1. HISTOLOGIC CONSIDERATIONS
All bones consist of peripheral cortical (compact) bone and central medullary (trabecular or cancellous) bone. In long bones, there is an inverse relationship between the amount of cortical and cancellous bone at any given site: in the diaphysis, the cortical bone is thick whereas the trabecular bone is sparse; conversely, metaphyseal and epiphyseal regions are characterized by thin cortical bone and prominent cancellous bone. In addition to bone trabeculae, the medullary cavity contains bone marrow, including yellow marrow (housing fat and connective tissue) and red marrow (consisting of hematopoietic cells, fat and connective tissue). The distribution of hematopoietic and fatty marrow is dependent on age and metabolic state (Ricci et al. 1990). The outer surface of cortical bone is invested by the periosteum—a dense fibrous connective tissue layer that is anchored to the cortical bone by means of perforating Sharpey fibers—which plays a role in allowing rapid healing of fractures. The periosteum thickness varies depending on age: it is thicker and more active in children. Nutrient arteries and emissary veins cross the cortical bone through the nutrient foramina. In mature long bones, they are most often observed at the diaphysis level. In terms of histogenesis, the bone develops from two distinct processes referred to as intramembranous and endochondral ossification (Erickson 1997). Intramembranous ossification occurs through direct mineralization of vascular connective tissue and is responsible of the growth of flat bones; it also contributes to the width of the shaft of long bones. Endochondral ossification arises within a cartilage model and is responsible for the longitudinal growth of long bones and the formation of the axial skeleton (Fig. 1).
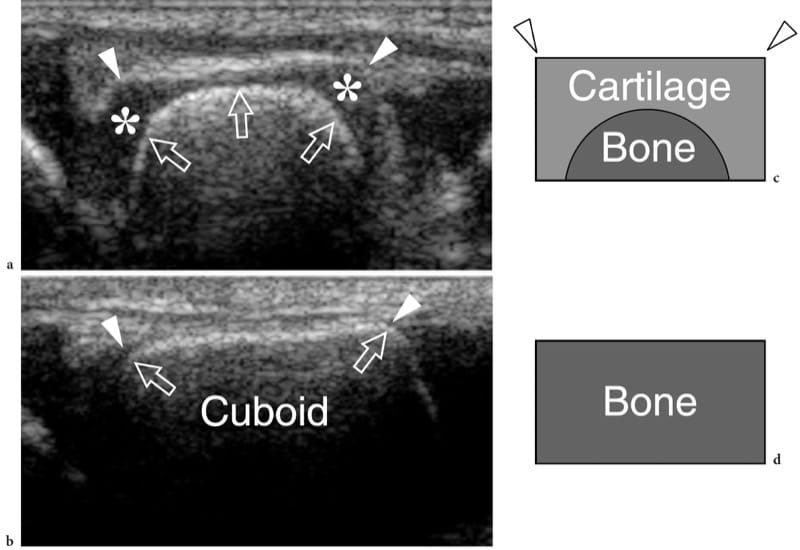
Figure 1. a–d. Endochondral ossification. a,b Coronal 12–5 MHz US images over the lateral midfoot with c,d schematic drawing correlation show the growing cuboid at a,c 1 year of age and b,d at the end of development. The cuboid is a square bone with right angles (arrowheads). Initially, the cartilage (asterisks) forms a square model reflecting the definitive appearance of bone. The primary center of ossification is visible in the center of the future bone as a hyperechoic rounded image (arrows). During growth, endochondral ossification advances toward each end of the cartilaginous model. At the end of this process, the primary center has reached the ends of the cartilaginous model and assumes the definitive square shape.
2. NORMAL US ANATOMY AND SCANNING TECHNIQUE
There is no doubt that radiography is the first-line imaging modality for assessment of bone disorders: it allows a panoramic, low-cost and reproducible evaluation of bone. More accurate analysis can be obtained by means of CT, especially if complex anatomic areas must be examined. While CT allows an optimal assessment of the bone cortex, MR imaging is the technique of choice to evaluate the bone marrow. US has intrinsic limitations in the assessment of bone. In some applications, however, it can be useful to assess selected bone disorders, especially if performed as a complement to standard radiographs (Cho et al. 2004). With US, the interface between soft tissue and cortical bone is highly echogenic because of an inherent high acoustic impedance mismatch (Erickson 1997). The bone cortex appears as a regular continuous bright hyperechoic line with strong posterior acoustic shadowing and some reverberation artifact (Fig. 2). Deeper structures, such as the internal cortical architecture, the endosteum and the underlying trabecular bone, remain inaccessible with US, except for rare pathologic conditions in which the cortex is extremely thinned or destroyed in its full thickness. In normal adults, the periosteum cannot be detected as a separate structure with US. Using very high frequency probes, it may appear as a thin hypoechoic line apposed to the bone cortex at certain sites in children.
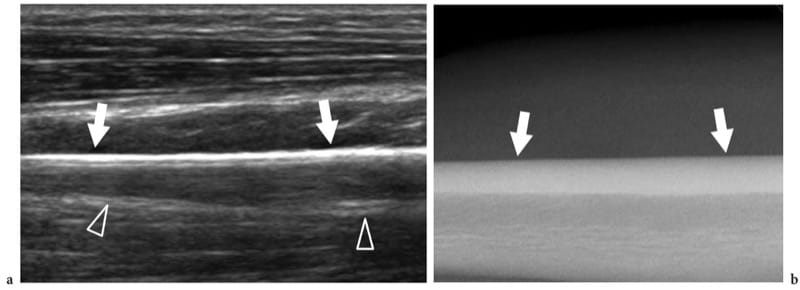
Figure 2. a,b. US appearance of normal bone: surface echotexture. a Longitudinal 12–5 MHz US image obtained over the diaphysis of the radius with b radiographic correlation demonstrates the bone surface as a continuous straight hyperechoic line (arrows) produced by a strong reflection of sound due to the marked difference in acoustic impedance of the soft tissues and bone. Reverberation artifact (arrowheads) projecting in the shadow beyond the bone can be seen.
Given the straight and continuous appearance of the bright echo of the bony cortex, subtle surface irregularities and sites of penetration of nutrient vessels can be visualized (Fig. 3). A careful scanning technique and Doppler imaging allow easy depiction of the vessels entering the bone. The posterior acoustic shadowing of sesamoids or calcifications located in close relationship with the bone surface can mimic cortical breaks. Growth plates in the immature skeleton may also resemble a focal discontinuity of the bone surface: they can be distinguished from fractures due to their peculiar anatomic location (Fig. 4). Marginal osteophytes or bone spurs can project over the cortex mimicking focal breaks. Previous surgery may also affect the continuity of the cortex. Focal interruptions of the hyperechoic cortical line are seen after construction of bone tunnels, such as in ligament reconstruction surgery or following ablation of screws and pins. Close correlation with standard radiographs allows a definitive diagnosis in nearly all the circumstances described above.
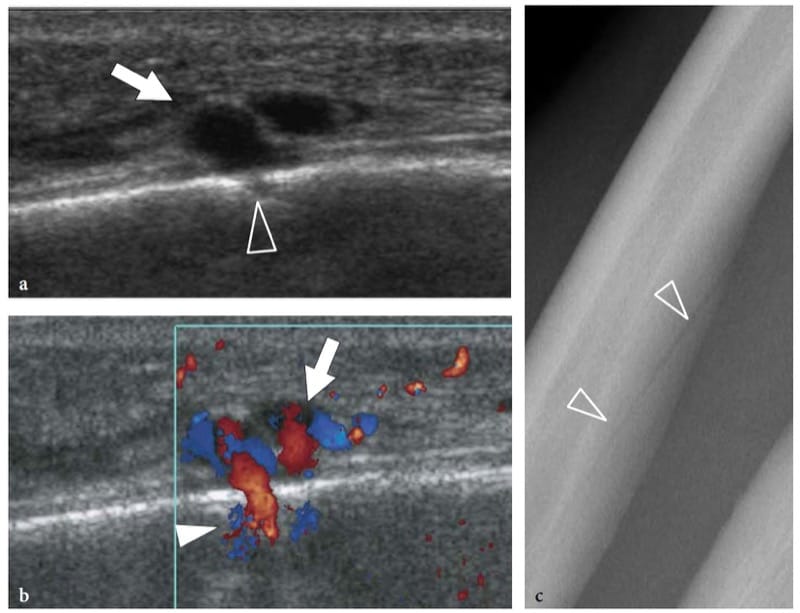
Figure 3. a–c. US appearance of normal bone: nutrient vessels. a,b Longitudinal a gray-scale and b color Doppler 12–5 MHz US images over the diaphysis of the ulna reveal a small break (arrowhead) in the bone surface crossed by nutrient vessels (arrow). This finding should not be mistaken for fractures or erosions. c Radiographic correlation demonstrates a nutrient channel (arrowheads) piercing the ulnar shaft obliquely.
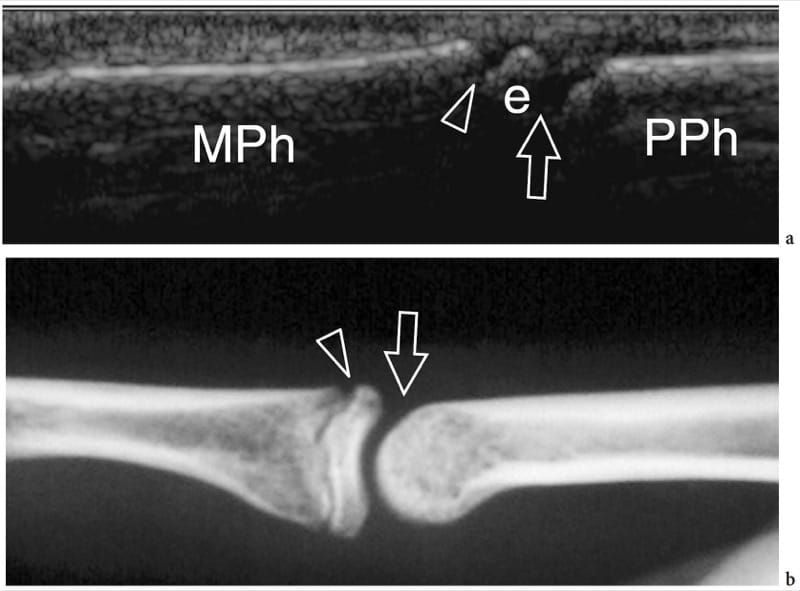
Figure 4. a,b. Developing bone. a Longitudinal 12–5 MHz US image over the proximal interphalangeal joint in a 10-year-old boy with b radiographic correlation demonstrates the base (e) of the middle phalanx (MPh) separated from the head of the proximal phalanx (PPh) by a large break in the cortical bone due to the joint space (arrow). Another smaller hypoechoic break (arrowhead) separates the shaft from the base of the middle phalanx. It is related to the growing plate (physis), which is responsible for allowing the shaft of the bone to lengthen until full growth is obtained. The epiphysis fuses to the diaphysis by bone when the cartilaginous growing plate becomes ossified.
A variety of focal projections (tuberosities, ridges, etc.) and defects (fossae, sulci) of bone modulate the cortical surface; they are often associated with tendon or ligament insertion (tuberosities, ridges) or tendon and nerve reflection (sulci). US can appreciate them as outgrowths or depressions of the otherwise straight cortical outline (Fig. 5). In some instances, these alterations are useful landmarks to orient the probe more appropriately when scanning articular and para-articular structures. Anatomic variation of the shape and size of sulci and tubercles may also have clinical relevance and can be readily appreciated with US. Shallow bony grooves predispose to tendon instability during muscle contraction and joint motion (Levinsohn and Santelli 1991). Hypertrophy of tubercles that act as reflection pulleys for tendons results in local friction during movement and development of stenosing tenosynovitis. In the lower limb, measurement of torsion angle of long bones has clinical value because pathologic states may affect the joint function and predispose it to degenerative changes. Torsion angle measurements can be performed by collecting serial transverse US images at the proximal and distal epiphyses. Assessment of tibial retroversion and femoral anteversion has been reported with US: in this setting, the US findings were found to correlate well with the CT findings (Kumar et al. 1992; Pasciak et al. 1996; Ehrenstein et al. 1999). After closed intramedullary nailing of femoral fractures, intraoperative detection of torsion abnormalities with US can avoid a second surgical look for the patient (Ehrenstein et al. 1999).
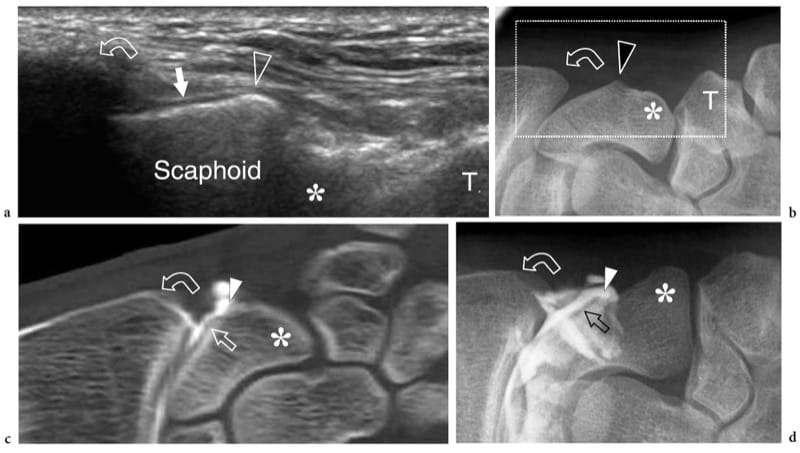
Figure 5. a–d. US appearance of normal bone: surface details. a Coronal 12–5 MHz US image obtained over the lateral aspect of the scaphoid with b correlative anteroposterior radiograph of the radial wrist demonstrates a blunt focal projection of bone (arrowhead) at its waist emerging from underneath the radial styloid (curved arrow) and separating the proximal articular surface covered by hyaline cartilage (arrow) from the extra-articular portion of bone. Distally, note the scaphoid tubercle (asterisk) in a deeper location. T, trapezius. The field-of-view of the US image is indicated by a dashed rectangle in b. c Coronal reformatted CT-arthrographic image and d anteroposterior conventional arthrogram obtained after intra-articular injection of contrast material within the radiocarpal joint show the relationship of the landmarks described above with the intra- and extra-articular portions of the scaphoid surface.
Abnormalities of the bone surface displayed with US may reflect underlying bone pathology (Fig. 6). On the basis of the US findings these abnormalities can be arbitrarily divided into: “outgrowths” or “plus images”, which are focal projections of bone extruding into the soft tissues (Fig. 6b); “irregularities of the cortical outline”, which are focal breaks or step-off deformities of the cortical echo (Fig. 6c); and “defects” or “minus images”, which are irregularities of the cortical outline associated with lesions developing from inside the bone or lesions expanding outside the bone and invading it later (Fig. 6d). Although the diagnosis of bone pathology relies primarily on conventional radiographs, CT and MR imaging, sonologists should be aware of the US appearance of basic bone abnormalities because radiographs are not always available at the time of US examination; in addition, on radiography subtle lesions may be obscured by the curvature of bone and overlying structures. These are the main reasons why bone should invariably be checked during a standard US examination of the musculoskeletal system. Bone abnormalities seen at US can easily be correlated with clinical findings and can suggest the requirement for additional radiographic views or other imaging studies if further evaluation is warranted.
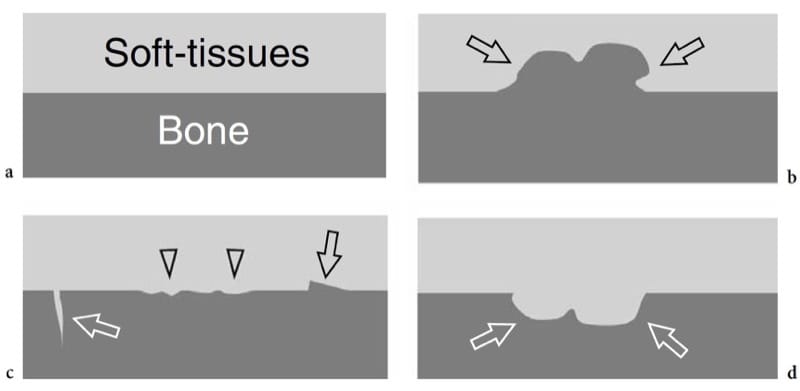
Figure 6. a–d. Bone surface abnormalities that are detectable with US. a Normal bone: a straight regular interface separates the bone from the soft-tissues. b Outgrowths or “plus images”: a focal projection of bone (arrows) is observed in the soft tissues. c Irregularities of the cortical outline: the bone–soft tissue interface is rough (arrowheads); focal breaks (white arrow) or step-off deformities (black arrow) can be seen. d Defects or “minus images”: a focal loss of bone (arrows) is observed. Soft tissues intervene within the defect.
3. OUTGROWTHS
Anatomic Variants
Plus lesions can be related to normal anatomic variants that may become symptomatic because of compression exerted on the adjacent soft-tissue structures. The role of US in the assessment of bone variants is twofold: to detect them and to reveal associated pathologic changes in the adjacent soft tissues. US is not only able to demonstrate the relationship between the abnormal bony outgrowth and the surrounding soft tissues, but can also evaluate tendon or nerve impingement during dynamic scanning. Among possible examples of bone outgrowths that represent anatomic variants, the supracondylar process is a rare bony outgrowth that arises from the medial aspect of the distal humeral shaft (Sener et al. 1998; Subasi et al. 2002). It can give rise to a thick fibrous band (Struthers ligament) inserting into the distal humeral epiphysis. Due to the close relationship with the median nerve, the process and the adjacent ligament can cause a nerve entrapment syndrome. The peroneal tubercle of the calcaneus is a small bone ridge that gives insertion to the inferior peroneal retinaculum and separates the peroneus brevis from the peroneus longus tendons. Congenital enlargement of the tubercle appears at physical examination as a firm mass located just inferior to the tip of the lateral malleolus. Chronic friction of a hypertrophied tubercle with the adjacent tendons can cause stenosing tenosynovitis or tendon rupture (Bruce et al. 1999; Wang et al. 2005).
4. BONE EXOSTOSES
Bone exostoses (osteochondromas) are benign tumors arising, in most instances, from the metaphysis of long bones. They consist of a bony spur whose cap is covered by hyaline cartilage. Exostosis can be solitary or multiple, the latter condition being known as multiple hereditary exostosis (Murphey et al. 2000; Stieber and Dormans 2005). Most solitary osteochondromas occur in the distal femur, proximal tibia and proximal humerus. They may become symptomatic because of impingement on the adjacent soft-tissue structures, such as nerves, tendons and vessels or, more rarely, because of neoplastic changes (chondrosarcoma) occurring in the cartilaginous cap. In other instances, exostoses may lead to formation of an inflamed synovial bursa as a result of chronic friction. US demonstrates exostoses as outgrowths of hyperechoic bone covered by hypoechoic cartilage (Fig. 7). The bone component of the exostosis appears as a continuous hyperechoic line, whereas the cartilaginous cap consists of a hypoechoic layer that may contain some hyperechoic foci with posterior acoustic shadowing related to cartilage calcifications (Murphey et al. 2000). US has been shown to allow accurate measurement of the cartilaginous cap thickness, a factor related to the risk of sarcomatous degeneration (Malghem et al. 1992). The main limitations of US are its inability to evaluate deep lesions inaccessible to the probe and the analysis of the osseous component of the lesion (Murphey et al. 2000). Local compression exerted on the adjacent soft tissues can be diagnosed with US. Deep venous thrombosis, arterial insufficiency and synovial bursa formation and bursitis (bursa exostosica) are associated findings detectable with gray-scale US and Doppler imaging (Fig. 8) ( El – Khoury and Bassett 1979; Keeling et al. 1993; de Matos et al. 1983).
