1. ADVANCES IN US TECHNOLOGY
US technology is rapidly advancing and being refined, and is aimed at both increasing image quality and opening new fields of applications. This chapter will review the main advances in US technology and address the clinical impact they have had or are likely to have in the future in the field of the musculoskeletal system. New developments in transducer technology and advances in the quality and presentation of US images will be discussed.
2. TRANSDUCERS
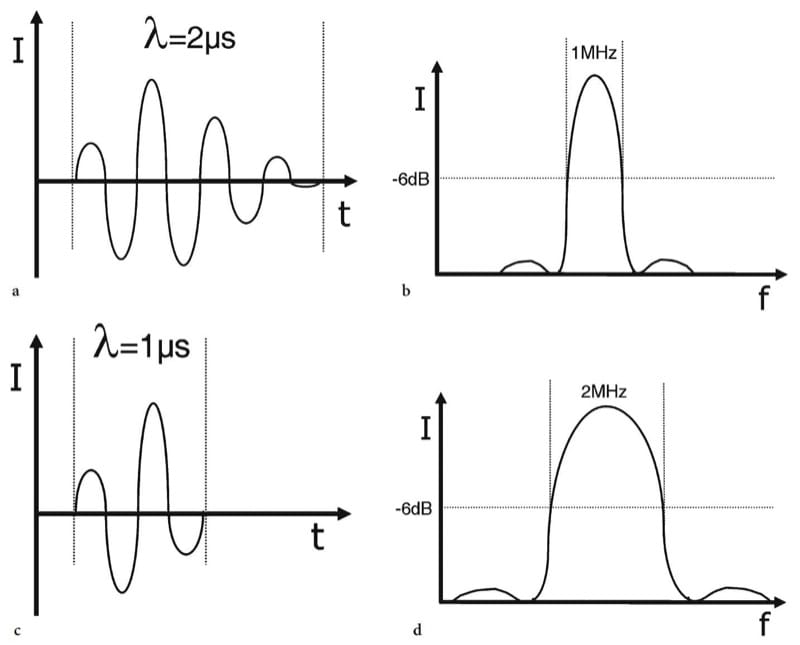
Figure 1. 1a–d. Relationship between spatial pulse length and frequency spectrum. a,b Intensity versus time diagrams illustrate different pulse lengths (λ). Two sine wave pulses are shown lasting 2 µs (four-cycle) and 1 µs (two-cycle) respectively. c,d Corresponding Fourier power (intensity versus frequency) diagrams show the spectrum of frequencies present in the pulses shown in a and b. The bandwidth is measured between the 6 dB points on each side of the spectrum. The longer pulse in a generates a narrower bandwidth (1 MHz) than the shorter pulse (2 MHz) in b.
3. BROADBAND TRANSDUCERS
One of the original objectives in designing broadband transducers was to improve axial resolution without changing the emission frequency. This is related to the fact that the shorter transmission pulses used in a broadband emission generate shorter echo pulses which can be faithfully converted into electric signals (Whittingham 1999b). Because short pulses suffer attenuation to a greater extent and are characterized by less penetration than long pulses, some specific techniques have been introduced by different manufacturers to compensate for these drawbacks, including single-pulse and multi-pulse techniques (Claudon et al. 2002). Among single-pulse techniques, the emission of a long, peculiarly shaped transmission pulse, which varies in frequency and amplitude within the duration of the pulse itself, has been used instead of a simple sinusoidal pulse (Fig. 2). When the signal is received, a filter analyzes the signal frequencies as a short pulse, erasing the components introduced to make it long (chirp): the result is increased image penetration with an improved signal-to-noise ratio, without compromising axial resolution. Other multi-pulse techniques make use of a coded-emission mode consisting of transmission of an integrated sequence of many short, high-frequency transmission pulses which vary in terms of phase and are modulated in a code sequence. When the signal is received, the signal frequencies are compared with the transmission pulses by a matching decoding filter working at a high sampling rate. The subtraction process results in increased image penetration without loss of axial resolution or an increase in emission peak pulses (Claudon et al. 2002).

