic nerve block for outpatient inguinal herniorrhaphy: an expanded case report of 22 patients. Reg Anesth Pain Med 1998;23:306–310.
- Wassef MR, Randazzo T, Ward W: The paravertebral nerve root block for inguinal herniorrhaphy—a comparison with the field block approach. Reg Anesth Pain Med 1998;23:451–456.
- Murata H, Salviz EA, Chen S, Vandepitte C, Hadzic A. Case report: ultrasound-guided continuous thoracic paravertebral block for outpatient acute pain management of multilevel unilateral rib fractures Anesth Analg. 2013 Jan;116(1):255–257.