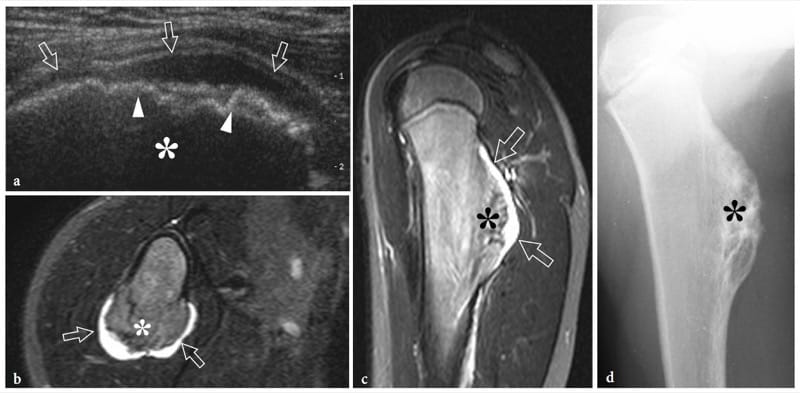
Figure 7. a–d. Sessile osteochondroma. a Longitudinal 12–5 MHz US image over the proximal right arm in an 8-year-old child with local painless swelling reveals a broad-based projection of the humerus (asterisk) characterized by irregular outlines (arrowheads) and a continuous hypoechoic cap (arrows) merging without interruptions with the external surface of bone. Correlative b transverse and c sagittal T2-weighted MR images reveal a broad-based osteochondroma arising from the posterior cortex of the proximal diaphysis of the right humerus covered by an area of high signal intensity (arrows) related to the cartilaginous cap. Both b,c MR imaging and d plain film confirm that the cortex of the host bone merges without interruption with the cortex of the tumor and that the medullary portion (asterisk) communicates with the medullary cavity of the adjacent humerus.
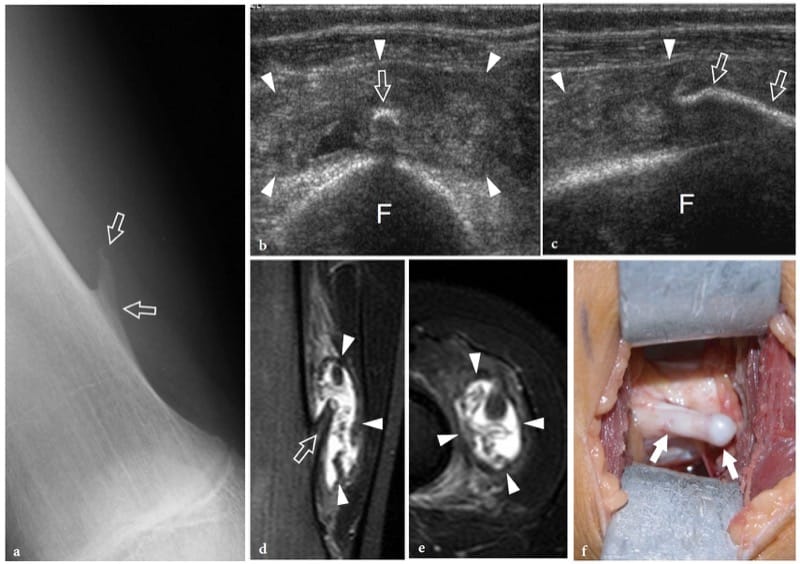
Figure 8. a–f. Bone exostosis. a Anteroposterior radiograph of the distal right femur demonstrates a sting-like exostosis (arrows) arising from the medial side of the diaphysis in a 13-year-old boy with local swelling, tenderness and gradually increasing pain. b Transverse and c longitudinal 12–5 MHz US images obtained over the radiographic finding with d coronal and e transverse fat-suppressed T2-weighted MR imaging correlation show the exostosis (arrows) as an abnormal thin bony projection arising from the femoral (F) shaft. A huge bursal reaction (arrowheads) containing fluid and inflammatory tissue with fronds is seen surrounding the exostosis. Dynamic US scanning allows an easy assessment of the compressible nature of the bursa, excluding a possible malignant transformation to chondrosarcoma. f Gross operative view of the same case shows the exostosis (arrows).
5. DEFECTS
US can detect a variety of “minus” lesions ranging from small para-articular erosions caused by chronic synovitis to large post-traumatic defects. One of the most common bone defects is the Hill-Sachs lesion, a compressive fracture of the humeral head that follows anterior shoulder dislocation. The lesion derives from the traumatic action of the sharp anterior glenoid border against the posterolateral aspect of the dislocated humeral head. US has proved to be an efficient modality to detect a Hill-Sachs lesion and assess its size and depth (Cicak et al. 1998; Pancione et al. 1997; Farin et al. 1996). Compared with surgery, US has 96% sensitivity, 100% specificity and 97% accuracy in the diagnosis (Cicak et al. 1998). The McLaughlin fracture is the counterpart of the Hill-Sachs lesion in patients with posterior shoulder dislocation; it is located on the anterior aspect of the humeral head and results from impaction of the posteriorly dislocated humeral head against the posterior glenoid rim. A high degree of suspicion is necessary to detect Hill-Sachs and McLaughlin fractures because they can easily be overlooked during routine shoulder US. Both are detected as triangular notches of the osseous surface; because of their intra-articular location, they can be filled with synovial effusion. Once detected, a careful evaluation of the fracture size is mandatory because larger lesions are most likely associated with recurrent dislocation.
6. IRREGULARITIES OF THE CORTICAL OUTLINE
Bone surface irregularities usually refer to fractures and erosions.
7. ACUTE FRACTURES
It is accepted that US is not the first-line imaging modality to detect acute bone fractures. In the acute phase, plain films can diagnose the majority of fractures and assess the displacement of bony ends. CT may be performed in selected cases (tibial plateau, pelvic girdle or spine fractures, etc.) when the complex local anatomy causes overlapping of bones on conventional radiographs. Using volumetric scan acquisition algorithms, CT is able precisely to detect the number of fracture fragments and their position. Reformatted images in the sagittal and coronal planes can provide accurate map of the fracture lines. In addition, thin collimation CT scanning can identify small, nondisplaced fractures that can easily be missed on plain films (lateral talar process, transverse process fractures, etc.). Unlike CT, MR imaging has a marginal role in assessing acute fractures: in most cases, the fractures identified with MR imaging are incidental findings in patients examined for possible soft-tissue lesions. This technique seems, however, superior to CT for the assessment of nondisplaced fractures of the femoral neck and the proximal tibial epiphysis as well as for detecting stress fractures.
US may be useful for detecting fractures in those cases in which the fracture was undetected at the initial radiographic examination. In general, patients suffering persistent localized pain are submitted to US examination 2–3 weeks after trauma to rule out soft-tissue lesions. At US, fractures appear as focal breaks of the hyperechoic cortical line, usually associated with periosteal thickening and subperiosteal hematoma (Fig. 9): fractures should be not mistaken for cortical breaks related to the passage of nutrient vessels. In any case, US findings require comparison with the plain films, physical examination and patient’s history. In acute fractures, local pressure over the break, applied with either the probe or the fingers, generates pain. Several reports have described the usefulness of US in diagnosing nondisplaced fractures that are difficult to see on standard radiographs, such as those of the greater tuberosity (Patten et al. 1992), scaphoid (Hodgkinson et al. 1993; Munk et al. 2000; Herneth et al. 2001; Hauger et al. 2002; Senall et al. 2004), tibia, including the so-called Segond fracture (Boutry et al. 2005; Lewis and Logan 2006), cuboid (Wang et al. 1999; Enns et al. 2004), lateral talar process (Copercini et al. 2003), anterosuperior calcaneal process (Boutry et al. 2006) and os peroneum (Brigido et al. 2005) fractures. In the chest, US can help to differentiate rib fractures from metastases (Griffith et al. 1999; Hurley et al. 2004). Several articles have pointed out the utility of US for detecting fractures in the immature skeleton of children (Markowitz et al. 1992; Steiner and Sprigg 1992; Hubner et al. 2000; Kayser et al. 2003). In specific settings, US is an efficient means to detect interposition of soft tissues within the fracture line that can interfere with healing, as well as to identify nerve impingement between fracture fragments or inside a callus (Bodner et al. 1999, 2001; Tukenmez et al. 2006).
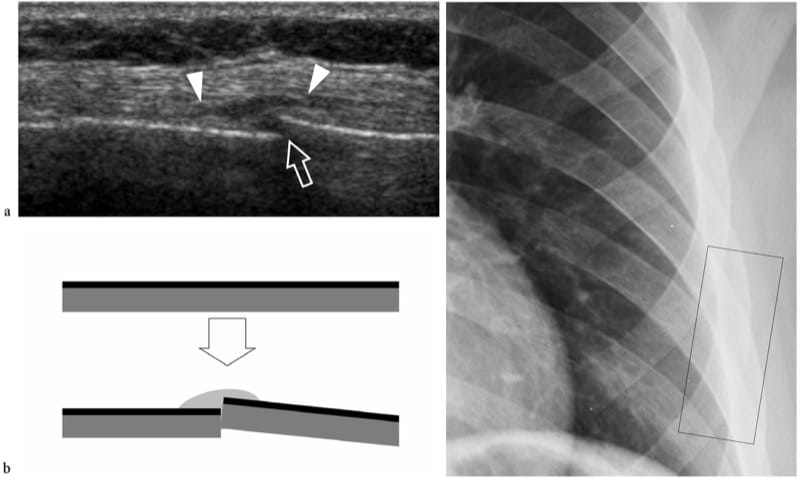
Figure 9. a–c. Occult fracture. a Long-axis 12 MHz US image over the left sixth rib with b schematic drawing correlation in a patient complaining of persistent local pain after a fall demonstrates a step-off discontinuity (arrow) of the rib surface with overlying soft-tissue edema (arrowheads) due to a fracture. The fracture site was extremely painful when applying pressure with the probe over it. c Chest radiograph obtained 1 week earlier did not demonstrate any bone abnormality. The black rectangle indicates US probe positioning.
8. STRESS FRACTURES
Stress fractures result from repetitive stress forces transferred to bone; they can be divided in fatigue fractures and insufficiency fractures. Fatigue fractures occur when normal bone undergoes repetitive overuse stressing distal to the fracture site. Insufficiency fractures involve weakened bones that are more vulnerable to normal stresses; these fractures are observed in aged women with osteoporosis and involve, in most cases, the calcaneus and the metatarsals. On the other hand, fatigue fractures typically involve the lower extremities in sportsmen; they may result from high impacts during running and jumping or from prolonged bicycling or walking. On plain films, an early diagnosis of undisplaced insufficiency fractures is not feasible because the callus is not yet calcified. US can be helpful in assessing early metatarsal stress fractures (Howard et al. 1992; Bodner et al. 2005). In stress fractures, US demonstrates soft-tissue swelling and local hyperemia, whereas the fracture line is not visible (preserved continuity of the cortical outline). A thin hypoechoic band overlying the cortex is often seen as a result of periosteal reaction and inflammation (Fig. 10). Later on, subtle calcified deposits may be observed over the bone reflecting initial callus formation (Fig. 11). In the appropriate clinical setting, we believe the US appearance of stress fractures rather specific. In positive cases, the patient is treated with rest and analgesic drugs until resolution of symptoms. MR imaging examination can be limited to patients with a negative US examination or with positive US findings that do not respond to appropriate treatment.
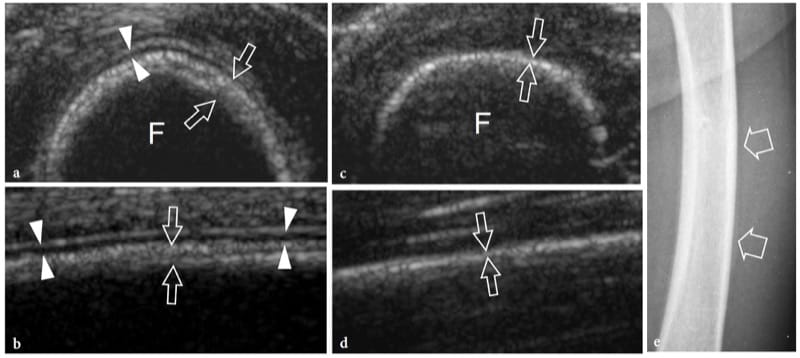
Figure 10. a–e. Stress reaction. a Transverse and b longitudinal 12–5 MHz US image over the mid-shaft of the left femur (F) in 1-year-old child after a blunt trauma shows a straight hypoechoic layer overlying the cortex related to elevation and thickening of the periosteum (arrowheads). A concomitant thickening and delamination of the underlying cortical echo (arrows) can be appreciated. These features indicate post-traumatic stress reaction of bone. c,d Corresponding US images obtained on the healthy side for comparison. Note the thinner appearance of the cortical surface (arrows) and the lack of periosteal visibility. e Anteroposterior radiograph of the femoral shaft does not reveal any bone abnormality.
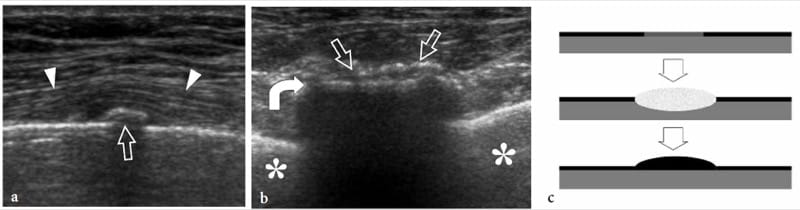
Figure 11. a–c. Stress fracture. a Long- and b short-axis 12 MHz US images over the seventh rib in a patient who suffered lateral chest trauma 2 weeks earlier show subtle periosteal new bone formation (straight arrows) over the bone surface (curved arrow) and slight local soft-tissue elevation (arrowheads) indicating the site of fracture. The initial radiograph was negative. Asterisk, lung. c Schematic drawing illustrates the pathomechanism of a stress injury involving a limited bone area in the absence of a frank cortical fracture line (upper image). The altered mechanics lead to focal periosteal and marrow edema formation (middle image) with final new calcific deposition in the periosteum over the involved site (lower image).
9. FRACTURE HEALING
In acute fractures of the long bones, US has proved to be more sensitive than conventional radiography for showing the early phases of organization of the callus, and its progression to bridging new bone, thus predicting whether the development of callus is normal or delayed (Craig et al. 1999). In displaced extra-articular distal radial fractures, US may assist the orthopaedic surgeon to assess bone alignment during closed reduction and cast immobilization (Chern et al. 2002). US can also assess the status of the fracture site in patients with non-union by showing disorganized soft tissue around the bony ends (Maffulli and Thorton 1995). After tibial nailing, US may provide prognostic information concerning fracture healing, thus helping selection of the most appropriate therapy (Moed et al. 1998). In addition, it is able to detect complications of orthopaedic treatment (Gibbon et al. 2002). Fracture fixation implants can be followed by complications, such as infection, impingement and mechanical failure. In infections, US can identify soft-tissue abscesses and sinus tracts, and assess their relationship with implants and vital structures (Fig. 12a,b) (Gibbon et al. 2002). In addition, US can be used to guide needle aspiration of fluid collections for cultural purposes. Recently, extensor pollicis longus tendon tethering following K-wire insertion to treat unstable distal radius fractures has been described with US (Harrison et al. 2004). After volar plate osteotomy for Colles fracture, tenosynovitis and tears of this tendon following impingement on the screw can be demonstrated with US as well. Ankle tendon impingement due to orthopaedic hardware has also been reported (Fig. 12c–e) (Shetty et al. 2002). In children with percutaneous cross-pin fixation for displaced supracondylar humeral fractures, dynamic US can evaluate altered gliding and impingement of the ulnar nerve in the cubital tunnel (Karakurt et al. 2005).
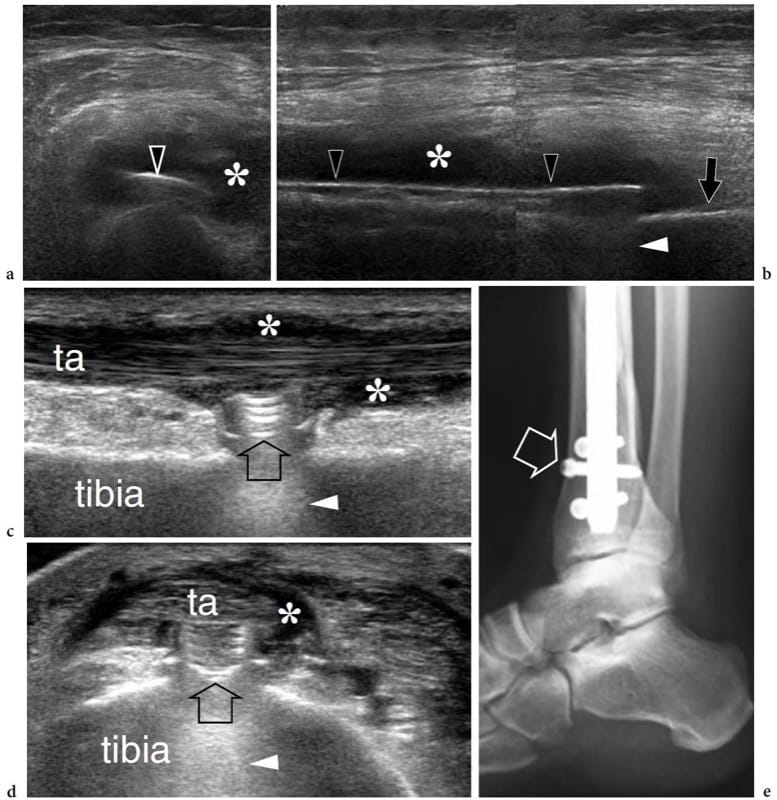
Figure 12. a–e. Complications of orthopaedic treatment of fractures. Two different cases. a,b Transverse and b longitudinal 12–5 MHz US images of the left femur in a patient who was previously treated for a femoral shaft fracture with placement of a metal implant (open arrowheads) demonstrate a hypoechoic fluid collection (asterisks) surrounding the compression plate. Subsequent surgery disclosed an abscess. Note the posterior reverberation artifact (white arrowhead) of the plate compared with the femoral cortical bone (arrow). c Long- and d short-axis 17–5 MHz US images over the anterior cortex of the distal
tibia with e radiographic correlation in a patient previously operated on for a tibial fracture reveal the surface contours of an interlocking screw head (arrow) impinging on the tibialis anterior tendon (ta). Reverberation (arrowhead) is shown deep relative to the screw head. Note the associated tenosynovitis (asterisks) of the tibialis anterior tendon.
Some authors have suggested that the process of fracture healing can be followed with color Doppler imaging and spectral analysis (Caruso et al. 2000). The rationale is based on the fact that, at the time of trauma, the blood supply to the fracture site is interrupted; then, blood vessels reach the periosteal portion of the callus from adjacent soft tissues forming a new circulation to the callus (Postacchini et al. 1995). US is able to follow the formation of new vessels at the fracture site and to assess flow characteristics in them during development of fracture callus (Fig. 13a) (Caruso et al. 2000). In patients with normal callus development, Doppler spectral analysis reveals an initial decrease in the resistive index as a result of the neoangiogenetic process occurring during the early weeks after fracture (Fig. 13b). Over time, the arterial resistance progressively increases, reflecting a physiologic decrease in the degree of local vasculature that accompanies the mature phase of the callus. On the other hand, patients with non-union and delayed healing have higher resistances early, related to a poor formation of neovasculature. Although these features need further experience in larger series, Doppler imaging seems a promising modality for predicting normal or delayed fracture healing based on defective vasculature at the fracture site about 1 month after trauma (Caruso et al. 2000). However, standard radiographs remain the primary imaging technique for evaluating callus formation.