Figure 2. a,b. US pulse shaping. a Intensity versus time diagram illustrates a short pulse wave (arrow) characterized by a few oscillations rapidly dampened by the backing material of the transducer. This short-duration pulse is associated with a broad bandwidth but, when transmitted through tissues, it is rapidly attenuated and absorbed resulting in a poor penetration of the US beam. b Intensity versus time diagram illustrates a chirp pulse. This pulse has a longer duration to increase the penetration of the US beam. It is not a simple sine wave: it is modulated in terms of phase and frequency to include a central component (arrow) – that a receive filter reads as a short pulse to obtain high axial resolution – and two sine queues (arrowheads) on each side of the central component to give penetration capabilities. Example of Chirped Emission (Siemens).
Apart from advances in emission pulse technology, broadband transducers use a spectrum of frequency distribution (i.e., 12–5 MHz) instead of a single fundamental frequency (i.e., 10 MHz): the high-frequency components tend to increase the intensity maximum in the focal zone but cause a prompt decrease in intensity with depth, whereas the low-frequency components extend the penetration depth (Whittingham 1999b). In multiple-frequency imaging, the available broad bandwidth is subdivided into multiple frequency steps for trans-mission and reception of sound waves: these transducers enable selection of the optimal frequency range in a given scanning plane as though two or more independent transducers – each with a different center frequency – were available (Fig. 3). Other systems use the total transducer bandwidth for the transmitted pulse and then adjust the receiver bandwidth to lower frequencies as deeper depths are sampled. These systems give increased flexibility to the US examination, enabling the same transducer to change the image acquisition parameters during scanning based on the desired clinical information. In musculoskeletal imaging, this is particularly important when the study focuses on both superficial (i.e., subcutaneous tissue planes) and deep (i.e., muscle tissue layers) tissues in the same study and body area to be explored.
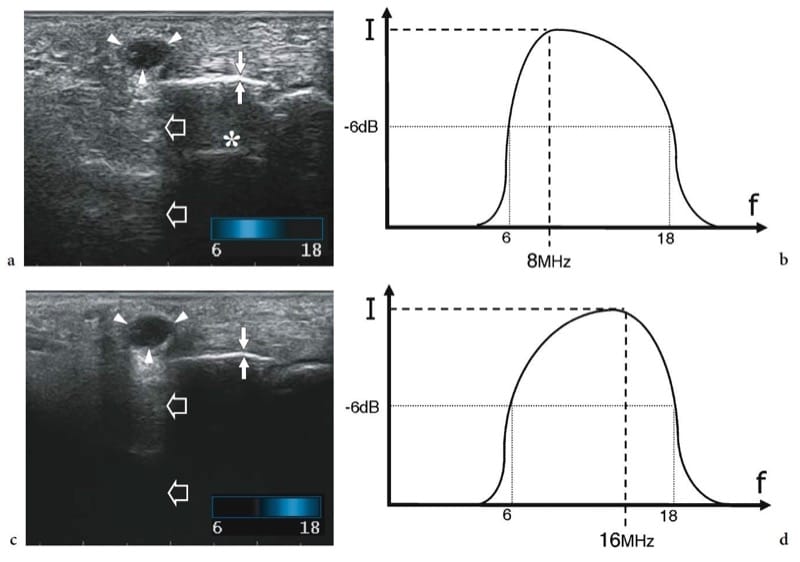
Figure 3. a–d. Multiple-frequency transducers. a,b Longitudinal US images obtained over the palmar aspect of the hand with a 18–6 MHz multiple frequency transducer by setting the center frequency at a 8 MHz and b 16 MHz respectively. Shifting on the lower frequencies of the bandwidth, penetration (large open arrows) of the field-of-view is achieved; on the other hand, the small superficial cyst (arrowheads) overlying metacarpal bone (thin white arrows) does not appear completely anechoic, subcutaneous tissue echoes are coarse and reverberation artifacts (asterisk) appear deep to the bone. Shifting the frequency band upward, a more defined echotexture is appreciated in the superficial part of the image as a result of an increased resolution. In contrast, a strong attenuation affects the deep part of the US image, which loses intensity. c,d Corresponding intensity versus frequency diagrams illustrate how the frequency band is modulated in multiple-frequency transducers. Example of “eXtreme High-Frequencies imaging” technology (Esaote).
4. FOCUSING
Reducing the width and thickness of the US beam has definite advantages in terms of contrast and spatial resolution. In modern linear-array transducers, focusing is currently not obtained by means of a fixed lens as in the old mechanical sector probes in which degrading of the image quality occurred at a short distance from the focal zone (Fig. 4a). Focusing is now produced electronically by activating a series of elements in the array with appropriate delays, so that the trigger pulses to the inner elements are delayed with respect to the pulses to the outer ones. In this way a curved wavefront results from constructive interference bringing the US beam toward a focus. By adjusting the values of the delays applied to the trigger pulses, the curvature of the wavefront and, therefore, the focal depth can be changed dynamically. As the resulting wavefront has the characteristics of a short excitation pulse, the axial resolution is preserved. When the pulses are received, the US machine continuously refocuses them according to the position from which the echoes come, thus giving real-time focal tracking along the depth axis: synchronization of the received signals is essential to minimize out-of-axis echo interference. An important factor influencing the lateral resolving power of the system is the dynamic aperture: this is achieved by activating variable numbers of elements dynamically to optimize focusing at many depths. As a rule, the higher the number of channels (electric pathways) involved in this process to activate the elements in a combined mode and with appropriate delays, the higher the complexity and the cost of the equipment, but the more accurately the beam can be focused. Recently, the introduction and refinement of matrix (1.5D probes) transducers led to further progress. In these transducers, the single row of long piezoelectric elements found in a conventional probe is replaced by more layers (three to seven) incorporated into a single thin layer to produce parallel rows of short elements. The slice thickness of the US image is improved by performing dynamic focusing in the elevation plane (Fig. 4b). This leads to better spatial and contrast resolution and reduction of partial-volume averaging artifacts (Rizzatto 1999). A less expensive alternative to 1.5D probes is the use of peculiar acoustic lenses –Hanafy lenses –placed in front of the piezoelectric elements. The Hanafy lens has non-uniform thickness and resonance properties: it produces a narrow and uniform image slice thickness and, simultaneously, a very broad band-width pulse. The inner portion of the lens is thinner, resonates at higher frequencies and focuses in the near field, whereas its outer portions resonate at lower frequency and are focused in both transmission and reception at the deepest part of the image providing better penetration (Claudon et al. 2002).

Figure 4. a,b. Elevation focusing. a Schematic drawing shows mechanical focusing of an electronic linear-array transducer (in gray) with a single row of elements (arrows) by an acoustic lens (in black). Note that focusing is applied uniformly to each crystal of the array. As shown on the right, a side view of the transducer illustrates the resulting slice thickness of the US beam. Using mechanical focusing, the beam has non uniform thickness throughout the scanning plane: it is narrow at a given depth but soon diverges away from the focal zone. b Schematic drawing shows a 1.5D array transducer made of three rows of elements (arrows) instead of a single row. Beam width reduction is achieved by electronic focusing control in the z-plane by introducing appropriate delays of crystal activation. The resultant slice thickness is uniformly narrow throughout the scanning plane.
5. TRANSDUCER SELECTION AND HANDLING
A variety of linear-array transducers, including large (>40 mm), medium-sized (<40 mm) and small-FOV (hockey-stick-shaped) probes, are currently available in the frequency range used for musculoskeletal examinations. Selection of the most appropriate transducer primarily depends on the frequency but is also related to other factors. Hockey-stick probes are the best choice for imaging small superficial structures at sites in which the skin surface does not allow adequate contact with larger probes (i.e., soft tissues adjacent to bony prominences) or while performing dynamic maneuvers: they are, however, characterized by a restricted field-of-view which often allows only an incomplete evaluation of the structure of interest and surrounding anatomy. Compared with small transducers, high-frequency large-diameter transducers tend to have a large near-field beam width leading to a poor lateral resolution at shallow depths. Because they maintain beam shape to greater depths with less divergence of the US beam, they have the best potential for imaging deep-seated structures. During evaluation of the musculoskeletal system, probe handling has need of maximum stability over the region of interest; compression is never required, and the mobility of the probe to cover wide body areas is considerably less than in abdominal studies. Because pathologic findings may be very small in size and are often evaluated by placing the probe over curvilinear (i.e., humeral head) and irregular surfaces (i.e., cubital tunnel), stability of the transducer is a main factor required for high-quality examinations. In our experience, the best grip to obtain probe stability can be obtained by placing the ulnar fingers (long, ring, little) directly on the patient’s skin while holding the probe with the radial fingers (so that the probe hangs between the thumb and the index finger). This grip allows easy translation of the probe along its short axis at a given angle minimizing rotational changes. When possible, the examiner being in a lower position than the patient (i.e., the examiner seated on a chair and the patient supine on the bed at the level of the examiner’s shoulder) may also help to achieve probe stability.