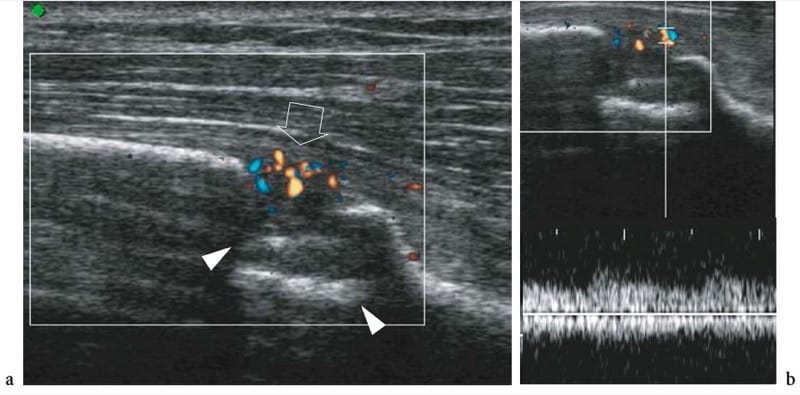
Figure 13. a,b. Early callus formation following fracture of the distal tibia. a Color Doppler 12–5 MHz US image obtained 12 days after treatment shows a bone defect (arrowheads) related to the fracture site and multiple blood flow signals (arrow) in the periosseous soft tissues superficial to the fracture. b Spectral analysis reveals low-resistance (RI <0.50) arterial flow in the vessels surrounding the fracture. These features indicate initial normal development of fracture callus.
10. EROSIONS
In patients who have rheumatoid arthritis, US has proved to be an excellent modality for detection of early bone erosions, with a sensitivity superior even to plain films (Wakefield et al. 2000). Erosions typically occur in the hand, the capitate being the bone most commonly affected, followed by the triquetrum, hamate, scaphoid and trapezoid; the second and third metacarpal heads are also a common location (Cimmino et al. 2000). US demonstrates erosions as oval or rounded well-defined cortical breaks with an irregular floor visible in longitudinal and transverse planes (Fig. 14a,b). They initially affect the bare areas of the joint surface and share a common appearance in rheumatoid arthritis and other seronegative arthropathies. Hypoechoic synovial pannus and Doppler signals of flow are often detectable within them. Loss of definition of the articular cartilage and widening of the joint spaces are associated findings. Compared with standard radiographs, US can be considered a more sensitive, effective and reliable means for detecting erosions in rheumatoid arthritis (Wakefield et al. 2000; Alarcon et al. 2002; Weidekamm et al. 2003). In early disease, it has been shown able to detect 6.5-fold more erosions than did radiography in 7.5-fold the number of patients. In advanced disease, these differences were 3.4-fold and 2.7-fold, respectively (Wakefield et al. 2000). Depending on their location, US has proved to be superior to radiography for depiction of erosions in the first, second and fifth metacarpophalangeal joints, but inferior in the fourth metacarpophalangeal joint due to the problem of access (Schmidt 2001). Erosions being most commonly found along the radial and ulnar sides of the joints, the main drawback of US is related to the evaluation of the third and fourth metacarpopha-langeal joints as the defects are masked by bone and not sufficiently exposed to the US beam (Fig. 14c). This is the main reason why MR imaging or CT are, in this particular application, more sensitive. When an adequate acoustic window exists, US can appreciate even minimal erosions.
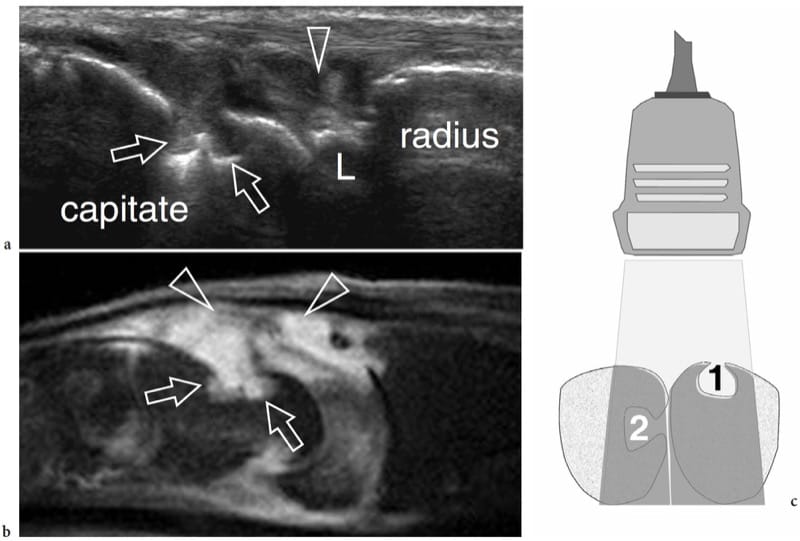
Figure 14. a–c. Bone erosions in rheumatoid arthritis. a Longitudinal 17–5 MHz US image over the dorsal aspect of the wrist with b sagittal fat-suppressed T2-weighted MR imaging correlation demonstrates two adjacent erosions (arrows) on the capitate as well-defined focal cortical defects filled by hypertrophied synovium (arrowheads). L, lunate. c Schematic drawing illustrates the site-dependent ability of US to detect bone erosions. Two erosions are shown: one placed on a bone surface facing the probe (1), the other on the side surface of an adjacent bone (2). US is able to display only the first one, because the second is masked by the acoustic shadowing (intermediate gray) coming from intervening bone.
11. OSTEOMYELITIS
Osteomyelitis is the inflammatory response of bone secondary to infection. Depending on the pathomechanism involved, osteomyelitis can derive from hematogenous spread versus direct seeding of bacteria to the bone. Hematogenous osteomyelitis tends to involve the highly vascularized metaphysis of growing bones in the pediatric age group, with a bimodal distribution affecting children younger than 3 years of age and older than 7 years of age. It is more often caused by Staphylococcus aureus and, to a lesser extent, by Enterobacteria, group A and B streptococci and Haemophilus influenzae (Jbara et al. 2006). Direct osteomyelitis may be secondary to penetrating trauma, foreign bodies, open fractures and iatrogenic procedures. Compared with the hematogenous form, direct infection more usually affects an older age group, consisting of adolescents and adults. Staphylococcus aureus, Enterobacter and Pseudomonas are the most common causative agents (Jbara et al. 2006). Finally, contiguous spread of infection from adjacent tissues is often encountered in immunocompromised and diabetic patients. In osteomyelitis, diagnostic imaging helps to make an early diagnosis and establish an appropriate therapy (Sammak et al. 1999). Radiography is routinely used as the first-line imaging modality but, in general, does not show any abnormality during the first 1–2 weeks of infection (Sammak et al. 1999). Subtle periosteal reaction may indicate initial disease. Owing to problems of access, US cannot assess bone marrow and trabecular bone involvement, but is an excellent means of identifying abscess formation and adjacent soft-tissue involvement (Mah et al. 1994; Davidson et al. 2003). In the pediatric age group, deep soft-tissue swelling has been described as the earliest sign of disease followed by periosteal elevation and formation of a thin layer of subperiosteal fluid (Mah et al. 1994). At US, periosteal elevation can be appreciated as single or multiple linear echoes surrounding the cortical bone, whereas subperiosteal fluid appears as an anechoic or hypoechoic collection separating the periosteum from the cortical bone as the result of superficial extension of the intraosseous process (Fig. 15a) (Steiner and Sprigg 1992; Sammak et al. 1999). Detection of blood flow within or around the infected periosteum demonstrated by Doppler imaging can be useful in distinguishing early from advanced acute osteomyelitis (Chao et al. 1999). Doppler US has also been found valuable in assessing the efficacy of antibiotic therapy (Chao et al. 1999). One should be aware, however, that a normal US examination does not exclude bone infection (Bureau et al. 1999). Later stages of disease are characterized by cortical irregularities and erosions, which are typically found in patients with symptoms lasting for more than 1 week (Fig. 15b–e). Then, subperiosteal collections may expand and form abscesses that can be drained under US guidance when medical therapy alone is inadequate (Abiri et al. 1989; Bureau et al. 1999; Craig 1999). US guidance contributes to reducing complications related to the procedure, such as the inadvertent contamination of uninvolved compartments and traumatic damage to vessels and nerves along the needle path (Bureau et al. 1999; Craig 1999). An opening (cloaca) connecting the infected bone with the abscess or a channel between the infected bone and the skin (sinus tract) can be seen as a defect of the cortical layer in continuity with the hypoechoic collection. Generally speaking, the value of US appears even more relevant in the post-operative phase when the use of MR imaging may be hampered by the presence of orthopaedic metallic implants. In this instance, US can reveal the fluid collection apposed to the implant, which appears as a bright linear structure with posterior reverberation artifact surrounded by hypoechoic fluid. Finally, it is important to point out that evaluation of osseous involvement requires composite imaging algorithms for specific clinical scenarios, with combined use of plain films, nuclear medicine, CT and MR imaging (Sammak et al. 1999).
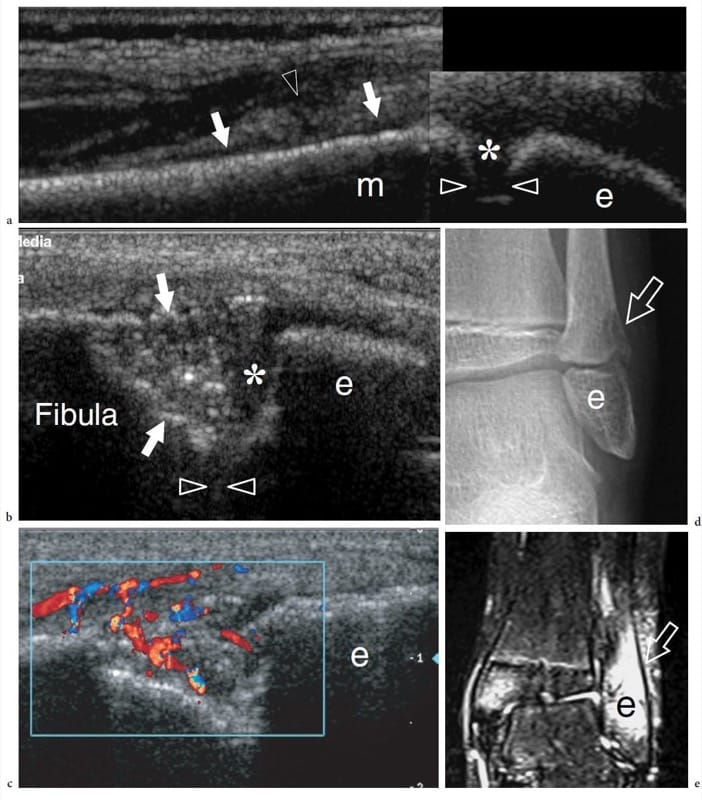
Figure 15. a–e. Acute osteomyelitis. a Longitudinal 12–5 MHz US image over the distal fibula in a 7-year-old child with fever, left lateral ankle pain and swelling demonstrates an echogenic juxtacortical soft-tissue thickening (arrowhead) associated with subtle subperiosteal fluid (arrows) overlying the metaphysis of the fibula. Note the growth plate (asterisk) intervening between the epiphysis (e) and the metaphysis (m). The initial radiographic examination was negative. The patient underwent a course of antibiotic therapy. b,c Longitudinal b gray-scale and c color Doppler 12–5 MHz US images obtained 2 weeks later on the same scanning plane shown in a with d radiographic and e coronal fat-suppressed T2-weighted MR imaging correlation demonstrate a deep defect (arrows) at metaphyseal level with focal bone resorption. A hypervascular blood flow pattern and marked T2-hyperintensity is found within and around the defect. Note the residual layer of physeal cartilage (asterisk) of the growth plate (arrowheads).
12. JOINT
Histologic Considerations
Joint anatomy is variable depending on specific functional requirements. Based on their anatomic structure, joints can be divided in three main groups: fibrous, cartilaginous and synovial (Erickson 1997). In fibrous joints, the bone ends are linked by intervening solid connective tissue, including a sutural ligament (sutures), a collagenous interosseous ligament or membrane (syndesmoses) or cartilaginous periodontium (gomphoses). Cartilaginous joints are divided into symphyses—which contain a fibrocartilaginous disk—and synchondroses—which are formed by bony ends covered by cartilage but lacking synovium. Synovial joints are formed by adjacent bones connected by a cavity lined by synovial membrane. The above types of joints allow different degree of motion, which is minimal in the first group (fibrous) and maximal in the latter (synovial). Because synovial joints are the most commonly examined with US, we will specifically discuss their normal anatomy.
Synovial joints are formed by articulating bone surfaces, fibrous capsule and ligaments, synovium and other intra-articular structures (menisci, labra, ligaments, fat pads, etc.) (Fig. 17). The subchondral bone plate is a thin layer of dense bone linked to the cancellous and cortical bone of the metaphysis that acts as a support for the articular cartilage. The main function of bone plates is to adsorb part of the load from the cartilage and transfer it to the cortical bone through the metaphysis. The microstructure of subchondral bone, with its peculiar orientation of trabeculae, reflects this function. The articular surfaces of bone are covered with hyaline cartilage (Fig. 16a). The cartilage thickness varies among joints: thicker cartilage is found in larger joints subjected to considerable loading, such as the weight-bearing joints of the lower limb. The cartilage thickness also varies in different sites of the same joint as an expression of local differences in load. The hyaline cartilage is formed by cells—the chondrocytes, which account for 0.1% of cartilage volume—and chondroid matrix consisting of collagen and proteoglycans. From the histologic point of view, four cartilage layers can be recognized from superficial to depth, based on a different architecture and orientation of collagen fibers. In the superficial layer, the collagen fibers run tangential to the surface; in the transitional layer, the collagen fibers are arranged randomly; in the deep layers (the radial zone and calcified layer) the fibers are oriented vertically. The main function of the articular cartilage is absorption of load through graded deformation, and its distribution to the underlying bones. During loading, the cartilage is compressed and reduces its thickness due to transient squeezing of water and compression of collagen fibers. Following removal of the load, the induced deformity of the cartilage returns to normal. The hyaline cartilage is avascular and mainly nourished by synovial fluid; only its deepest layer receives arterioles from the subchondral bone plate (Erickson 1997). Marginal to the articular surfaces of bone, the joint capsule inserts into the cortical bone and periosteum (Fig. 17a). The thickness of the joint capsule varies among joints and among different parts of individual joints depending on local demand and functional requirement. The glenohumeral joint capsule, for instance, is very thin and lax to allow a wide range of movements, whereas the anterior capsule of the hip joint is thick to help in maintaining an erect posture. Focal discontinuities in the capsule allow the synovium to herniate into the surrounding soft tissues forming synovial recesses (Fig. 17b). The function of these recesses (synovial-lined bursae) is twofold: to facilitate gliding of para-articular tendons with the underlying joint, and to serve as reservoirs, limiting the damage to the intra-articular structures caused by fluid pressure in abundant joint effusions. The capsule may be reinforced and stabilized by ligaments, which are fibrous cords with some elastic properties connecting two bones just above the joint line. Ligaments can be completely independent of the joint capsule (i.e. the lateral collateral ligament of the knee), or represent merely focal capsular thickening (i.e. the glenohumeral ligaments) (Fig. 17c). Ligaments are oriented along specific anatomic planes to stabilize the joint in different positions (i.e. the proper collateral ligament of the interphalangeal joints acts as a stabilizer during flexion, whereas the accessory collateral ligaments play the same role during extension). The synovium, a mesodermal derivative, consists of a thin synovial lining supported by stromal tissue: it produces the synovial fluid, a clear or pale yellow viscous fluid that allows lubrication of the joint and cartilage nutrition. The synovial membrane lines the joint cavity except for the hyaline cartilage and the intra-articular fibrocartilage structures (Fig. 17d). It also invests some transitional zones extending from the peripheral boundaries of the hyaline cartilage and the fibrous capsule, the so-called bare areas. At these sites, the bone is covered by synovium without the protective layer of cartilage: this makes it particularly vulnerable to synovitis-induced bone destruction (Sommer et al. 2005). Different fibrocartilage structures can be found inside the joint space or related to the articular capsule: their main function is to increase the congruence of the articular surfaces by filling the space between them and to act as shock absorbers thus preventing damage to the hyaline cartilage (Fig. 17d). Some joints contain fat pads, which are adipose structures filling the space between the synovial membrane and the peripheral capsule (Fig. 17d). Intra-articular fat pads adapt their shape to joint movements and the amount of intra-articular synovial fluid; they absorb forces generated during joint motion.
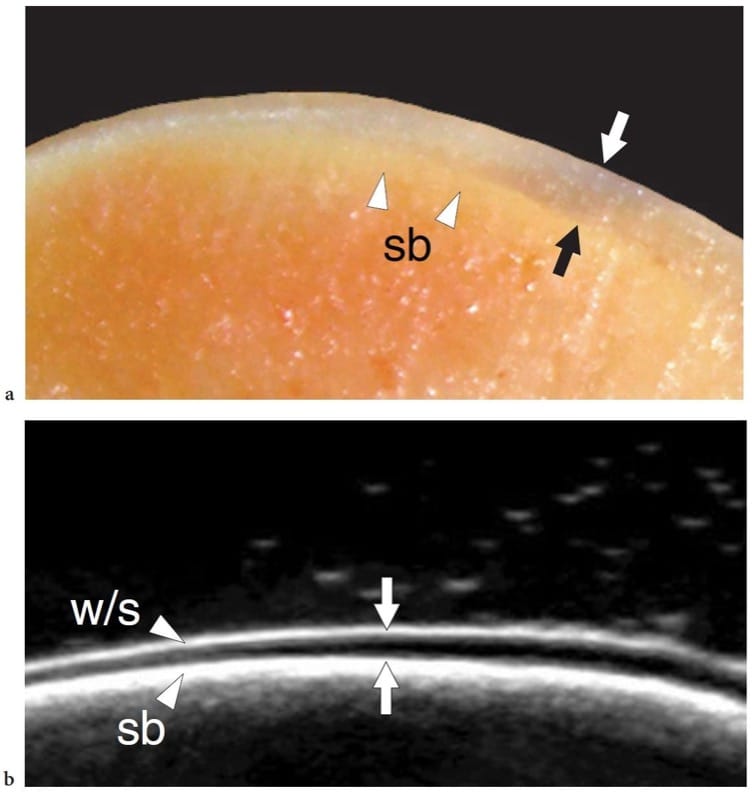
Figure 16. a,b. Normal hyaline cartilage. a Cadaveric cross-sectional photograph of the talar dome shows a superficial uniform layer of articular cartilage (arrows) overlying the subchondral bone (sb). b Corresponding in vitro 17–5 MHz US image demonstrates a superficial echogenic layer (w/s) related to an acoustical impedance mismatch between cartilage (solid) and adjacent fluid (water), an intermediate hypoechoic band (arrows) due to the hyaline cartilage and a deep echogenic layer at the cartilage–subchondral bone interface (sb).