6. IMAGING ALGORITHMS
Recent technologic innovations in US have resulted in improved diagnostic performance for the evaluation of the musculoskeletal system, including wide-band Doppler imaging, spatial compound imaging, extended field-of-view imaging, steering-based gray-scale imaging, elastography and 3D imaging. Because these new imaging procedures are many and characterized by different names depending on the manufacturer – so that considerable confusion may exist regarding how they work and should best be used – we provide here a brief description of these technologies, pointing out their main advantages in the musculoskeletal applications.
7. ADVANCES IN DOPPLER IMAGING
The ability of high-frequency color and power Doppler systems to detect low flow states in superficial tissues fed by small vessels and to correlate hyperemic changes with structural abnormalities has opened new perspectives in the evaluation of a variety of musculoskeletal disorders. The introduction of broadband Doppler technology has led to some advantages in imaging subtle blood flows such as those encountered in the extremities. Unlike conventional Doppler systems, in which a long burst pulse containing a large number of cycles is used to reach high sensitivity in flow detection, and similar to B-mode emission, broadband Doppler technology makes use of short pulses to obtain wideband transmission. This creates a significant improvement in frame rate and axial resolution leading to a more defined display of tissue microvasculature as it exists in muscles, tendons and other soft tissue structures, thereby limiting “over-writing” of color signals on gray-scale structures (Fig. 5). Appropriate waveform shaping ensures penetration and avoids the reduction in Doppler signal sensitivity usually associated with short pulses. Among other advances in Doppler imaging modalities, directional power Doppler US has been developed to encode flow direction in real time with a two-color scale but continuing to estimate the signal intensity and not the mean Doppler shift as color Doppler mode does. This system should add the advantages of better sensitivity to flow and lower dependence on angle of the power mode to estimation of flow direction.

Figure 5. a,b. Wideband Doppler imaging. a Power Doppler imaging obtained with a conventional long-pulse technique using a 14–7 MHz transducer performing Doppler imaging at 10 MHz frequency. b Wideband Doppler imaging obtained with a short-pulse technique and the same parameters used in a. Both images display a swollen Achilles tendon (arrowheads) with degenerative changes (asterisk). Note the subtle intratendinous vessel (arrow) branching within the tendon substance: comparison of the two scans reveals that the wideband Doppler image shown in b has higher spatial resolution (smaller vessel caliber) and better sensitivity (depiction of secondary branches). Ca, calcaneus. Example of Advanced Dynamic Flow (Toshiba).
8. COMPOUND IMAGING
Spatial compound imaging indicates an acquisition mode in which the information is obtained from several angles of insonation and combined to produce a single image (Entrekin et al. 2001; Lin et al. 2002).
Different from conventional B-mode in which the US images are obtained from a single angle of insonation (perpendicular to the transducers array), in compounding mode the digital beamformer steers the US beam at several (up to nine) steering angles during real-time acquisition rates (Claudon et al. 2002). When the signal is received, the lines of sight are rendered according to the rectangular geometry of the field-of-view of the US image. The advantages of compound mode are many, including reduction of image artifacts (e.g., speckle, clutter, noise, angle-generated artifacts), sharper delineation of tissue interfaces and better discrimination of lesions over the background as well as improvement in detail resolution and image contrast. In the musculoskeletal system, compound imaging leads to an improved delineation of structures composed of specular echoes, such as tendons and muscles (Lin et al. 2002). This derives from the fact that, when these structures are imaged, the highest echo amplitude is obtained at the point at which the US beam is perpendicular to them as a result of anisotropy (i.e., fibrillar echotexture of tendons, curved surfaces). With spatial compounding, images are generated from different view angles: therefore, the likelihood is greater that one of these angles will be perpendicular to the tendon or the muscle fibers to generate a higher echo amplitude even at insonation angles that cause anisotropy on conventional mode (Fig. 6a) (Lin et al. 2002). Edge shadows resulting from the boundaries of subcutaneous fat lobules, tendons, muscles, nerve fascicles, fascial planes and vessel walls are also erased because they reflect only weakly at oblique angles (Fig. 6b–e) (Claudon et al. 2002). Another advantage of compound imaging is reduction of speckle noise, a random artifact causing a grainy appearance of the US images as a result of scattering from tissue reflectors (Lin et al. 2002). Speckle reduction obtained by averaging frames from different angles of insonation leads to improved image definition and better signal-to-noise ratio. The resulting image appears smoother with better tissue-plane definition. Compounding with a high number of averaged frames worsens temporal resolution (Lin et al. 2002): this does not seem to be a problem in musculoskeletal US as the examination is free from respiratory and cardiac motion and, in most cases, static. In general, dynamic maneuvers during passive tendon or joint movement are not significantly affected by frame averaging. Recently, some compound mode systems have been developed using simultaneous emission of two different frequencies instead of one to improve contrast resolution (transmit frequency compound). Adaptive algorithms which perform real-time analysis of patterns at pixel level and refine the image by emphasizing patterns within the tissue texture and de-emphasizing artifacts and noise, can be combined with spatial compound imaging to further sharpen borders and tissue interfaces. Similarly, color B-mode imaging systems with contrast optimization (photopic imaging) can be applied to improve overall image contrast and definition of deep soft-tissue boundaries (Sofka et al. 2005).

Figure 6. a–e. Spatial compound imaging. a Schematic drawing illustrates the image acquisition process in compound mode. The US beam is steered out of axis providing multiple lines of sight at several angles during real-time acquisition. Signal processing renders the steered frames into a final image in real time as each new frame is acquired. With this system, a clearer delineation of borders (in black) and interfaces is obtained even when they are oriented at unfavorable angles. The acoustic shadow posterior to calcifications is usually thinner and less delineated than in the conventional mode. b,c Conventional cross-sectional b 12–5 MHz and c 17–5 MHz images of the median nerve at the mid-forearm. Deep to the flexor carpi radialis tendon (arrowheads), the nerve (arrow) appears as a rounded structure composed of many small hypoechoic dots related to the fascicles. Note how the fascicles are more clearly depicted as the frequency increases. The muscle tissue of the flexor digitorum profundus (dashed square) appears coarse and grainy. d,e Corresponding d 12–5 MHz and e 17–5 MHz compound images. The fascicles are better delineated compared with the images acquired in conventional mode. The best result is obtained with the combined use of spatial compounding and the 17–5 MHz US probe. Muscles (dashed square) exhibit a more homogeneous echotexture as a result of better suppression of speckle artifact and an increased signal-to-noise ratio. Example of SonoCT Imaging (Philips).
9. EXTENDED FIELD-OF-VIEW IMAGING
One of the main drawbacks of linear-array transducers to image the musculoskeletal system is the limited extension of the field-of-view (often < 4 cm wide). With these probes, displaying the full extent of an abnormality and showing its relationship with adjacent structures on a single image may be problematic: this creates inadequate reproduction of the full lesion on prints and difficulties for colleagues and the referring physician when reading the US images. Somewhat similar to the compound systems produced in the middle and late 1970s, extended field-of-view technology uses specific image registration analysis to track probe motion and reconstruct a large composite image during real-time scanning over long distances and curved body surfaces without using external positional devices. After selecting a scanning plane of interest, the examiner slides a standard probe along the skin surface in the direction of the scan plane while monitoring the image on the screen. During lateral probe motion, there is an advancing real-time portion of the image and a static portion which displays what has been scanned (Fig. 7). The reconstruction process is based on the fact that image features of a given frame and the next frame are very similar, except that the second image is slightly shifted or rotated relative to the first one (Weng et al. 1997). Successive frames are registered and blended with the previous ones based on an autocorrelation algorithm and an advanced parallel processing architecture requiring intense digital work. As determined on phantoms, geometric measurement of extended field-of-view US is accurate to within < 5% (Weng et al. 1997; Fornage et al. 2000). Particularly in the examination of the musculoskeletal system, this technique seems able to provide accurate data because of the absence of respiratory movements or pulsatility of large vessels (Weng et al. 1997; Barberie et al. 1998; Lin et al. 1999). Extended field-of-view imaging can show the abnormality (most often large fluid collections, muscle injuries, tumors, etc.) in association with the appropriate landmarks, such as joints, tendons and muscles, which may even be remote from the structure of interest. Although training is important to obtain accurate images, the extended field-of-view technique contributes to an improved presentation of the US information for the referring physician (Weng et al. 1997; Barberie et al. 1998; Lin et al. 1999; Sauerbrei 1999).