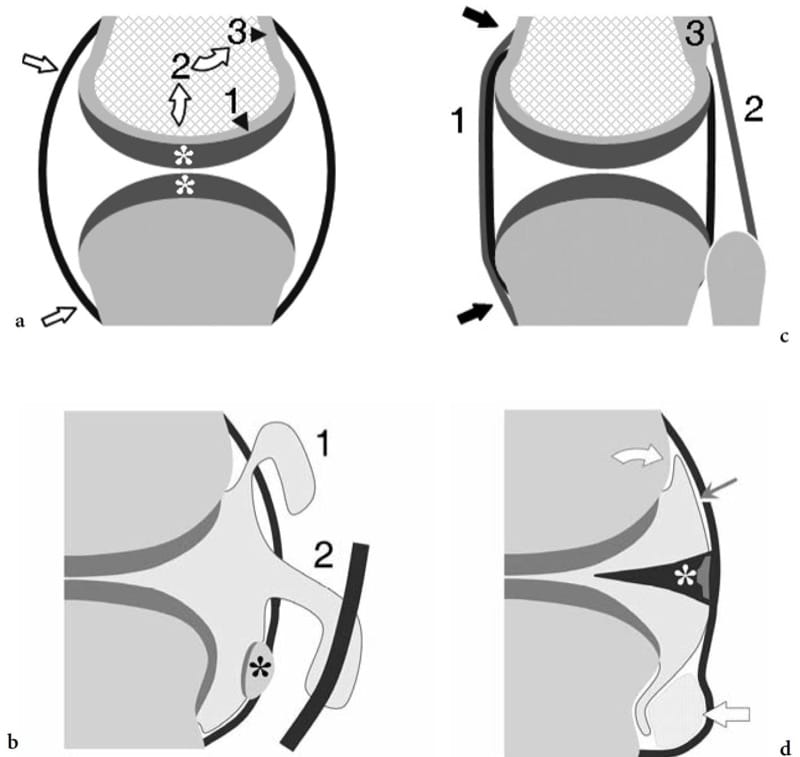
Figure 17. a–d. General anatomy of synovial joints. Schematic drawings of a cross-sectional view of a synovial joint. a Joint capsule and articular cartilage. The joint capsule (straight arrows) is a fibrous sac that inserts beyond the articular surfaces of articulating bones. The thickness of the articular cartilage (asterisks) may vary among parts of the same joint depending on the different demands of loading and weight-bearing (arrowheads). The cartilage transmits loading to the subchondral bone plate (1) which, in turn, transfers part of it (curved arrows) to the cortical bone (3) through the metaphyseal region (2). b Synovial recesses and sesamoids. The synovial recesses arise from focal discontinuities of the capsule, allowing the synovium to extrude into the surrounding soft tissues. Synovial herniation may form communicating synovial pouches (1) or may link the joint cavity with adjacent synovial tendon sheaths (2). Sesamoids (asterisk) are small ossicles embedded in the fibrous capsule or the plantar plate. They can or cannot articulate with the joint surfaces. c Ligaments. These are fibrous bands formed by focal thickening of the capsule (1) or lying at a certain distance from it (2). The strongest ligaments insert into para-articular bone ridges or tubercles (3); these are appropriately oriented to counteract joint instability. d Synovium, fibrocartilages and fat pads. The synovial membrane (thin arrow) invests the joint cavity with the exception of fibrocartilaginous structures (asterisk) and intra-articular extrasynovial fat pads (thick arrow). Between the peripheral boundaries of the hyaline cartilage and the capsule, the synovium invests the bone directly. These zones are called “bare areas” (curved arrow).
13. NORMAL US ANATOMY AND SCANNING TECHNIQUE
The indications for joint US are rapidly expanding due to the refinement of high-resolution transducers and to the fact that both radiologists and clinicians are increasingly aware of the potential of US in this field. US can reliably identify and quantify intra-articular effusions in all appendicular joints, including those, such as the hip, in which physical examination does not yield enough information. Detection of joint effusion indicates the articular origin of symptoms and virtually rules out referred pain or para-articular disorders. US-guided needle puncture of joint spaces allows less painful and more rapid sampling of synovial fluid for analysis and culture than use of a blind technique. In addition, the correct intra-articular injection of medicaments, such as lidocaine or steroids, can easily be confirmed on real-time US scanning. Depending on the individual joint examined, US can visualize only limited portions of the joint surfaces. Tight joints are difficult to examine with these technique. On the other hand, imaging of large and lax joints can be improved by different maneuvers that help to reposition the articular surfaces from underneath the bone. The articular surface of the metacarpal head, for example, can be almost completely evaluated if scanning is obtained in different degrees of flexion. Similarly, forceful flexion of the knee joint allows evaluation of the trochlea by placing the probe over the suprapatellar region (Martino et al. 1998). In the absence of the joint space, as occurs in congenital tarsal coalition, US can demonstrate fibrous or osseous bridging at the level of the joint line (Lee et al. 2002). Detection of the coalition is clinically relevant as it may cause symptoms related to an altered loading to adjacent joints. Once suspected at US examination the diagnosis must be confirmed on CT (Herzenberg et al 1986).
In general, US examination of the joint surfaces reveals a homogeneously hypoechoic smooth linear band due to the hyaline cartilage (Martino et al. 1998; Grassi et al. 1999). Deep to it, the subchondral bone appears as a regular, continuous bright hyperechoic line (Fig. 18). Using in vitro specimens and very high frequency probes (50 MHz), some investigators have demonstrated that the articular cartilage is composed of three layers, characterized—from superficial to deep—by a hypoechoic, hyperechoic and anechoic appearance, respectively (Kim et al. 1995). Although there has been speculation that a somewhat similar pattern can be discerned in infants and young children in vivo using conventional equipment, daily experience suggests that, in normal conditions, the adult articular cartilage appears, and has to be considered, homogeneously hypoechoic (Erickson 1997). Changes in cartilage thickness are well depicted with US and can be accurately measured. In normal states, the synovial membrane is too thin to be discriminated with US: it can be appreciated only in pathologic states that lead to its thickening and hypertrophy. Many joints contain fibrocartilaginous structures, such as the meniscus in the knee, the labrum in the hip and the shoulder, the triangular fibrocartilage in the wrist and the volar and plantar plates in the hand and foot. All these structures appear homogeneously hyperechoic and adherent to the bone or the joint capsule (Fig. 19) (Gerngross and Sohn 1992). The fibrocartilage echotexture differs significantly from that of the hyaline cartilage: in the fibrocartilage, the predominant component is collagen fibers and these cause increased reflectivity. Because of their deep location and close contact with the bone, these structures can be evaluated only in part and, in most cases, not reliably with US. In many joints, dynamic scanning during joint motion may induce changes in the fibrocartilage shape. US demonstrates the joint capsule as a hyperechoic line bordering the joint cavity and merging with the para-articular tissues. Recently, the US appearance of a meniscal ossicle has been reported (Martinoli et al. 2000).
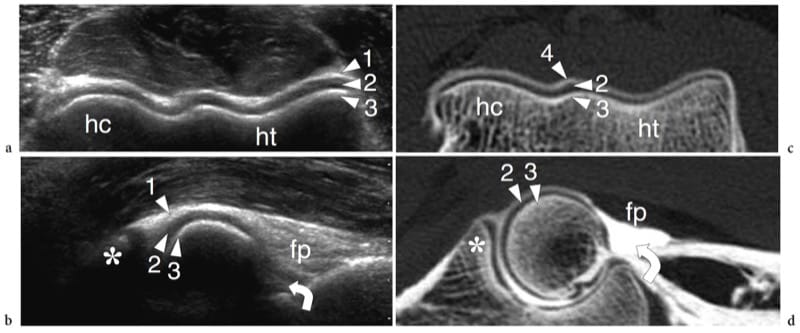
Figure 18. a–d. US appearance of a normal synovial joint. a Transverse and b sagittal 12–5 MHz US images of the distal humeral epiphysis with c,d CT-arthrographic correlation demonstrate the normal hyaline cartilage (arrowheads) as a uniform hypoechoic band (2) covering the hyperechoic subchondral bone (3) of the humeral capitellum (hc) and the humeral trochlea (ht). The joint capsule (1) appears as a hyperechoic line overlying the cartilage. In the sagittal image, the coronoid recess (curved arrow) and the anterior fat pad (fp) are demonstrated. In the CT-arthrographic images, the intra-articular contrast medium (4) helps to delineate the superfi cial boundaries of the cartilages. Asterisk, coronoid process.
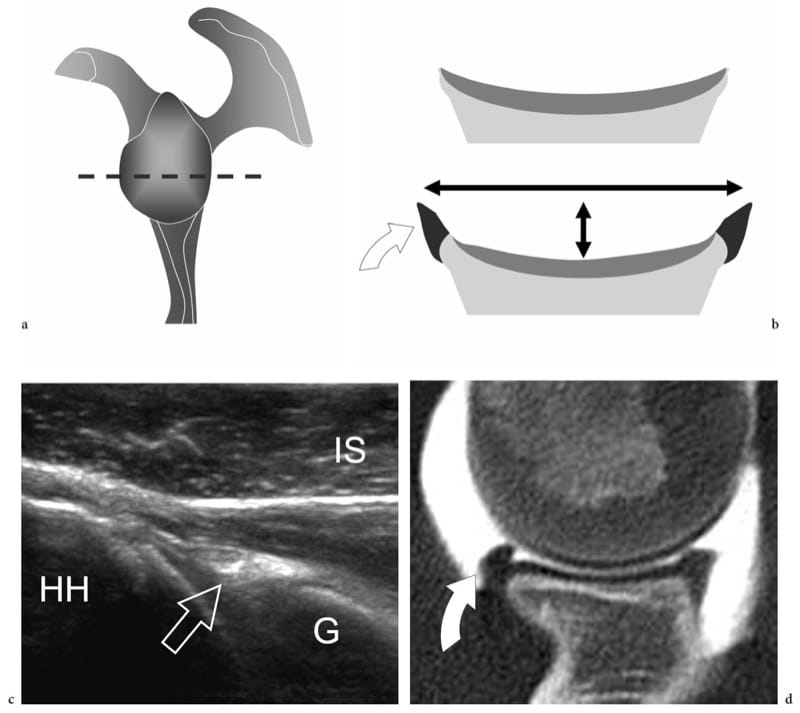
Figure 19. a–d. Articular fibrocartilages: glenoid labrum. a Schematic drawing indicates the cross-sectional plane (dashed line) represented in b. The glenoid labrum (curved arrow) is a fibrocartilaginous ring attached to the peripheral rim of the glenoid cavity. Its main function is to make the glenoid fossa wider (long double-headed arrow) and deeper (short double-headed arrow). c Transverse 12–5 MHz US image obtained over the posterior aspect of the glenohumeral joint with d CT-arthrographic correlation demonstrates the posterior glenoid labrum (arrow) as a homogeneously hyperechoic triangular structure with its base adherent to the glenoid rim (G). HH, humeral head; IS, infraspinatus muscle.
Ligaments appears as hyperechoic fibrillar bands joining two opposite bone surfaces (Fig. 20) (Lee et al. 1996; Mathieu et al. 1997; Brasseur et al. 1994; Morvan et al. 2000; Ward et al. 2003; Miller et al. 2004; Finlay et al. 2004; Peetrons et al. 2004; Boutry et al. 2005). Somewhat similar to tendons, ligaments are anisotropic structures. Therefore, care should be taken to place the probe as parallel as possible to them to avoid artifactual hypoechoic patterns that can mimic pathology. Often, changing the position of the joint improves ligament visualization. Small probes that can better hug the curves and bulges of the bony landmarks are preferred for imaging ligaments. Some ligaments located in the central portion of joints (i.e., the interosseous tarsal sinus ligaments and the cruciate ligaments of the knee) cannot be visualized with US because of the overlying osseous structures. Complex ligaments (i.e., the medial collateral ligament of the knee, the deltoid ligament of the ankle) are made up of individual components that can be distinguished with US as individual structures. In general, ligaments that stabilize a joint are best evaluated while stretched. For example, in the relaxed state, the calcaneofibular ligament of the ankle has a concave course which makes the evaluation of its cranial insertion difficult; with the ankle in dorsiflexion the ligament tightens, pushing the peroneals superficially, and is better depicted (Peetrons et al. 2004). Intra-articular fat pads appear at US as fat-like hyperechoic structures (Fig. 18c). The most important are recognized in the knee (Hoffa pad) and the elbow (anterior and posterior fat pads) (Miles and Lamont 1989; Ferrara and Marcelis 1997). In most joints, small amounts of normal intra-articular fluid can be detected in the articular cavity by means of high-resolution US.

Figure 20. a–d. Normal ligaments. a–c Schematic drawings illustrate the relationship of a ligament (large straight arrows) with the underlying joint structures, including the hyaline cartilage (thin straight arrow), the joint cavity (asterisk) and the synovial membrane (s). The position of the fibrous capsule relative to the ligament may be variable: a internal (between the ligament and the synovium, i.e., the lateral collateral ligament of the knee); b bending to it (i.e., the glenohumeral ligaments of the shoulder, the anterior talofi bular ligament of the ankle, the medial collateral ligament of the knee); c external (outside the ligament and the synovium, i.e., the anterior cruciate ligament of the knee). d Long-axis 17–5 MHz US image over the lateral ankle demonstrates the normal anterior talofi bular ligament as a thick fibrillar band (arrowheads) joining the lateral malleolus (LM) and the talus. The deep surface of the anterior talofi bular ligament is merged with the ankle joint capsule. Note the joint fluid (asterisk) in close contact with the ligament. Thin arrow, articular cartilage.
14. PATHOLOGIC CHANGES
Joint Effusion
Demonstration of an intra-articular effusion is a major step in the investigation of musculoskeletal disorders, as it points the clinician’s attention toward a joint problem and excludes other extra-articular sources of pain and disability. A joint effusion can derive from traumatic or mechanical causes as well as from inflammatory or infectious synovitis; more rarely, it can be related to neoplastic conditions. At physical examination, detection of synovial effusion depends on the overall amount of fluid and the type of joint is involved. Accurate palpation allows detection of medium to large effusions in the elbow, hand and knee. Based on clinical findings, joint effusions in the wrist and ankle are more difficult to be appreciated and discriminated from para-articular soft-tissue swelling and tenosynovitis: distinguishing selective involvement of one joint among adjacent normal ones (e.g., the radio-carpal from the midcarpal) is often problematic at these sites. Shoulder and hip effusions also cannot be identified with certainty based on physical findings alone. Standard radiographs allow diagnosis of significant intra-articular effusion in the elbow, knee and ankle joint. The radiographic landmark of joint effusions is related to direct visualization of distended synovial recesses and secondary shifting of intracapsular extrasynovial fat pads (Smith and Lee 1978). Radiographs are, however, inaccurate for diagnosing joint effusions in the shoulder, wrist and hip. Identification of intra-articular effusions is one of the main reasons why US examination of joints is requested. US has proved to be extremely sensitive and reliable in the detection of even small amounts of intra-articular fluid by scanning not only the level of the joint line but also the recesses and para-articular bursae which are, in general, better exposed to the US beam (Jacobson et al. 1998; Moss et al. 1998; Iagnocco and Coari 2000; Delaunoy et al. 2003). If the amount of fluid is small, examination of the contralateral joint may be helpful to establish whether synovitis is present. In some rheumatologic disorders (e.g., rheumatoid arthritis), however, the usual rule of comparing the opposite side does not invariably help because the disease can have a symmetrical presentation. Although US is highly sensitive in detecting intra-articular fluid, it cannot definitively differentiate among different types of effusions (Wilson 2004). Depending on its nature, a non-infected non-hemorrhagic joint effusion can be completely anechoic with absence of internal echoes (Fig. 21a); or it may contain scattered echogenic spots as a result of proteinaceous content, fibrin, crystals or cellular debris (Fig. 21b) (Farina et al. 2002). The role of US in assessment of joint effusion is not limited to detection of fluid, but can also assist in percutaneous needle placement. Demonstration of an intra-articular effusion affecting a single joint (monoarthritis) is a definite indication for sampling, analysis and cultural procedures in order to rule out microcrystal arthritis and infection. For injecting small joints, US guidance allows significantly greater accuracy than a blind approach (Raza et al. 2003). In our practice, US-guided aspiration of joint fluid significantly reduces the pain associated with needle puncture. In addition, real-time monitoring of the needle reduces the risk of potential damage to adjacent structures, including arteries and nerves. In the traumatic setting, hemorrhagic joint effusions may appear highly echogenic in the first few hours after the trauma (Fig. 21c). Lipohemarthrosis is a condition in which blood and bone marrow fat are found inside the synovial cavity. While blood usually derives from tears of the synovial membrane, fat comes from yellow bone marrow as a result of bone fracture or, more rarely, from periligamentous fat. In most cases, lipohemarthrosis can be considered a confident indicator of an intra-articular fracture: it is characterized by a multilayered appearance made up of a superficial hyperechoic layer reflecting fat and a deep hypoechoic layer due to sedimentation of the red blood cells. After 10–15 minutes of joint immobilization, a thin intermediate band due to the serum can be noted between the fat and the red blood cells (Fig. 21d,e) (Bianchi et al. 1995).
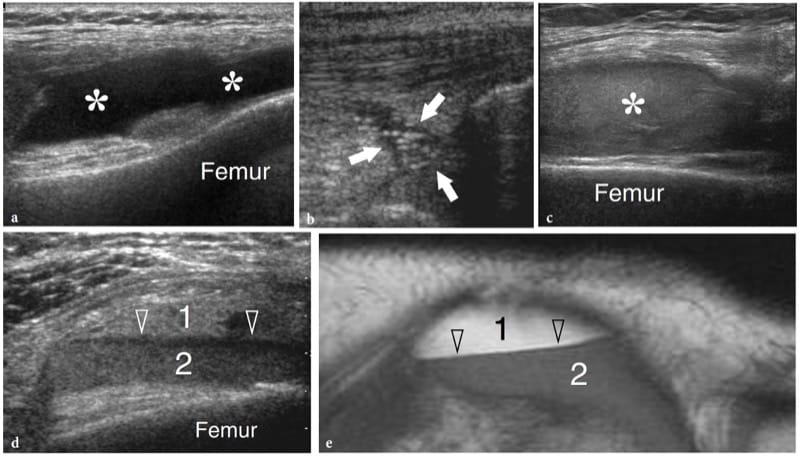
Figure 21. a–e. Joint effusion: spectrum of US appearances in different pathologic conditions. US images were obtained over the suprapatellar recess of the knee with a 12–5 MHz transducer. a Mechanical or inflammatory effusions (asterisks) usually exhibit an anechoic appearance. b Scattered echogenic spots (arrows) can occasionally be encountered within mechanical or inflammatory effusions as a result of a highly proteinaceous content, fibrin, crystals or cellular debris. c Recent hemorrhagic effusion (asterisk) may appear homogeneously hyperechoic following leakage of blood within the synovial cavity. d,e Comparison of d US and e T1-weighted MR images in lipohemarthrosis demonstrates hyperechoic fat (1) in suspension floating over a deep layer (2) of medium-level echoes due to blood. A third thin hypoechoic band (arrowheads) corresponding to the serum can be appreciated in between the other two layers.
15. RHEUMATOID ARTHRITIS AND OTHER INFLAMMATORY ARTHROPATHIES
US has been proposed for the early detection and follow-up of several chronic inflammatory disorders of joints, including rheumatoid arthritis (Wakefield et al. 2000; Keen et al. 2005; Scheel et al. 2006; Gibbon 2004) and seronegative arthropathies (Gibbon 2004; Milosavljevic et al. 2005; Kane 2005). Rheumatoid arthritis is a chronic systemic disease that affects approximately 0.5–1% of the population and has a definite prevalence (2:1 to 3:1) in women. The etiology of rheumatoid arthritis is unknown but it seems to be multifactorial, with any genetic susceptibility, expression of HLA-DR4 and environmental factors believed to play a role (Sommer et al. 2005). The diagnosis requires a spectrum of disease manifestations and can be made according to established clinical criteria, the description of which is, however, beyond the scope of this chapter (Arnett et al. 1988; Sommer et al. 2005). From the pathophysiologic point of view, synovial hyperemia is the first step of the inflammatory process in rheumatoid arthritis that can be identified with diagnostic imaging modalities, including power Doppler contrast-enhanced US (Sommer et al. 2005). Then, the immune response mediated by cytokines (TNFα and IL-1) and the subsequent infiltration by inflammatory cells lead to edema and swelling of the synovium. This causes widening of the joint space, which may be further expanded by effusion (Fig. 22a). It is assumed that the above stages of the disease may be fully reversible. Later, the inflammatory response leads to hypertrophy of the synovial membrane by invasive granulation tissue with proliferation of synoviocytes, macrophages, lymphocytes, plasma cells and mast cells. As synovial hypertrophy continues, the hypertrophied synovium—usually referred to as “pannus” (the Latin for “cloth”)—undergoes villous transformation and expands concentrically into the joint space leading to damage of the central portion of the articular cartilage and the subchondral bone (formation of subchondral cysts and erosions) (Fig. 22b). Tenosynovial sheath involvement coexists in many instances. From the clinical point of view, the above abnormalities are encountered not only in rheumatoid arthritis but also in other forms of chronic arthritis. The hallmark of rheumatoid arthritis is bilateral symmetrical involvement of more than three joints. Early in its course, the disease usually affects the small hand joints, the second and third metacarpophalangeal and the third proximal interphalangeal joints being the more typically affected (a characteristic finding of rheumatoid arthritis is sparing of the distal interphalangeal joints, which are commonly involved in osteoarthritis and psoriatic arthritis). In more advanced disease, synovitis involves the larger joints of the limbs and extremities. The destructive action of the pannus is responsible for progressive joint surface damage, ligament and capsule tearing and, finally, joint instability and deformities (Fig. 22c,d). When imaging rheumatoid arthritis, one should consider that the disease progresses in a nonlinear fashion and that joint involvement is non-uniform, particularly in the early stages. Although a consensus has not been reached on which joints must be systematically checked, the symptomatic ones and those typically involved in rheumatoid arthritis (i.e., wrist and hand joints) should be examined (Sommer et al. 2005). For follow-up purposes, wrist and hand joints are the preferred sites for assessing the efficacy of therapy (Sommer et al. 2005). In terms of treatment, among drugs that have an influence on the course of disease are the so-called biologic response modifiers (i.e., anti-TNFα drugs) that inhibit certain cytokines, thus reducing the inflammatory activity. These drugs are expensive, have important side effects and must be used in patients with erosive aggressive arthritis in whom conventional drugs (NSAIDs, steroids, analgesics, etc.) do not produce a positive response. Early diagnosis of synovitis is, therefore, required to start adequate aggressive therapy before occurrence of structural damage to the joint (Herburn 1988).