Figure 7. a–c. Extended field-of-view imaging. a–c Formation of a panoramic extended field of view image over the gluteus minimus muscle (arrowheads). During real-time scanning, the probe is moved caudally (arrows). The box indicates where the current frame is obtained. Image frames are translated and rotated according to the estimated probe motion by means of image registration. The final panoramic image shows the whole length of the gluteus minimus from the iliac crest (Ic) to its insertion into the great trochanter (Gt). The relationships of the gluteus medius tendon (open arrows) with the gluteus minimus tendon (white arrow) are shown. The photographs at the upper left side of the figures indicate probe positioning. Example of Extended-FOV Imaging (Siemens).
10. STEERING-BASED IMAGING
In addition to spatial compound and Doppler systems, the beam steering function has recently been applied to B-mode imaging to obtain a parallelogram format with lateral sides parallel but oblique instead of a rectangular field-of-view. This function is obtained by activating consecutive elements in the array with increasing delays so that a wavefront resulting from constructive interference sends oblique lines-of-sight along the depth axis. In musculoskeletal US, this function seems to be useful when anisotropic structures, such as tendons or ligaments, are examined with an incidence angle far from 90° due to their oblique course from surface to depth (distal biceps tendon, Achilles and supraspinatus tendon insertion, etc.). Beam steering may optimize depiction of the fibrillar echotexture in an otherwise hypoechoic tendon area, thus helping to avoid confusion between artifact and disease (Fig. 8). Given that many pathologies of the musculoskeletal system are larger than the small field-of-view of linear-array transducers, a steering technology (wide field-of-view) able to increase the lateral size of the image in the far-field has been developed recently. The resultant trapezoid shape of the field-of-view leads to reproduction of large lesions in their full extent without the requirement for extended field-of-view algorithms (Fig. 9).

Figure 8. a–d. Beam steering for gray-scale imaging. a,b Long-axis 14–7 MHz US images over the insertion of the Achilles tendon (white arrowheads) on the calcaneus (Ca) acquired a on conventional mode and b by steering the beam (void arrowheads) to produce an oblique wavefront. In b, note suppression (open arrow) of the artifactual hypoechoic intratendinous area (white arrow) due to steering the beam perpendicularly to the tendon insertion. c,d Correlative schematic drawings. Example of B-mode steering function (Toshiba).

Figure 9. a–e. Wide field-of-view technology. a,b Transverse 12–5 MHz US images over the anterior thigh with c,d schematic drawing correlation in a 25-year-old patient who suffered a strain injury of the distal aponeurosis of the rectus femoris muscle resulting in an extensive peripheral hematoma (white arrows). a Using a conventional rectangular field-of-view, the hematoma cannot be displayed in its full extent: part of it (arrowheads) is out of the field-of-view of the US image. b With a trapezoidal field-of-view, the full width of the hematoma is depicted, including its more lateral portion (open arrows). Curved arrow, central aponeurosis. e Corresponding extended field-of-view imaging obtained on a transverse plane over the anterior thigh. In the panoramic view, the relationships of the muscle injury with adjacent anatomic landmarks, including the vastus lateralis (VL) and the vastus medialis (VM), are shown. Example of Wide-FOV (Philips).
11. THREE-DIMENSIONAL IMAGING
The improvement in fast digital computer processing and memory storage capacity has recently improved the possibility of applying 3D technology to US (Brandl et al. 1999; Wallny et al. 2000; Claudon et al. 2002). Three-dimensional acquisition can be achieved with US using either 2D conventional transducers equipped with a small electromagnetic positional sensor or dedicated “3D-volume transducers,” which are larger than standard probes and more difficult to handle but have the advantage of providing more exact assessment of each scanning plane (Fig. 10). These latter transducers sweep the US beam throughout the tissue volume by tilting the scan-head with a mechanized drive along the z-axis. During this procedure, serial slices are recorded resulting in a pyramid-shaped volume scan: for each slice, the angle between slices is known, minimizing distortion in the final image. Following volume scan acquisition, the monitor displays reconstructed slices according to longitudinal, transverse and coronal planes. Each plane can be oriented within the volume block for detailed analysis by parallel or rotational shifting around any of the three spatial axes (Brandl et al. 1999). Data can also be displayed as true 3D images using various rendering algorithms, including maximum intensity projection, transparent, surface and Doppler renderings (Brandl et al. 1999). Recently, volume transducers in the frequency range suitable for analysis of the musculoskeletal tissue have been introduced, opening new interesting perspectives for evaluation of a variety of disorders, including rotator cuff tears, infant hip, congenital clubfoot and bone lesions (Gerscovitch 1997; Wallny et al. 2000; Hünerbein et al. 2001). As well as dedicated systems, software programs for 3D rendering of power Doppler images are now available in many scanners, involving capture of a series of sequential images while the transducer is translated manually without the necessity for specific hardware. Although some inaccuracies occur if the motion is not uniform, the available technology seems able to produce vascular images of acceptable quality in the musculoskeletal system (Doria et al. 2000).