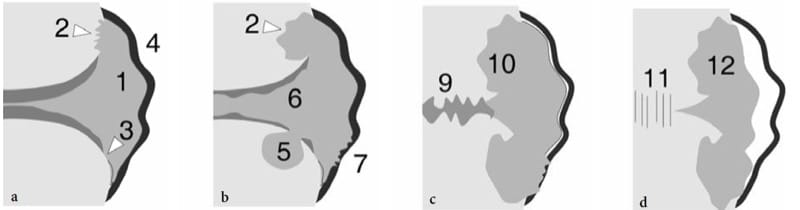
Figure 22. a–d. Rheumatoid arthritis. Schematic drawings showing progression of joint damage during the course of disease. a Early involvement is characterized by joint effusion and pannus formation (1) associated with marginal erosions (2), cartilage thinning (3) and loosening of the capsuloligamentous structures (4). b As the disease progresses, the erosions increase in size, subchondral cysts become evident (5) and the hyaline cartilage appears increasingly thinned (6). Partial tears of the para-articular structures (7) may occur leading to joint instability. c Later on, fibrous ankylosis (9) of the joint can take place with more evident destruction of the bone ends (10). d In some joints (carpal and tarsal joints) bone ankylosis (11) is the end stage. Inactive fibrous pannus (12) may replace active erosive pannus in the chronic phase.
Because early changes in rheumatoid arthritis are nonosseous in nature, US has proved superior to conventional radiography in terms of disease detection (Gibbon 2004; Clement et al. 2005: Keen et al. 2005). In patients with rheumatoid arthritis and other seronegative arthropathies, US is an effective means for detecting early signs of synovitis, thus allowing prompt institution of an appropriate treatment (Grassi et al. 1993, 2001; Brown et al. 2004). As stated before, US is able to detect joint effusion which accompanies acute inflammatory phases or exacerbation of disease – even in small synovial joints and can distinguish affected from adjacent normal joints. It can define synovial changes, allowing evaluation of pannus as hypoechoic vegetations protruding inside the synovial fluid or completely filling the articular space (Fig. 23a,b). Using MR imaging as the reference method, US has proved to have higher sensitivity and accuracy in detecting signs of inflammation in finger joints than do clinical and radiographic examinations, without loss of specificity (Szkudlarek et al. 2006). In other series, it was even more sensitive than MR imaging in detecting synovitis (Backhaus et al. 1999). The integrated use of Doppler imaging can help to distinguish hypervascular (active) from hypovascular (inactive) pannus, to monitor the response to therapy based on a decreased hyperemia (reflecting improvement in terms of symptoms and disease activity variables) and to differentiate active pannus from echogenic effusion (Fig. 23c–f) (Spiegel et al. 1987; Newman et al. 1996; Hau et al. 1999, 2002; Backhaus et al. 1999; Stone et al. 2001; Szkudlarek et al. 2001; Klauser et al. 2002; Fiocco et al. 2005; Kiris et al. 2006). In addition, Doppler US may have value in distinguishing noninflammatory synovial proliferation in osteoarthritis from inflammatory arthritis (Breidahl et al. 1996). Similar to gadolinium-enhanced MR imaging, some attempts have been made with US to obtain a quantitative estimate of the synovial volume. Although a correlation among the histologic findings, clinical markers of disease activity and synovial volume seems to exist, such measurements are time-consuming and, therefore, not currently applicable in routine practice. More recently, microbubble-based US contrast agents seem to be a promising adjunct to assess the activity of the disease process (Magarelli et al. 2001; Klauser et al. 2002). There have been many reports in the literature on power Doppler rather than on color Doppler imaging to detect synovial hyperemia. Current US technology indicates, however, that the sensitivity of color Doppler systems to detect slow and low blood flow signals is now at least equal to or even superior to that of power Doppler imaging. The main limitations of both techniques are essentially related to the lack of standardized examination technique, reproducibility, operator experience and choice of the equipment (Cardinal et al. 1996; Koski et al. 2006).
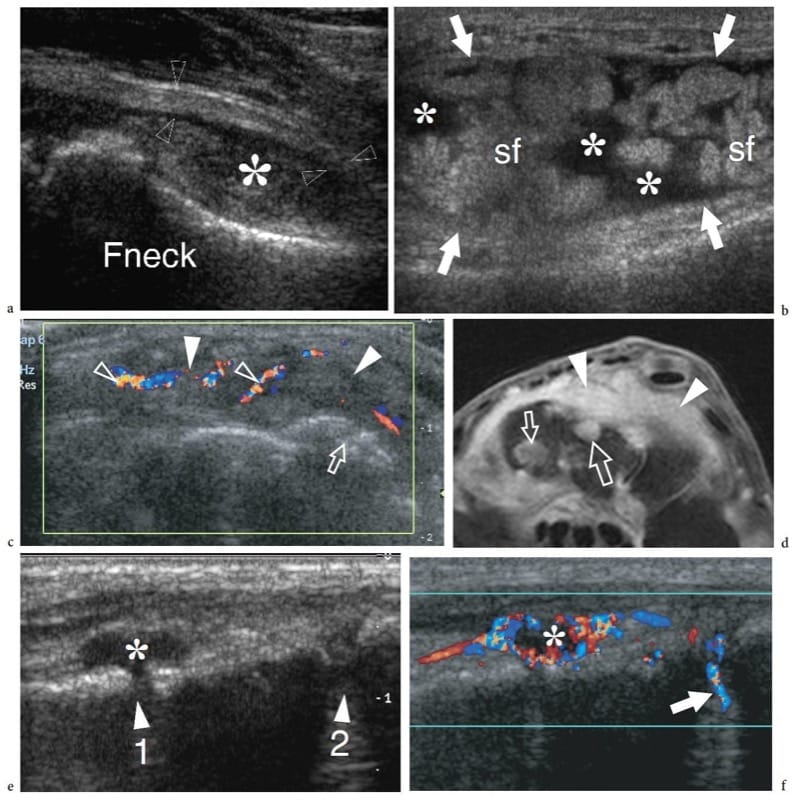
Figure 23. a–f. Rheumatoid arthritis: spectrum of US appearances of synovitis in active disease. Different cases. a Longitudinal 12–5 MHz US image over the anterior femoral neck (Fneck) demonstrates a distended anterior recess (asterisk) with thickened synovial walls (arrowheads). The space of the recess is filled with effusion mixed with hypoechoic synovium. b Longitudinal 12–5 MHz US image over the suprapatellar recess (arrows) of the knee shows prominent synovial fronds (sf) within the effusion (asterisks), partially filling the articular space. The synovial fronds can be seen moving back and forward during alternate compression and release of the probe over the recess. c Transverse 12–5 MHz US image over the dorsal wrist with d fat-suppressed T2-weighted MR imaging correlation reveals marked distension of the dorsal carpal recesses by hypervascular (open arrowheads) active synovial pannus (white arrowheads). Note the multiple erosions (arrows) affecting the bone surfaces. e,f Longitudinal e gray-scale and f color Doppler 17–5 MHz US images over the dorsal midfoot in a patient complaining of pain and local swelling demonstrate the navicular-cuneiform (1) and the cuneiform-metatarsal (2) joint spaces. The first joint is characterized by hypoechoic distension of the joint space (asterisk) reflecting synovitis. Note the marked hyperemia around it on the color Doppler image. The second joint may appear normal on gray-scale US but shows increased blood flow (arrow) on color Doppler imaging. In this particular case, color Doppler increased the sensitivity of gray-scale US for detecting joint involvement at the midtarsal level.
In advanced disease, the inflammatory process may lead to massive erosions and extensive bone damage with disintegration of structures of and around joints, fibrosis, subluxation or dislocation and, at the end stage, bone ankylosis. Based on cartilage thickness measurements, US has proved able to estimate the amount of cartilage destroyed (Grassi et al. 1993; Grassi et al. 1999). US can also depict joint space abnormalities at an earlier stage than conventional radiography. A characteristic feature of rheumatoid arthritis is the release of a subset of loose bodies, called “rice bodies,” within the joint cavity. These particles are considered to be the result of sloughed fibrinogen-coated infarcted synovial tissue or aggregates of fibrin, fibronectin or multinuclear cells (McCarthy and Cheung 1982; Popert 1985). US may demonstrate rice bodies as hypoanechoic spherules measuring a few millimeters in size (Martini et al. 2003). In many instances, however, distinguishing them from hypertrophied synovium with US may be difficult (Fig. 24).
Among the seronegative (rheumatoid factor negative) arthropathies, US has proved useful to examine patients with psoriatic arthritis (Kane et al. 1999; Fiocco et al. 2005; Ory et al. 2005; Kane 2005). Like rheumatoid arthritis, psoriatic arthritis is a chronic disorder with significant joint damage at an early stage of the disease process (Husted et al. 2001). The distal interphalangeal joints are typically affected in an asymmetric pattern. Characteristic radiographic features include joint erosions, joint space narrowing, bony proliferation including periarticular and shaft periostitis, osteolysis with “pencil-in-cup” deformity and acro-osteolysis, ankylosis, spur formation and spondylitis. In psoriatic arthritis, synovitis, enthesitis and tenosynovitis can be reliably assessed with US (Barozzi et al. 1998; Kane et al. 1999; Fiocco et al. 2005; Ory et al. 2005; Kane 2005; Falsetti et al. 2003). In general, the US findings are nonspecific as they may also occur in patients with rheumatoid arthritis and osteoarthritis (Fiocco et al. 2005; Ory et al. 2005). In psoriatic dactylitis (sausage digit), US may show subcutaneous soft-tissue enlargement and, to a lesser extent, tenosynovitis and joint synovitis, the latter sign correlating with joint space narrowing and periostitis on plain films (Fig. 25) (Barozzi et al. 1998; Kane et al. 1999). In seronegative arthropathies, unenhanced and contrast-enhanced color Doppler imaging are able to demonstrate active sacroiliitis by showing a hypervascular pattern around the posterior aspect of the sacroiliac joints (Arslan et al. 1999; Klauser et al. 2005). In ankylosing spondylitis, there is predominant involvement of large joints, such as the knee, the shoulder and the hip, with uniform joint space narrowing and low-grade subchondral sclerosis and synovitis. Reiter arthritis is characterized by prevalent distal lower extremity involvement and conspicuous new bone deposition. Arthritis in inflammatory bowel disease is, for the most part, transitory and not destructive; the most commonly involved joints are the knee and the ankle. The features of juvenile idiopathic arthritis are discussed in detail elsewhere.
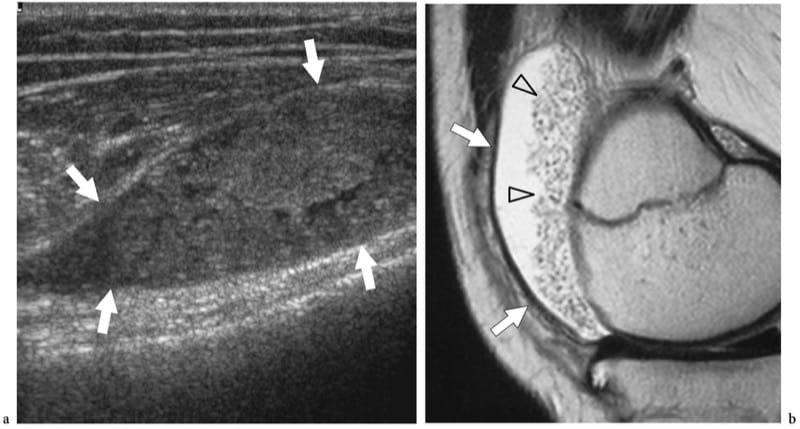
Figure 24. a,b. Rice bodies in rheumatoid arthritis. a Longitudinal 12–5 MHz US image over the anterior knee shows a distended suprapatellar recess (arrows) filled with heterogeneous solid tissue resembling synovial pannus. b Corresponding sagittal T2-weighted MR image reveals multiple hypoechoic dots (arrowheads) in the fluid related to rice bodies. The US appearance of rice bodies may be virtually indistinguishable from synovial pannus.
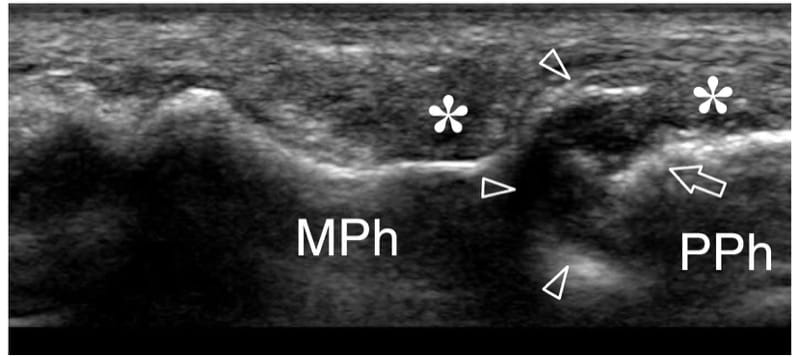
Figure 25. Psoriatic dactylitis. Longitudinal 17–5 MHz US image over the proximal interphalangeal joint of the middle finger in a 45-year-old woman with psoriatic arthritis shows extensive destruction of the articular surface (arrowheads) of the middle phalanx (MPh). Coexisting deformity (arrow) of the head of the proximal phalanx (PPh) and heterogeneous appearance of para-articular soft-tissues (asterisks) is found. The findings correspond to the radiographic sign referred to as the “pencil-in-cup” deformity.
16. SEPTIC ARTHRITIS
Septic arthritis is a serious condition leading to rapidly destructive joint disease (Goldenberg 1998; Mohana-Borges et al. 2004). This condition is most commonly caused by Staphylococcus aureus (in adults and children older than 2 years) and Neisseria gonorrheae (in young adults), which have a definite tropism for the synovium (Craig et al. 2003; Mohana-Borges et al. 2004). A variety of streptococci, including S. viridans and S. pneumoniae, group B, aerobic Gram-negative rods, viruses, mycobacteria and fungi may also produce joint infection in isolation or as a result of poly-microbial association (Jbara et al. 2006). Possible pathomechanisms of infection are: hematogenous seeding of the synovium from a distant focus or an adjacent area of osteomyelitis; spread from a contiguous infected site, such as the soft tissues in the diabetic foot; and inadvertent implantation during arthrocentesis or secondary to penetrating wounds and postoperative infection (Mohana-Borges et al. 2004). The most common pattern of presentation of septic arthritis is monoarticular. The most commonly involved joints are the hip, the knee, the shoulder, the elbow and the ankle (Mohana-Borges et al. 2004; Chau and Griffith 2005). Infection causes lysis of the articular cartilage, joint space narrowing and periarticular osteopenia. Late complications of arthritis include joint sub-luxation, premature osteoarthritis, osteonecrosis, fibrous or bony ankylosis, and limb shortening. In the acute setting, US is a reliable way to detect early septic arthritis before the occurrence of substantial cartilage lysis and when radiographs are still noncontributory (Bureau et al. 1999). The main US sign of septic arthritis is detection of a joint effusion in a patient with clinical signs of joint infection (pain, redness, heat, soft-tissue swelling about the involved joint). As regards fluid echotexture, septic effusions often contain a diffuse pattern of low-level echoes and are clearly demarcated from the thickened synovial walls (Chau and Griffith 2005). Highly hyperechoic effusions with debris and septations are often encountered. This appearance might confuse the inexperienced examiner as the collection appears to be solid on static scans; however, dynamic examination and probe compression may show swirling of echoes indicating fluid (Fig. 26). Gas bubbles may also be found in joint infection. On the other hand, completely anechoic collections of infected joint fluid are rare. However, these characteristics are too subtle to allow a definitive diagnosis and needle aspiration of fluid, possibly obtained under US guidance, is needed to confirm the infectious nature of the effusion (Bureau et al. 1999; Widman et al. 2001). Power Doppler imaging almost invariably shows a synovial hyperemic flow pattern of hypertrophied synovium and para-articular tissues. Even though the absence of fluid in a joint does not exclude adjacent osteomyelitis, a negative US and Doppler imaging examination makes diagnosis of septic arthritis unlikely (Zawin et al. 1993).
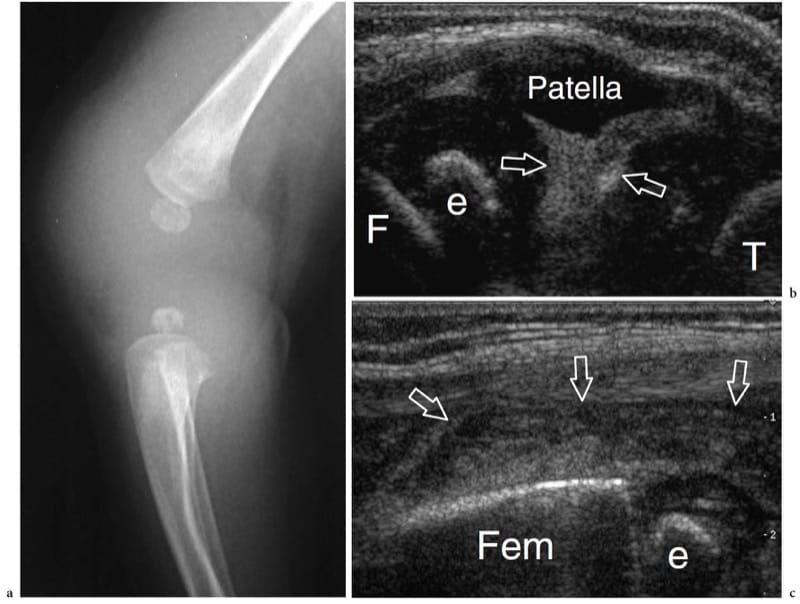
Figure 26. a–c. Septic arthritis. a Lateral radiograph of the knee in a newborn with fever and painful swollen knee suggesting an infection reveals diffuse soft-tissue swelling and an enlarged knee joint space. b,c Longitudinal 12–5 MHz US images obtained over b the patella and c the suprapatellar recess demonstrate the joint cavity filled with highly echogenic dense fluid (arrows) related to purulent material and debris. Note the hypoechoic appearance of the unossified patella and the epiphyseal cartilages of the femur (F) and tibia (T). e, ossification center of the distal femoral epiphysis. Aspiration of the joint fluid revealed Staphylococcus aureus infection.