Figure 10. a–d. Three-dimensional imaging. a Schematic drawing of a coronal view through the metatarsal bones demonstrates a conventional 2D scanning plane obtained along the x-axis (coronal) by placing the probe over the dorsal forefoot. b Corresponding drawing shows a reconstructed plane oriented over the z-axis (axial) by means of 3D technology. c,d Three-dimensional volume acquisitions over the forefoot using a high-frequency dedicated probe. Conventional US scans (upper images) reveal the metatarsal bones (M) as hyperechoic images with posterior acoustic shadowing. With 3D imaging, two reconstructed axial planes (lower images) have been obtained at the level of c the subcutaneous tissue and d the metatarsal bones according to the white bars shown in the upper images as reference. In c, the fat globules appear as confluent hypoechoic areas embedded in a homogeneous hyperechoic background; in d, the metatarsals (M) and the interosseous muscles (asterisks) are displayed in their long axis. Example of 3D-Voluson Technology (General Electric).
12. ELASTOGRAPHIC IMAGING
In many clinical settings, physical examination provides essential information in detecting abnormalities and monitoring changes related to worsening or healing of disease. Manual palpation is part of the physical examination, with the aim of providing qualitative assessment of changes in tissue softness/stiffness that often accompany pathologic states. Generally speaking, findings at palpation depend on the difference in stiffness between normal and pathologic tissues based on their histologic composition and supramicroscopic architecture. In many instances, however, the lesion may lie too deep or be too small to be detected by palpation despite a large difference in stiffness with the surrounding tissues. For these reasons interest is growing in developing methods for recognizing abnormal tissues based on shear elastic properties (Bamber 1999). US-based elastography measures tissue displacement (strains) responses to an external force on the assumption that the strain is smaller in harder than in softer tissues. The method is based on comparison of US radiofrequency waveforms obtained before and after light tissue compression with a conventional probe using a free-hand technique (Itoh et al. 2006). Analysis of strain is based on automated segmentation of continuous US images obtained during tissue compression. Color pixels are assigned to the elastographic image depending on the magnitude of strain, with a scale range from red (soft components) to blue (stiff components). In the musculoskeletal system, preliminary experience indicates that elasticity assessment may be promising to separate structures (i.e., degenerated from partially torn tendons) that are indistinguishable on gray-scale US imaging, as well as to disclose occult disease in otherwise normal-appearing tissue, such as compartment syndromes (Fig. 11). It is obvious that lesions containing fat, fluid or synovium will be softer than fibrotic and collagen-containing disease processes. With future improvements in technology and experience, we expect that elastography will become an important tool for the diagnosis of musculoskeletal disorders in selected clinical settings.