17. TRAUMATIC INJURIES
When affecting joint portions that are amenable to US examination, osteochondrosis and osteochondral fractures can be detected as surface irregularities of the cartilage or nidus formation involving the cartilage and the subchondral bone (Takahara et al. 1988). In degenerative osteoarthritis, the cartilage may appear progressively thinner and irregular, or even completely disintegrated, whereas the hyperechoic line of the subchondral bone shows irregularities. At US, osteophytes are usually depicted at the joint margins as beak-shaped hyperechoic bone projections covered by cartilage. Following joint surface fractures or other conditions leading to progressive derangement of joints (i.e., osteoarthritis, osteo-chondromatosis, neuropathic joint disease), loose bodies can be released into the joint cavity, possibly leading to intermittent locking of the joint and early degenerative changes. Intra-articular loose bodies are osseous, chondral or osteochondral fragments. They often have a three-layered structure composed of a superficial bright echo due to an artifact at the interface with fluid, an intermediate hypoechoic band due to the cartilage, and a deep hypoechoic surface with posterior acoustic shadowing due to the bone component (Bianchi and Martinoli 2000). In many instances, US can give a better delineation of loose bodies than can plain films and MR imaging (Fig. 27). On the other hand, MR imaging is superior in detecting the nidus of the fragment. A monolaminar appearance is observed in old extensively calcified fragments, which appear as hyperechoic images like gallstones without a detectable rim of hypoechoic cartilage (Bianchi and Martinoli 2000). During joint motion or while applying transducer pressure, loose bodies can be mobilized within joint recesses: this may be helpful for the differential diagnosis with either osteophytes or capsular and synovial calcifications.
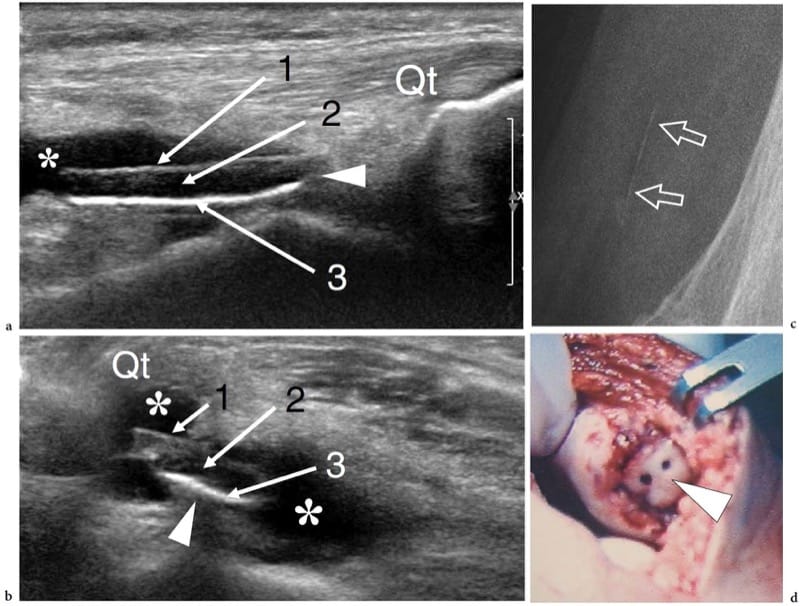
Figure 27. a–d. Osteochondral fracture. a Longitudinal and b transverse 17–5 MHz US images of the anterior knee in a patient with onset of painful swelling and locking of the knee following an episode of patellar dislocation show a distended suprapatellar recess (asterisks) containing an osteochondral loose body (arrowhead). The fragment is characterized by a trilayered structure composed of: a superficial bright echo (1) due to the acoustic impedance mismatch at the solid-fluid interface; an intermediate hypoechoic layer (2) due to the cartilage; and a deep bright echogenic surface (3) with slight posterior attenuation due to the detached subchondral bone. c Lateral radiograph is unable to reveal the loose body except for a subtle radio-opaque linear image (arrows) reflecting its bony component. Qt, quadriceps tendon. d Gross operative view demonstrates the loose body (arrowhead) fixed into the patellar nidus.
The diagnosis of joint instability basically relies on plain films. In some instances, however, the complex anatomy of joints and surrounding structures may make detection of subluxation and dislocation of joints difficult on standard radiographs. If undetected, joint instability may lead to chronic local pain, secondary osteoarthritis and altered joint function. In specific clinical settings in which the physical examination may be inconclusive, US can contribute to the detection of occult positional joint abnormalities, including posterior shoulder dislocation and mild acromioclavicular joint instability (Hunter et al. 1998; Bianchi et al. 1994; Bize et al. 2004; Borsa et al. 2005).
Many joints contain fibrocartilaginous structures, including the meniscus in the knee, the labrum in the hip and the shoulder, the triangular fibrocartilage in the wrist, and the volar and plantar plates in the hand and foot. Because of their deep location and close contact with the bone, these structures can be evaluated with US only in part and not reliably. Although different authors have reported a high sensitivity and specificity of US in diagnosing knee meniscal and shoulder labral tears (Sohn et al. 1987a, b; Schydlowsky et al. 1998; Hammar et al. 2001), further evidence has demonstrated that US cannot be considered an accurate technique for diagnosing fibrocartilage tears (Azzoni and Cabitza 2002). In particular, distinguishing tears from degenerative states is problematic on the basis of the US findings due to a similar echotextural pattern. However, some conditions involving the superficial part of these structures, such as an extruded meniscus, a meniscocapsular detachment with fluid intervening between the capsule and the fibrocartilage or a meniscal ossicle can be inferred on US. Initial experience is also available in the literature on US investigation of the normal triangular fibrocartilage of the wrist and the volar plates (Boutry et al. 2004; Chiou et al. 1988; Keogh et al. 2004). However, further studies are needed to establish the ultimate value of US in imaging pathologic conditions affecting these structures. In contrast to the results of fibrocartilage evaluation, US has proved to be an effective modality for diagnosing parameniscal and paralabral cysts (Peetrons et al. 1990; Rutten et al. 1998; Seymour and Lloyd 1998). These cysts are believed to derive from tangential or compressive forces that lead to trauma, degeneration and tearing of the fibrocartilage. Synovial fluid is extruded through the tear toward the peripheral margin of the fibrocartilage, expanding it and dis-placing the capsule outward into the surrounding tissues (McCarthy and McNally 2004). Because these cysts are almost invariably associated with a fibrocartilage tear, the US diagnosis of an associated meniscal or labral rupture is straightforward even in cases of unclear or doubtful findings (Fig. 28a,b). The cyst can track some distance from the fibrocartilage before becoming clinically palpable and US may show a narrow pedicle connecting it to the tear. Parameniscal and paralabral cysts appear as space-occupying masses with sharply defined borders. They often exhibit mixed internal echotexture as a result of mucoid degeneration or appear as undefined softening and swelling of the connective spaces in which they expand (Fig. 28c,d). Even if small in size, labral-related cysts may lead to neuropathy of adjacent nerves, such as the suprascapular nerve (posterior glenoid labrum cysts), the axillary nerve (inferior glenoid labrum cysts) and the femoral nerve (anterior acetabular labrum cysts) (Takagishi et al. 1991).
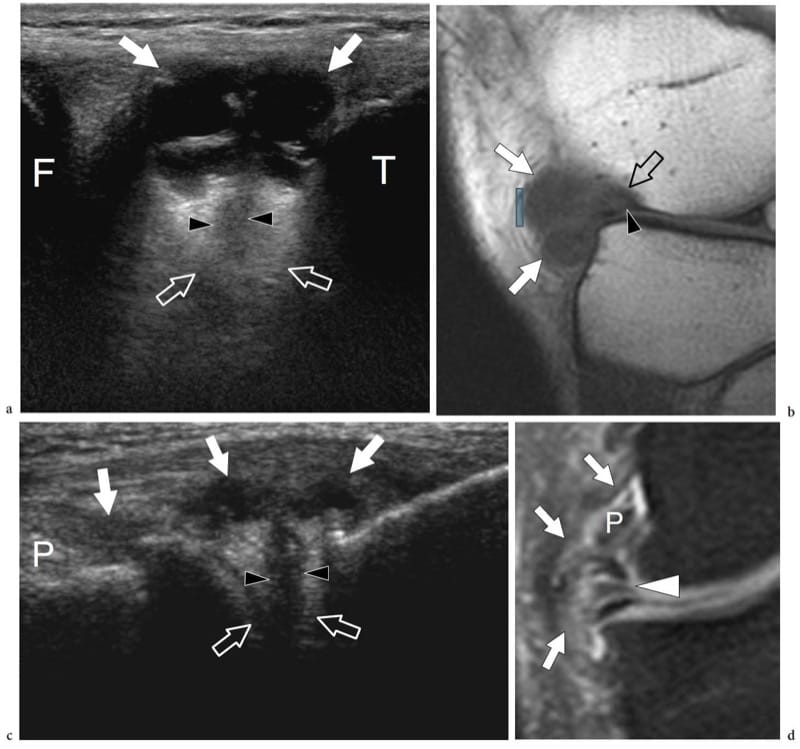
Figure 28. a–d. Cysts associated with fibrocartilage lesions. Spectrum of US appearances of parameniscal cysts in two different patients. a Oblique sagittal 17–5 MHz US image with b sagittal T1-weighted MR imaging correlation over the anterolateral knee shows a large cyst (white arrows) with internal septa lying adjacent to the anterior horn of the lateral meniscus (open arrows). A cleft (arrowheads) representing a horizontal meniscal tear is seen extending through the meniscus. F, femur; T, tibia. c Coronal 12–5 MHz US image obtained over the lateral knee with d fat-suppressed T2-weighted MR imaging correlation shows a horizontal degenerative tear (arrowheads) of the middle third of the lateral meniscus (open arrow) associated with extrusion of joint fluid in the adjacent soft tissues forming a irregularly shaped cyst (white arrows). Note the popliteal tendon (P) partially surrounded by fluid.
Ligament tears can be demonstrated with US at different sites, including the ankle and foot ( Campbell et al. 1994; Peetrons et al. 2004), the wrist and hand (Jones et al. 2000; Noszian et al. 1995; Finlay et al. 2004; Boutry et al. 2005), the knee (Ptasznik et al. 1995; Miller 2002; O’Reilly et al. 2003) and the elbow (Nazarian et al. 2003). The US features of a torn ligament vary depending on whether the lesion is acute or has healed. In acute phases, a partially torn ligament appears swollen and hypoechoic but continuous (Fig. 29a); an anechoic band over the superficial aspect of the ligament may be observed representing reactive soft-tissue edema (Peetrons et al. 2004). In complex ligaments, US can distinguish the abnormal hypoechoic portion of the ligament from the unaffected one retaining a normal appearance (Fig. 29b). In acute complete ruptures, a hypoechoic cleft reflecting the hematoma can be detected through the ligament substance and the free ends of the severed ligament may appear retracted and wavy (Fig. 30a,b). In doubtful cases, the ability to assess the ligament dynamically is a definite advantage of US: under stress, a normal ligament tightens preventing excessive widening of the joint space; if the ligament is torn, a paradoxical movement is obtained reflecting joint instability (Fig. 30) (De Smet et al. 2002; Brasseur et al. 2005). In chronic partial tears, the ligament always appears thicker than normal on US images. Calcifications within the ligament substance in old tears and irregularities of the bony insertions in avulsion injuries may be observed (Fig. 31) (Brasseur et al. 2005). A typical example is the Pellegrini-Stieda syndrome (calcification of the proximal end of the medial collateral ligament of the knee). In ligament lengthening following a partial tear, dynamic scanning during a stress maneuver obtained at rest and during stretching may be helpful to distinguish normal laxity from partial ligament tears based on widening of the joint space (Nazarian et al. 2003; Peetrons et al. 2004). The significance of these maneuvers has yet to be fully determined.
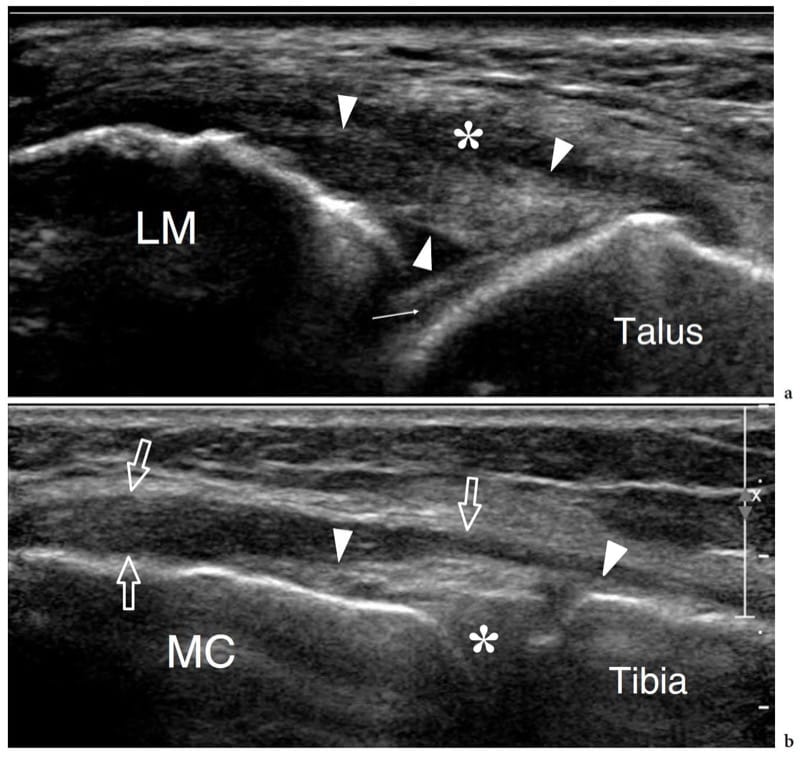
Figure 29. a,b. Partial ligament tear. a Long-axis 17–5 MHz US image over the anterior talofibular ligament (arrowheads) in a patient following an inversion injury of the ankle demonstrates a markedly thickened and hypoechoic but straight ligament without signs of macroscopic discontinuity. A hypoechoic band of fluid (asterisk) underlines the superficial aspect of the ligament representing reactive soft-tissue edema. LM, lateral malleolus. Arrow, articular cartilage of the talus. b Coronal 17–5 MHz US image over the medial aspect of the medial femoral condyle (MC) reveals diffuse hypoechoic thickening of the superficial component (arrows) of the proximal medial collateral ligament, whereas the deep meniscofemoral component (arrowheads) is unaffected. Asterisk, medial meniscus.

Figure 30. a–g. Complete ligament rupture. Spectrum of US appearances in a–d the anterior talofibular and e-g scapholunate ligaments. a Long-axis 17–5 MHz US image obtained at rest over the anterior talofibular ligament after an inversion injury with b schematic drawing correlation demonstrates the torn ends (1, 2) of the ligament in a wavy shape with hematoma insinuating between them. c,d While performing an anterior drawer test, there is opening (large arrows) of the joint space (asterisks) and the ligament ends are more clearly separated from each other. e Transverse 17–5 MHz US image over the dorsal aspect of the proximal carpal row shows a normal scapholunate ligament (arrows) joining the lunate (Lun) and the scaphoid (Scaph). f,g Transverse 17–5 MHz US images obtained in patient who underwent previous wrist injury. f In neutral position, there is absence of the scapholunate ligament with respect to the normal findings shown in e. The dashed lines demarcate the distance between
the scaphoid and the lunate. g During ulnar deviation of the wrist, widening (arrows) of the scapholunate distance can be seen: this can be considered a sign of ligament tear.
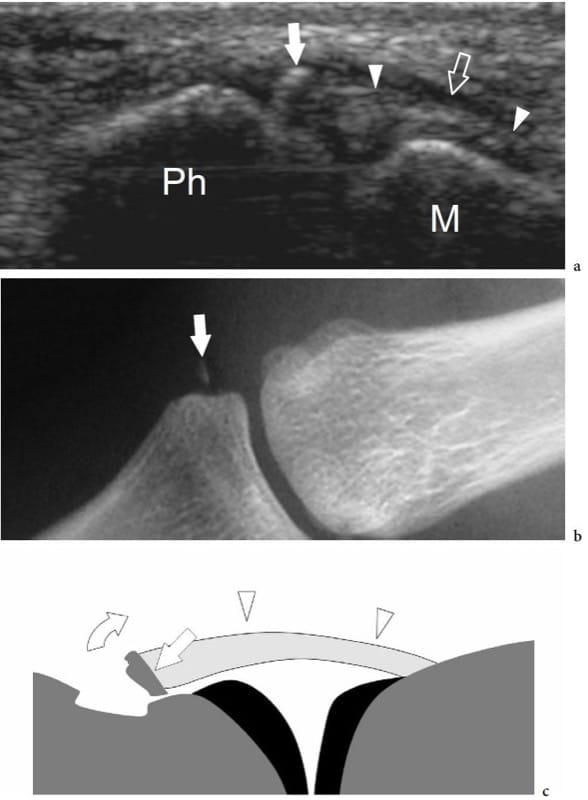
Figure 31. a–c. Avulsion injury of the ulnar collateral ligament of the thumb (gamekeeper thumb). a Coronal 15–7 MHz US image obtained over the ulnar aspect of the metacarpophalangeal joint of the thumb with b radiographic and c schematic drawing correlation shows a retracted ulnar collateral ligament (arrowheads) lying deep to the adductor pollicis aponeurosis (open straight arrow). Note a small distal hyperechoic fleck of bone (white straight arrow) corresponding to cortical avulsion (curved arrow) from the base of the proximal phalanx (Ph). The proximal ligament insertion onto the first metacarpal (M) appears normal.
18. DEGENERATIVE JOINT DISEASE (OSTEOARTHRITIS)
The term “osteoarthritis” (degenerative joint disease) includes a heterogeneous group of disorders which are characterized by defective integrity of the articular cartilage and related changes in the underlying bone and at the joint margins (Felson 2004; Gupta et al. 2004). Osteoarthritis is the most widespread form of joint disease in the Western world and can be divided into idiopathic and secondary forms (Gupta et al. 2004). The causes of osteoarthritis include various combinations of systemic risk factors (aging, inheritance, estrogen deficiency), local joint vulnerabilities (previous injuries, bone malalignment) and extrinsic factors acting on the joint (obesity, muscle weakness, occupational and sports-related repetitive overuse but also chronic underuse) (Felson et al. 2004). The initial abnormality of osteoarthritis occurs in the articular cartilage with edema followed by fibrillation and superficial and deep clefts possibly evolving toward ulcerations and production of new cartilage and bone. In severe forms, complete cartilage loss associated with pathologic changes of the subchondral bone (i.e., sclerosis, cysts) and marginal osteophytes can occur. Intra-articular loose bodies may develop as a result of detachment of small cartilage pieces or bone fragments which may activate new chondral or bone formation. The diagnosis of osteoarthritis is essentially based on clinical and radiographic data. US is able to detect joint surface and hyaline cartilage abnormalities (Grassi et al. 2005). Changes include progressive thinning and irregularity of the cartilage layer up to its complete disintegration and irregularities of the underlying subchondral bone (Fig. 32) (Grassi et al. 1999). One of the major limitations of US in evaluating osteoarthritis is the incomplete evaluation of the cartilage surface, which is, for the most part, masked by the ends of opposing bones. This is true for both tight and large joints. In the knee, for instance, articular cartilages that are vulnerable to tears and ulcerations are mainly located at the posteroinferior aspect of the femoral condyle and on the lateral facet of the patella: both surfaces are barely evaluated with US. Similarly, geodes (sub-chondral cysts) are not visible at US because they are completely surrounded by bone. On the other hand, osteophytes can be readily appreciated as beak-like bone projections covered by hypoechoic cartilage adjacent to the joint line (Fig. 33). They increase the surface area of the articular cartilage, thus lessening the stress and loading forces that are experienced by the joint and, at the same time, increasing its stability: typical locations of osteophytes are the posterior humeral head, the internal femorotibial and the anterior tibiotalar joints (Gupta et al. 2004). Finally, US can be useful in assessing para-articular soft-tissue abnormalities that can be responsible for pain in osteoarthritis and may help to guide intra-articular drug injection (Naredo et al. 2005; Migliore et al. 2005).
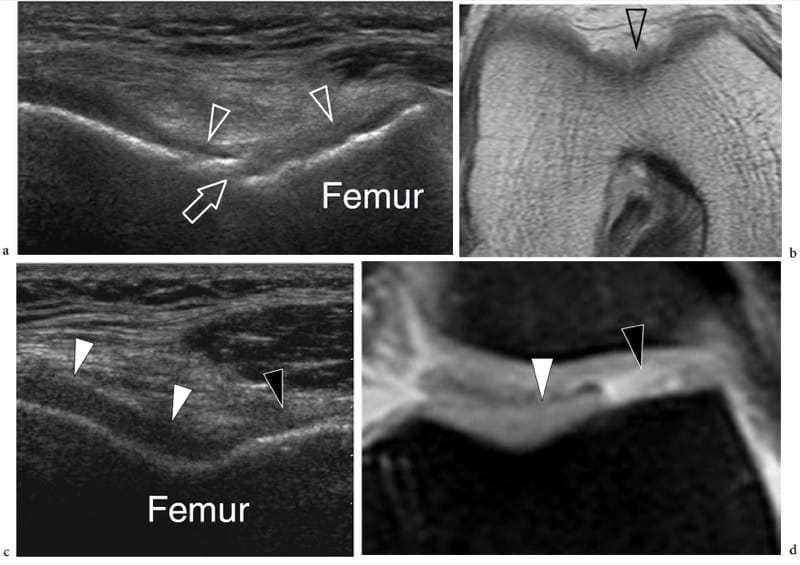
Figure 32. a–d. Degenerative osteoarthritis. Two different cases. a Transverse 12–5 MHz US image obtained over the trochlear cartilage with b proton density MR imaging correlation shows diffuse thinning (arrowheads) of the mid-third of the cartilage. Note the erosive changes (arrow) in the subchondral bone. c,d Loss of lateral compartment articular cartilage. c Transverse 12–5 MHz US image with d fat-suppressed proton-density MR imaging correlation shows a wide cartilage defect (black arrowhead) in the lateral facet of the trochlea. Note the normal-appearing cartilage of the medial trochlear compartment (white arrowheads).
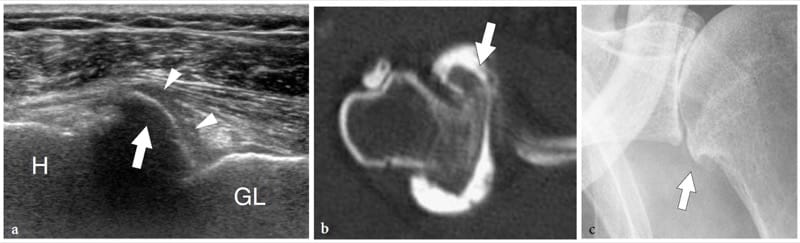
Figure 33. a–c. Degenerative osteoarthritis. a Transverse 12–5 MHz US image obtained over the anterior aspect of the glenohumeral joint with b CT-arthrography and c standard radiographic correlation in a patient with advanced degenerative joint disease demonstrates a large marginal osteophyte of the humerus (H) as a beak-like bone projection (arrow) lying adjacent to the joint line, covered by hypoechoic cartilage (arrowheads). GL, bony glenoid.
19. DEPOSITION DISEASES
The deposition of crystals of monosodium urate (gout), calcium pyrophosphate dehydrate (CPPD; chondrocalcinosis, pseudogout) and calcium hydroxyapatite (HADD; calcifying tendinitis) within synovium, articular cartilage, fibrocartilages and para-articular soft tissues may produce a spectrum of clinical conditions ranging from asymptomatic entities to severe rapidly destructive arthropathy (Steinbach 2004; Clement et al. 2005). The accumulation of crystals takes months to years to develop and leads to the establishment of a chronic synovitis with intermittent acute flares.
Gout is the most common form of microcrystalline arthropathy, with a peak incidence occurring in males between the third and fifth decades (Monu and Pope 2004). It results from a disturbance of urate metabolism and includes acute and chronic forms. Acute gout typically affects the first metatarsophalangeal joint of the foot (podagra), but almost any other joint can be involved. The initial attacks are monoarticular and usually involve the lower limb. Following intermittent flare-ups or exacerbations over a period of years, the disease becomes chronic and tends to involve multiple joints. An inflamed joint shares the usual presentation with an acute synovitis, including joint effusion, para-articular edema and hyperemia. In general, gouty crystals are not depicted with US unless larger aggregates have calcified (Farina et al. 2002). Basically, the US findings are not disease-specific (Filippucci et al. 2003). US may be used for short-term monitoring of acute gouty attacks (Filippucci et al. 2003). US findings in chronic tophaceous gout are described elsewhere.
Calcium pyrophosphate dehydrate crystal deposition disease is widespread in the elderly and has multifaceted clinical presentations ranging from asymptomatic disease to severe painful destructive arthropathy, with symptoms that often confound clinicians (Steinbach 2004). Patients can complain of either chronic joint pain mimicking osteoarthritis or a swollen inflamed joint mimicking gout (pseudogout) or a septic joint. The knee is the most commonly affected joint (articular cartilage and menisci), followed by the hip (symphysis pubis), the wrist (triangular fibrocartilage, intrinsic ligaments), the glenohumeral joint, the elbow and the ankle. During pseudogout attacks, shedding of pyrophosphate crystals into the joint fluid occurs. In general, these attacks are self-limiting, less painful than gout attacks, and can last up to several weeks causing joint stiffness and restricted joint motion. Standard radiographs can demonstrate small calcified deposits within degenerating hyaline cartilage, synovium and para-articular structures, including ligaments, tendons and bursae (Abreu et al. 2004; Steinbach 2004). A conclusive diagnosis is based on detection of pyrophosphate crystals in the synovial fluid by means of a polarizing microscope. US can demonstrate crystal deposition in the articular cartilage as multiple hyperechoic dots in the mid-thickness of the cartilage forming a dense echogenic line that runs parallel to the articular margin, particularly at the level of the femoral condyles (Fig. 34) (Kellner et al. 1990; Coari et al. 1995; Foldes 2002; Sofka et al. 2002; Frediani et al. 2005). Three main patterns of calcified deposits have been described (Frediani et al. 2005): thin hyperechoic bands parallel to the surface of the hyaline cartilage (more frequent in the knee); a ”punctate” pattern, composed of several thin bright echogenic spots (commonly seen in fibrocartilage and tendons); and homogeneous hyperechoic nodular or oval deposits (found in bursae and articular recesses).
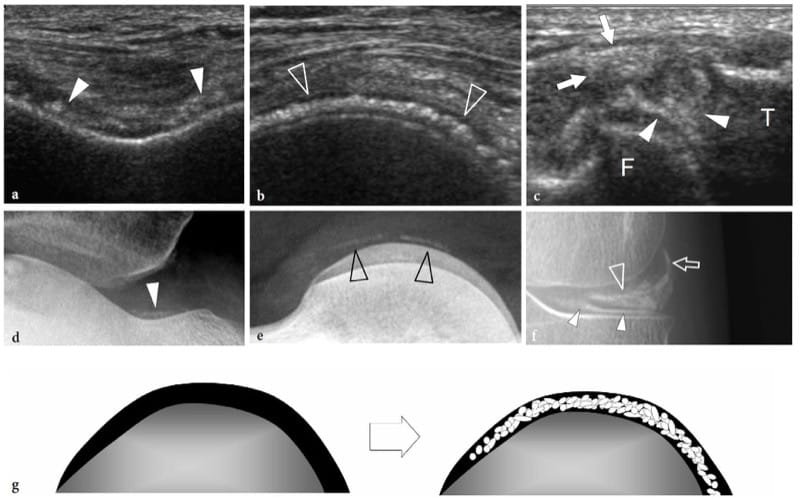
Figure 34. a–g. Calcium pyrophosphate deposition disease. a–c Transverse 12–5 MHz US images obtained over a the femoral trochlea, b the posterior aspect of the medial condyle and c the lateral meniscus in a patient with bilateral degenerative osteoarthritis of the knee reveal scattered hyperechoic foci (arrowheads) due to crystal deposition within the hyaline cartilage, the medial meniscus and the joint capsule (arrows). F, femur; T, tibia . Note that crystals tend to be deposited in the middle layer of the cartilage, parallel to the subchondral bone. d–f Radiographic correlation. g Schematic drawing illustrates the typical deposition pattern of pyrophosphate crystals within the cartilage.
The intra-articular involvement of calcium hydroxyapatite deposition disease—a condition more commonly referred to as “calcifying tendinitis” because of the involvement of tendons near to their osseous insertion—may produce an acute arthritis (chronic apatite arthropathy) possibly mimicking an infectious condition.
Amyloidosis is a major clinical complication with various subtypes that occur in association with multiple myeloma (primary disease), other chronic disorders (secondary disease) and in patients with chronic renal failure under long-term hemodialysis treatment (Jbara et al. 2006). Its prevalence increases with the duration of hemodialysis, ranging from approximately 20% of cases within 2 years of treatment to nearly all after 13 years (Dzido and Sprague 2003). This multiorgan disease is related to β2 microglobulin deposition in tissue. In the musculoskeletal system, amyloid deposition typically occurs on surfaces containing collagen, most often the synovium of joint and tendons. Such a predilection seems to be related to macrophage and cytokine activation, a process that might be involved both in the proteolytic processing of the molecule – a step that would enhance amyloid formation – and in the generation of pain, dysfunction and even destructive arthropathy (Bancroft et al. 2004). In its articular form, amyloidosis most often involves the hip, the knee and the wrist leading to development of large amyloid-filled subchondral cysts in the juxta-articular bone. The thickness of abnormal soft-tissue material measured with US in the anterior recess of the hip and the suprapatellar recess of the knee has been found to correlate positively with dialysis duration and radiological and histologic evidence of amyloidosis (Jadoul et al. 1993). Based on echotextural findings, amyloid deposits cannot be distinguished from other synovial pathology (Fig. 35a,b). MR imaging demonstrates amyloid with low to intermediate signal on T1- and T2-weighted sequences (Fig. 35c,d) (Cobby et al. 1991).
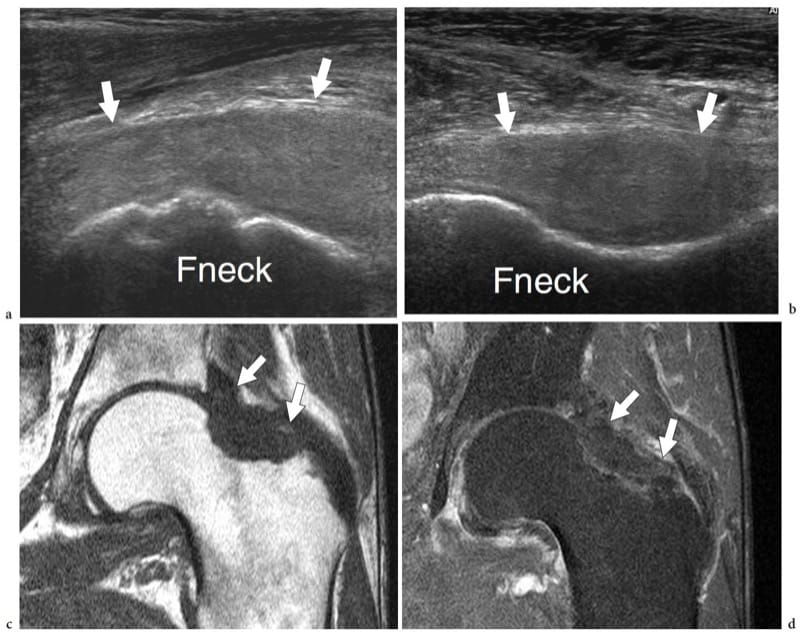
Figure 35. a–d. Amyloid arthropathy. a Transverse and b longitudinal 12–5 MHz US images obtained over the anterior hip in a patient affected by chronic renal failure demonstrate distension of the anterior joint recess (arrows) by solid homogeneously hypoechoic tissue. c,d Coronal c T1-weighted and d fat-suppressed T2-weighted MR imaging correlation confirm the presence of intra-articular amyloid (arrows), characterized by low signal intensities. Fneck, femoral neck.
20. POSTOPERATIVE COMPLICATIONS
Detection and localization of postoperative infection may be a challenging task. Septic arthritis complicating hip and knee joint replacement procedures is an important risk factor reported to involve approximately 2% of cases (Goldenberg 1998). Based on the clinical and radiographic findings, it may be impossible to distinguish mechanical loosening of a prosthesis from septic loosening (Bureau et al. 1999). Nuclear medicine, CT and MR imaging can be impaired around orthopaedic hardware by metallic shielding and artifact. US is less hindered in many of these respects and can be considered the first-line diagnostic modality in this setting, provided that an adequate probe access is available (Chau and Griffith 2005). In the postoperative hip, infection can be suspected with US by the presence of a large joint effusion (mean bone-to-pseudocapsule distance: normal, 3.2 mm; infected, 10.2 mm) associated with an extracapsular noncommunicating soft-tissue fluid collection and local inflammatory changes (van Holsbeeck et al. 1994). More recently, however, a retrospective study revealed some limitations of US as an indicator of adult hip joint effusion, even in the postoperative setting (Weybright et al. 2003). When a collection is present around a postoperative hip, US can effectively guide arthrocentesis to obtain material for Gram staining and bacterial culture ( van Holsbeeck et al. 1994; Gibbon et al. 2002). Doppler US may also be used to rule out deep vein thrombosis after total hip or knee arthroplasty, especially if routine perioperative pharmacologic antithrombotic prophylaxis is not practised (Ko et al. 2003).
Granulomatous synovitis can be seen as a result of reaction to small particles of silicone polymers or other synthetic components of prostheses secondary to shedding, so-called “silastic synovitis” or “detritic synovitis”. Often encountered in wrist implants, this condition derives from a kind of foreign-body response, in which activated macrophages provoke bone resorption and dissection around the implant (Bancroft et al. 2004). Standard radiographs show well-defined subchondral lucency and erosions usually associated with fracture and/or dislocation of the prosthesis (Rosenthal et al. 1983; Schneider et al. 1987). MR imaging confirms the radiographic findings and demonstrates multiple small hypointense particles that represent silicone fragments resulting from implant breakage and disintegration (Chan et al. 1998). US can demonstrate the implant-derived debris as hyperechoic spots embedded within hypertrophied synovium: these fragments should be differentiated from calcifications based on the presence of posterior reverberation and ring-down artifact (Fig. 36).
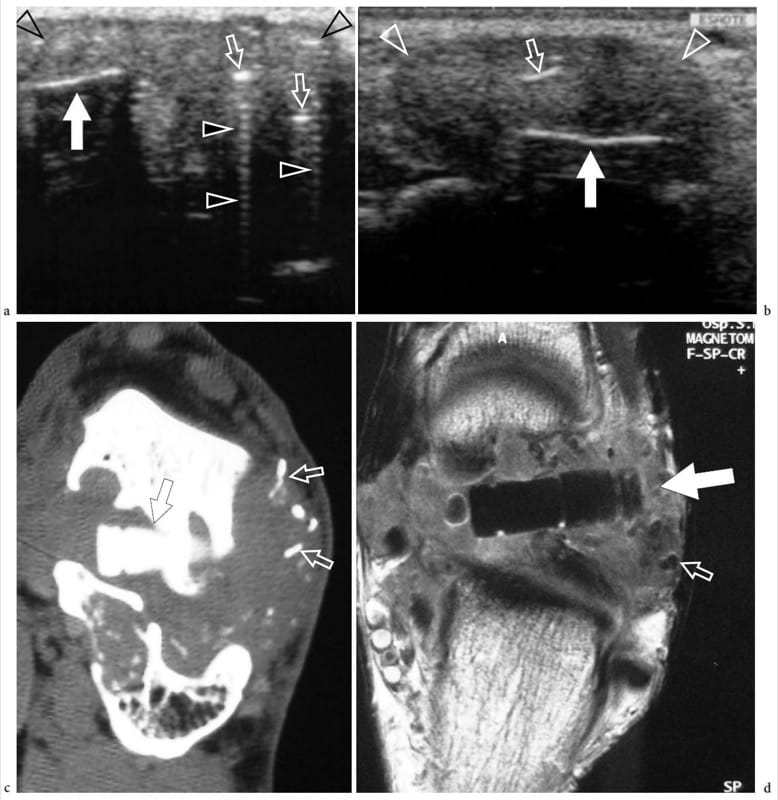
Figure 36. a–d. Silastic synovitis. a Coronal and b transverse 12–5 MHz US images obtained over the lateral aspect of the sinus tarsi in a patient who was previously operated on for flatfoot show a hypoechoic solid mass (open arrowheads) containing multiple hyperechoic spots (open arrows) with posterior reverberation artifact (black arrowheads). A large hyperechoic structure (white arrow) is also found protruding outside the sinus tarsi. c Reformatted coronal CT and d transverse T1-weighted MR images identify extensive bone resorption and striking synovial hypertrophy around a loosened silicone prosthesis (white arrow). Some particles (open arrows) representing silicone fragments are also found.
21. SPACE-OCCUPYING MASSES
Bone Tumors
The role of US for the detection and assessment of bone tumors is obviously poor, given its inability to define intraosseous processes. US can only detect lesions associated with considerable cortical thinning and/or large extraosseous spread, such as large tumors eroding the cortex (Saifuddin et al. 1998). While evaluating bone tumors, US can be useful in two main clinical situations: the detection of an otherwise unsuspected tumor, or as guidance for a percutaneous biopsy of a bone tumor already investigated with standard radiographs, CT and MR imaging. It is not uncommon for patients with a bone tumor, either primary or metastatic, to complain of nonspecific regional pain and to undergo US examination for a suspected soft-tissue abnormality before a radiographic study. Therefore, careful assessment of the bone surface must be part of a standard US examination of soft tissues and the US signs suggesting a possible abnormality of bone – even if minimal – must be known by the examiner.
In osteolytic tumors with extraosseous extension, US shows a periosseous soft-tissue mass arising from a break or a deep defect in the hyperechoic bony cortex (Fig. 37). Direct continuity of the mass from the inner bone into adjacent soft tissues is a sign of the intraosseous origin of the tumor. Although US has limitations in assessing infiltration of periosseous tissue planes, the boundaries of the extraosseous component of the neoplasm are usually well circumscribed. In general, the tumor echotexture ranges from solid hypoechoic to a mixed heterogeneous appearance due to internal anechoic areas related to necrosis. In most cases, US is unable to suggest the histologic diagnosis as well as to differentiate malignant from locally aggressive benign tumors. Based on their fluid-filled appearance, US can diagnose intraosseous ganglia extruding into the paraosseous soft tissues (Bianchi et al. 1995). Occasionally, a presumptive diagnosis can be made, such as in the case of aneurysmal bone cysts presenting marked cortical thinning and multiple fluid-fluid levels (Fig. 38) (Haber et al. 1993; Gomez et al. 1998). In these instances, changing the patient’s positioning can show respective changes in the disposition of fluid layers within the tumor compartments (Fig. 38a-d). Recently, the US appearance of osteoid osteoma located in the proximal metaphysis of the right tibia and left femoral diaphysis of adolescents has been described (Gil-Sanchez et al. 1999). Color Doppler imaging may be useful for detection of the hypervascular nidus and to guide percutaneous localization and biopsy. Doppler imaging should always be performed when evaluating soft-tissue tumors. It can show internal vasculature, thus helping to differentiate viable from necrotic tissue. When a bone tumor exhibits a large extraosseous component at MR imaging, US can be effective and reliable to guide percutaneous needle biopsy (Civardi et al. 1994; Saifuddin et al. 1998, 2000; Konermann et al. 2000; Yeow et al. 2000; Gil-Sanchez et al. 2001; Torriani et al. 2002). Biopsy is a fundamental step in the investigation of suspected bone tumors and must be obtained in specialized centers to avoid inadequate tissue sampling, which is the most common reason for the inability to perform limb-salvage surgery (Skrzynski et al. 1996; Saifuddin et al. 2000). US-guided biopsy must be performed only in patients in whom the feasibility of the procedure has been previously checked on MR imaging or CT scan. The biopsy of a bone tumor must be performed in agreement with the referring orthopaedic surgeon in order to decide the most appropriate needle path by consensus: this helps to avoid seeding of tumor cells out of the involved compartment. As described in Chapter 18, 14–18 gauge Tru-cut type automatic devices are recommended to achieve better diagnostic yield for histologic diagnosis. Fine-needle aspiration biopsy seems to have definite limitations in this field, even in detection of tumor recurrence or diagnosis of metastasis of a known primary tumor. Compared with CT guidance, the main advantages of US-guided biopsies of bone tumors rely on the ability of US: to recognize viable tumor and adjacent vessels with Doppler imaging; to allow continuous real-time visualization of the needle to avoid necrotic areas and the risk of injury to adjacent structures; and to perform the procedure more rapidly, reducing patient discomfort (Torriani et al. 2002). If the US biopsy is performed in experienced hands, complications are rare (Torriani et al. 2002). In rib lesions, local pain and hematoma, infection and pneumothorax have been reported as complications.