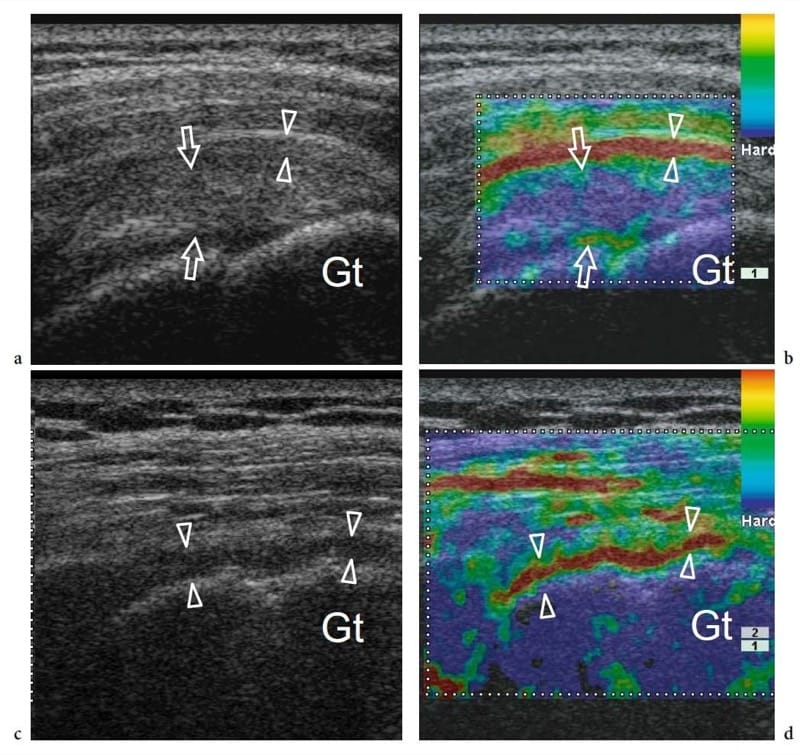
Figure 11. a–d. Elastographic imaging. Two different patients with shoulder impingement syndrome presenting with a,b cuff tendinosis and c,d supraspinatus tendon tear. a Long-axis gray-scale 13–6 MHz US image over the supraspinatus demonstrates a slightly swollen but intact tendon (arrows) associated with thickened bursal walls (arrowheads). Both structures are hypoechoic and cannot be clearly separated. Gt, greater tuberosity. b Corresponding elastographic image helps to distinguish the bursa from the underlying tendon on the basis of its greater compressibility. c Long-axis gray-scale 13–6 MHz US image over a torn and retracted supraspinatus shows residual hypoechoic bursal tissue and fluid (arrowheads) over the humeral head. d On the elasticity image, this tissue is compressible (imaged in red): this finding may help to distinguish it from residual intact tendon fibers. Gt, greater tuberosity. Example of Real-time Tissue Elastography (Hitachi).
13. ULTRASOUND CONTRAST MEDIA
The ability of US to enhance detection of blood flow with echo reflectors after the injection of a variety of fluids was first described approximately 40 years ago (Gramiak and Shah 1968). Once it was found that the source of the additional intravascular echoes was related to microbubbles developing during the injection process, the pharmaceutical industry started to develop stabilized microbubble preparations to be injected into the venous system in a safe way that would cross the pulmonary capillary bed and provide vascular enhancement for the whole duration of the clinical study. The technology used has been that of encapsulated bubbles of gas, smaller in size than the red blood cells: several gases have been used, ranging from air to less diffusible drugs, such as sulfur hexafluoride or perfluorocarbons. The gas was appropriately encapsulated in phospholipid shells of different thickness and stiffness to obtain stability and duration over scanning. US contrast agents serve as an active source of sound reflectors creating an echogenic pattern in the flowing blood. In pharmacologic terms, microbubble-based contrast agents are considered “blood pool agents” until metabolized, as they are neither filtered by the kidney nor able to enter the interstitial spaces: some have recently been shown to exhibit specific uptake in the liver and spleen after their loss from the blood pool. When microbubbles are contacted by a high-intensity high-pressure US beam, they collapse producing a transient strong broadband signal; on the other hand, when the intensity of the US beam is low, microbubbles oscillate in the US field and undergo a process of resonation, rapidly contracting and expanding in response to pressure changes of the US wave, emitting a spectrum of harmonic signals.
Specific US techniques have been developed to detect signals from microbubbles, including multipulse coded-emission modes and the so-called pulse or phase inversion in which consecutive pulses of opposite phase are transmitted along the same line: the signal subtraction leads to a relative increase in the nonlinear response from tissues by deleting the response from static structures which intrinsically have minor nonlinear components. In practice, two main imaging strategies are followed to optimize the microbubble response. With “destructive modes” (high mechanical index imaging), the signal derives from microbubble destruction produced by high-intensity US peaks: time intervals are needed for contrast replenishment between scans; with “non-destructive modes” (low mechanical index imaging), the harmonic response is collected from microbubble insonation at low-intensity US emission providing continuous imaging of microvessel perfusion (Claudon et al. 2002). Based on the latest advances, both techniques make use of gray-scale (and not Doppler) imaging to optimize detection of contrast enhancement. At present, the clinical use of US contrast agents is expanding but the experience is referred, in most cases, to abdominal applications. This is related to the fact that imaging of superficial tissues requires too high a transducer frequency band to induce a discrete harmonic response from the microbubbles. Recently, dedicated probes for use in contrast studies in superficial tissues and organs have overcome this limitation, leading to encouraging results in imaging arthritis and other rheumatologic conditions (Klauser et al. 2005).