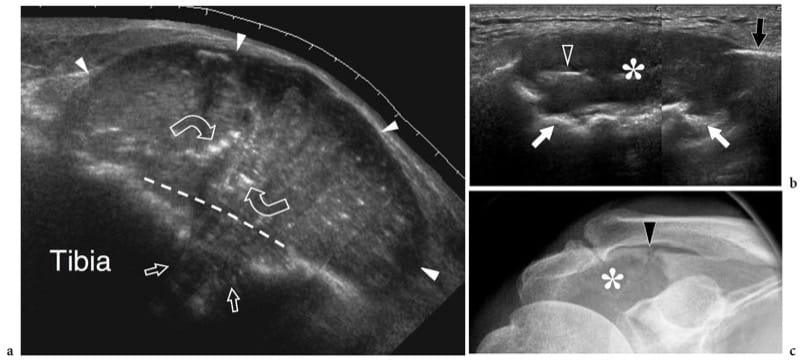
Figure 37. a–c. Bone tumors. Two different cases. a Extended field-of-view 12–5 MHz US image over a slow-growing palpable pretibial mass in a patient with breast cancer shows a large well-circumscribed solid neoplasm causing osteolysis (straight arrows) and extending into the adjacent extraosseous spaces (white arrowheads). Dashed line indicates the level of destroyed tibial cortex. Residual small bone fragments (curved arrows) displaced by the growing neoplasm are seen. Biopsy revealed breast metastasis. b Longitudinal 12–5 MHz US image obtained over the shoulder in a patient with suspected rotator cuff disease reveals a large hypoechoic mass (asterisk) causing extensive osteolysis (white arrows) of the acromion (black arrow): some bone fragments are visible within the mass (arrowhead). c Anteroposterior radiograph confirms the osteolytic lesion (asterisk) and an associated pathologic fracture (black arrowhead). Biopsy revealed metastasis from lung cancer.
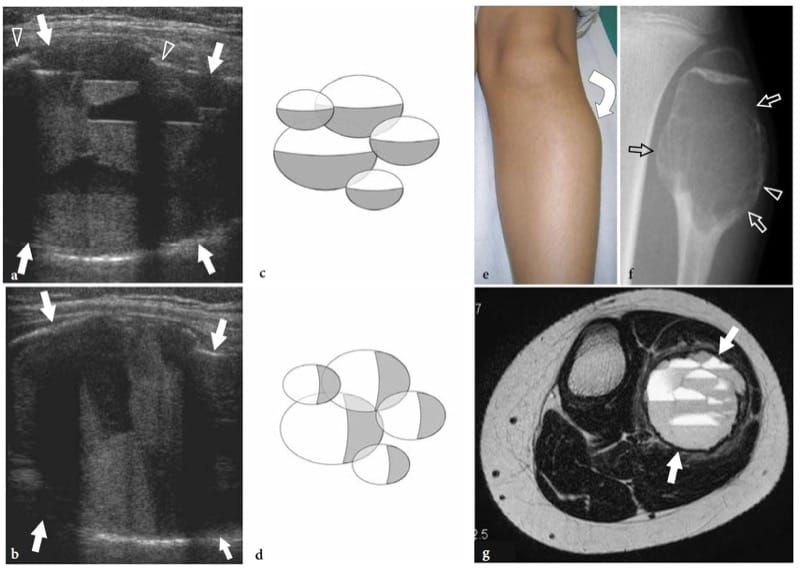
Figure 38. a–g. Aneurysmal bone cyst in a 12-year-old boy with a stiff slow-growing painless swelling over the fibular head and neck. a,b Coronal 12–5 MHz US images obtained over the fibular head a with the patient supine and b standing reveal a complex mass (arrows) which creates a bulge on the surface of the bone with cortical thinning (arrowheads) sufficient to allow US beam penetration. The mass is characterized by multiple dual fluid levels, an appearance fairly specific for an aneurysmal bone cyst. When the patient’s position is changed, fluid levels rearrange parallel to the floor according to gravity. c,d Diagrams show the disposition of fluid levels as seen in a and b. e Photograph shows focal bulging (curved arrow) over the fibular head. f Anteroposterior radiograph and g transverse T2-weighted MR imaging confirm the US diagnosis.
22. PIGMENTED VILLONODULAR SYNOVITIS
Pigmented villonodular synovitis is an uncommon benign proliferative disease of the synovium that affects joints, bursae or tendon sheaths (Dorwart et al. 1984; Yang et al. 1998; Bianchi et al. 1998; Lin et al. 1999; Middleton et al. 2004). From the pathogenetic point of view, the etiology of pigmented villonodular synovitis remains controversial. Of the two most widely accepted theories, one implicates a chronic inflammatory process, the other a benign neoplasm (Byers et al. 1968; Mukhopadhyay et al. 2006). The presence of histiocytes that contain fat or hemosiderin (a breakdown product of hemoglobin), multinucleated giant cells and plasma cells seems suggestive of inflammation, whereas the high cellularity, tendency for recurrence and recent cytogenetic studies revealing clonal cell proliferation favor a neoplastic process (Mukhopadhyay et al. 2006). The clinical presentation of articular pigmented villonodular synovitis depends on the morphologic form of disease, including a diffuse and nodular type. The more common diffuse pigmented villonodular synovitis grossly appears as a widespread proliferation of the synovium that shows fingers-like masses and villosities. It usually occurs in the third and fourth decades of life as a monoarticular arthritis affecting the knee (80% of cases) , the hip and the ankle. Patients’ complaints include insidious onset of progressive joint swelling, discomfort, pain, mechanical derangement and decreased range of motion. Focal pigmented villonodular synovitis more frequently occurs in the fifth and sixth decades, affecting joint, para-articular bursae and synovial tendon sheaths (giant cell tumor of tendon sheath), the latter being more common. It has a female predominance and affects the digits of the hand and foot, presenting as a slow-growing painless mass (Rao and Vigorita 1984).
Plain radiographs can be normal or show intra-articular effusion, an ill-defined para-articular soft-tissue mass and, in longstanding disease, juxta-articular bone erosions and subchondral cysts caused by pressure and hypertrophied synovium. CT shows hemosiderin and fat deposits, and is able to detect bone erosions that are not manifest on radiographs.
MR imaging shows synovial effusion and hypertrophied synovitis containing scattered areas of hemosiderin that exhibits low signal intensity on T1- and T2-weighted sequences—darker on T2* gradient-echo sequences due to susceptibility artifact (Fig. 39). Although similar appearances can be observed in hemophilic and rheumatoid arthritis in the proper clinical setting, these findings are often considered to be diagnostic of pigmented villonodular synovitis (Hughes et al. 1995; Jelinek et al. 1989; Narváez et al. 2001). The US appearance of diffuse pigmented villonodular synovitis is nonspecific as it appears either as an intra-articular area of hypoechoic synovial thickening or as an irregular mass located within the joint cavity usually associated with a joint effusion (Fig. 39a). In some cases, US can detect pressure erosions on the bone cortex as defects of the joint surfaces filled with hypertrophied synovium. Doppler imaging can show a hypervascular pattern within the synovial mass (Yang et al. 1998; Lin et al. 1999). A pattern of relatively increased flow in the periphery of the synovial capsule may be present (Lin et al. 1999). Local recurrence after surgical or arthroscopic synovectomy takes place in almost 50% of cases (Sheldon et al. 2005). The nodular type of pigmented villonodular synovitis (Bianchi et al. 1998; Middleton et al. 2004).
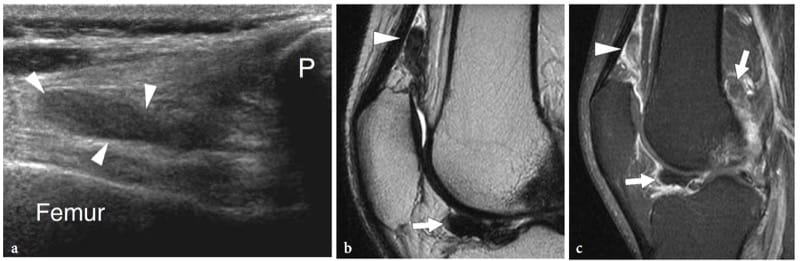
Figure 39. a–c. Pigmented villonodular synovitis: diffuse type. a Longitudinal 12–5 MHz US image obtained over the suprapatellar recess demonstrates hypoechoic thickening of the synovium (arrowheads), a fairly nonspecific appearance. P, patella. b Sagittal T2-weighted and c fat-suppressed postcontrast T1-weighted MR images confirm the US finding (arrowhead) and reveal additional lesions (arrows) lying in the anterior and posterior joint recesses. The hypointense pattern of these lesions on T2-weighted sequences strongly suggests pigmented villonodular synovitis.
23. LIPOMA ARBORESCENS
Lipoma arborescens, also referred to as diffuse synovial lipoma or villous proliferation of synovium, is a rare monoarticular reactive lesion giving rise to a focal fat-containing villous proliferation. It can be primary or, more frequently, associated with other joint disorders, such as degenerative joint disease, rheumatoid arthritis or previous trauma (Al-Ismail et al. 2002; Murphey et al. 2004; Davies and Blewitt 2005; Sheldon et al. 2005). Although occasionally reported in the hip, shoulder and elbow (Doyle et al. 2002; Nisolle et al. 1999; Bejia et al. 2005), lipoma arborescens most commonly occurs in the knee, particularly in the suprapatellar recess. From the clinical point of view, it presents with long-standing painless synovial thickening and intermittent joint effusions. US demonstrates lipoma arborescens as a mass with tree-like contours and villous projections related to replacement of subsynovial tissue by mature fat (Learch and Braaton 2000). In general, the mass is more echogenic than synovial tissue and is associated with joint effusion (Fig. 40a). During joint manipulation, dynamic examination allows real-time demonstration of to-and-fro bending and waving of villous projections (Learch and Braaton 2000; Sheldon et al. 2005). If lipoma arborescens is suspected, MR imaging should be obtained to confirm the diagnosis based on the frond-like architecture and signal intensity similar to that of fat in all pulse sequences (Fig. 40b,c) (Vilanova et al. 2003). Synovectomy is the definitive treatment.
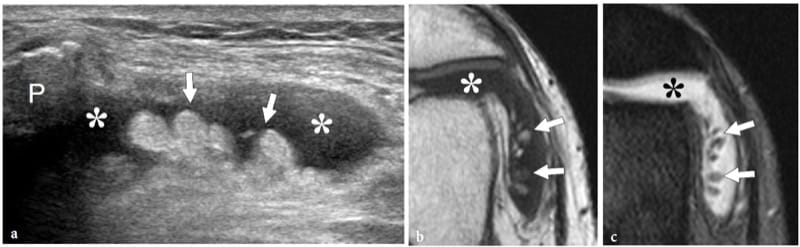
Figure 40. a–c. Lipoma arborescens. a Transverse 12–5 MHz US image obtained over the lateral parapatellar recess of the knee shows an intra-articular relatively hyperechoic fronded mass (arrows) outlined by synovial effusion (asterisks). P, patella. b Transverse T1-weighted and c fat-suppressed T2-weighted MR imaging correlation confirm the fatty nature of the frond-like projections of the mass.
24. SYNOVIAL OSTEOCHONDROMATOSIS
Primary synovial osteochondromatosis (synovial chondromatosis) is a benign condition affecting the synovial membrane that leads to proliferation of intrasynovial chondral or osteochondral nodules. It most commonly affects the knee (50% of cases) of middle-aged men. In order of decreasing prevalence, other commonly involved joints are the elbow, the hip and the shoulder. In early disease (type 1), there is active synovial proliferation with cartilaginous masses embedded within the synovium; then (type 2), osteochondral nodules become pedunculated and are released within the joint cavity; finally (type 3), intra-articular bodies lie free with regression of synovial hypertrophy (Milgram 1977). Clinical symptoms include local pain and swelling, followed by stiffness, intermittent joint locking due to loose bodies trapped in between the joint surfaces, and premature degenerative joint changes. Synovial chondromatosis should not be confused with other causes of intra-articular osteocartilaginous bodies (secondary chondromatosis). US can detect thickened synovium containing hypoechoic chondral nodules that can or cannot undergo calcification. When mineralized, these nodules appear as bright hyper-echoic fragments with posterior acoustic shadowing and are better delineated with US (Fig. 41) (Pai and van Holsbeeck 1995; Leekam and Voorneveld 1996; Campeau and Lewis 1998; Cho et al. 2000). In conglomerate heavy calcified masses, the posterior acoustic shadowing from superficial calcified deposits can mask the rest of the synovial abnormality (Roberts et al. 2004). It is not uncommon that fragments migrate outside the joint space into communicating synovial bursae, such as the semi-membranosus-gastrocnemius bursa or the iliopsoas bursa (Moss and Dishuk 1984). When the diagnosis is suspected on US findings, standard radiographs must be obtained to confirm the presence of punctate or dense lobular calcifications, which occur in approximately two-thirds of cases. Treatment is surgical synovectomy; however, the recurrence rate is over 25% (Sheldon et al. 2005).
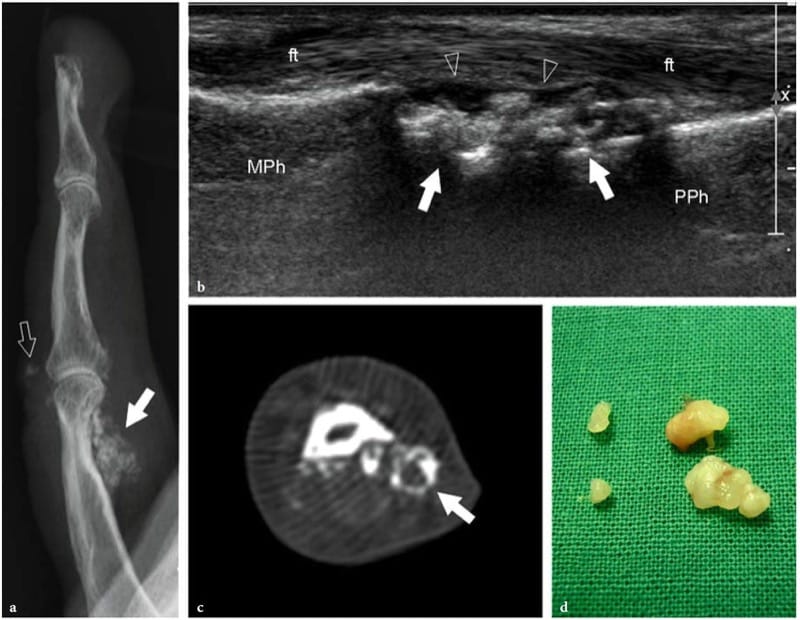
Figure 41. a–d. Synovial chondromatosis. a Plain lateral radiograph of the index finger shows a small soft-tissue calcified mass on the volar (white arrow) aspect of the first phalanx. Some calcifications are also visible on the dorsal aspect of the proximal phalangeal joint (void arrow). Lack of bone erosion and of periosteal reaction suggests excluding bone involvement. b Longitudinal 17-5 MHz US images obtained at the level of the proximal interphalangeal joint reveal a conglomerate of calcifications (arrows) filling the ventral joint recess (arrowheads) between the bone and the flexor tendons (ft). MPh, middle phalanx; PPh, proximal phalanx. c Axial CT imaging correlation. d Surgical specimen of the same case shows the loose bodies.
25. SYNOVIAL HEMANGIOMA
Synovial hemangioma is a rare intra-articular benign tumor arising from the synovium that is difficult to diagnose because of its nonspecific clinical presentation of nontraumatic recurrent swollen painful knee (Narváez et al. 2001; Okahashi et al. 2004). The knee of young adults and adolescents is usually affected. Clinical symptoms are related to the mass effect of the tumor, which leads to a decreased range of motion and repetitive episodes of bleeding causing joint pain and swelling and possibly mimicking other conditions, such as hemophilic arthropathy, arthritis or medial shelf syndrome. Hemangiomas arising from the synovium have a similar US appearance to other soft-tissue hemangiomas, but the presence of hypoechoic tissue with fluid-filled areas related to pooling of blood within vascular spaces may generate confusion with more common synovial conditions. Therefore, the examiner must be aware of this condition to avoid making a wrong diagnosis and wasting time for the patient. In the cavernous type of synovial hemangioma, slow-flowing blood can occasionally be appreciated within the anechoic spaces of the mass on gray-scale and color Doppler US (Fig. 42a–c). MR imaging of synovial hemangioma is pathognomonic, with intermediate T1-signal intensity and marked hyperintensity on T2-weighted images and after gadolinium administration (Fig. 42d,e). Intratumor fat overgrowth, low T2-signal vascular channels and phleboliths can also be found (Fig. 42e,f). Circumscribed masses can be resected on arthroscopy, whereas diffuse lesions need open surgery (Sheldon et al. 2005).
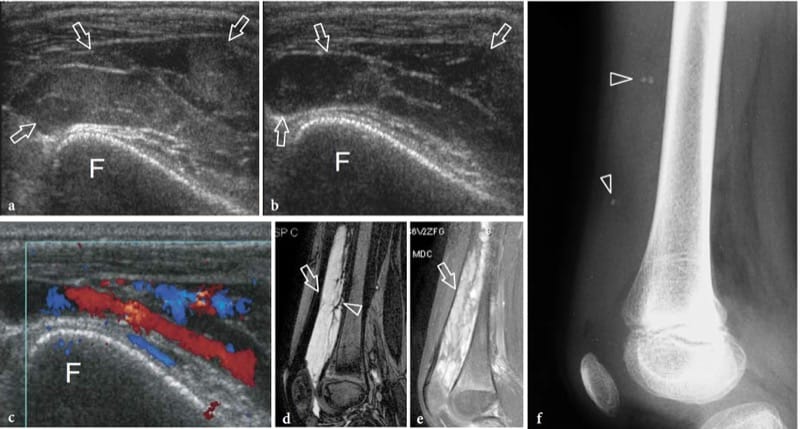
Figure 42. a–f. Synovial hemangioma. a,b Transverse 12–5 MHz US images obtained over the suprapatellar region a without and b with compression with the probe demonstrate a fluid-filled suprapatellar recess (arrows) containing slow-flowing swirling echoes that change their echogenicity depending on the pressure exerted over them. F, femur. c Color Doppler imaging displays venous flow filling the fluid-filled cavities contained in the recess. d Sagittal T2-weighted and e fat-suppressed postcontrast T1-weighted MR imaging correlation show a markedly hyperintense lesion (arrow) containing linear low-signal structures (arrowhead) representing fibrofatty septations and intratumor vascular channels. f Lateral radiograph reveals fullness of the suprapatellar recess and scattered phleboliths (arrowheads), a feature of value for the diagnosis of synovial hemangioma.