INTRODUCTION
Compartment syndrome is an orthopedic emergency. It is an acute condition of the limbs in which the pressure of isolated or groups of poorly compliant muscle compartments increases dramatically and limits local soft tissue perfusion to the point of motor and sensory impairment and neuronal and tissue ischemic necrosis. Although regional anesthesia is often thought to delay diagnosis and treatment of acute compartment syndrome (ACS), there are only isolated case reports and a lack of evidence-based information to guide the clinical practice.
Regardless, practitioners should be aware of the patient risk factors, clinical presentation, and management of this potentially limb-threatening condition. The musculoskeletal structures of the limbs are enclosed within compartments created by investing fascial layers with a limited ability to stretch. These compartments enclose skeletal muscles along with the neurovascular structures that pass through the compartments. If missed, compartment syndrome can be a limb- and life-threatening condition.
Compartment syndrome is most common in the lower leg and forearm, although it can also occur in the hand, foot, thigh, and upper arm. In theory, the upper leg muscles are at a lower risk for injury than are the smaller muscles of the lower leg, because the muscles of the thigh can dissipate the large forces of direct trauma, causing less muscle injury and resultant edema. Acute compartment syndrome occurs more commonly in one of the four smaller compartments of the lower leg.
The consequences of persistently elevated intra-compartmental pressures were first described by Richard von Volkmann, who documented nerve injury and late muscle contracture from compartment syndrome after supracondylar fracture of the distal humerus. Jepson described ischemic contractures in dog hind legs, resulting from limb hypertension after experimentally induced venous obstruction. Only after about 40 years (since the 1970s) has the importance of measuring compartmental pressures become apparent.
Etiology
Any condition that can reduce the volume of the compartment or increase the size of the contents of the compartment can lead to an acute compartment syndrome. Examples of factors leading to these changes are presented in Table 1.
Pathophysiology
Compartment syndrome is essentially soft tissue ischemia, generally associated with trauma, fracture with subsequent casting, prolonged malpositioning during surgery, or reperfusion injury. However, the entire mechanism of compartment syndrome is unclear. Because various osseofascial compartments have a relatively fixed volume, introduction of excess fluid or external constriction increases pressure within the compartment and decreases tissue perfusion (Figure 1). As the compartmental pressure increases, the tissue hypoperfusion results in tissue hypoxia impeding cellular metabolism. If prolonged, permanent myoneural tissue damage occurs. Under physiologic circumstances, the venous pressure exceeds that of the interstitial tissue pressure, sustaining venous outflow. However, as tissue pressure increases, extrinsic venous luminal pressure is exceeded, resulting in vein collapse. The pressure at which this occurs is not known; however, it is generally agreed that compartmental pressures greater than 30 mm Hg require emergent intervention because ischemia is imminent. Hypoxic injury causes cells to release free radicals, which increases endothelial permeability. This, in turn, leads to a vicious cycle of continued fluid loss, further increasing tissue pressure and injury. Diminished blood flow to local nerves first manifests as sensory changes.
TABLE 1. Factors leading to compartment syndrome.
| Conditions that Increase the Compartment Volume |
| • Direct soft tissue trauma with or without long bone fracture (10%–20% incidence after closed fracture) • Closed tibial shaft fractures (40%) and closed forearm fractures (12%) • Soft tissue crush injuries without fractures in 23% of cases of compartment syndrome5,6 • Open fractures, which should theoretically decompress the adjacent compartments, may lead to compartment syndrome7 • Hemorrhage: Vascular injury, coagulopathy • Anticoagulation therapy8 • Revascularization of limb after ischemia • High-energy trauma, as from high-speed motor vehicle accident or crush injury • Increased capillary permeability after burns (especially circumferential) • Infusions or high-pressure injections (eg, regional blocks, paint guns) • Extravasations of arthroscopic fluid (eg, after routine knee arthroscopy9) • Reperfusion after prolonged periods of ischemia • Anabolic steroid use, resulting in muscle hypertrophy10 • Decreased serum osmolarity (eg, nephritic syndrome11) • Strenuous exercise, especially in previously sedentary people |
| Conditions that Lead to a Reduction in Volume of Tissue Compartments |
| • Tight circumferential dressings (eg, can occur with cotton cast padding alone) • Closure of fascial defects12 • Cast or splint, especially if placed before removal of surgical tourniquet • Prolonged limb compression, as in Trendelenburg and lateral decubitus positions6,13 or in patients obtunded from alcohol or drug abuse • Excessive traction to fractured limbs14 |
Paresthesias develop within 30 minutes of onset of ischemia. Irreversible nerve damage begins after 12–24 hours of total ischemia. Irreversible changes in the muscles begin after only 4–8 hours, leading to muscle fiber death and late myocontracture.
DIAGNOSIS OF COMPARTMENT SYNDROME
Compartment syndrome is often a diagnosis based primarily on variation in the patient’s clinical signs and symptoms in sequential examinations. Pain out of proportion to the injury, especially with passive stretch of the muscles in the suspicious compartment or limb, is one of the most significant indicators.
A palpably tense extremity compared with the uninjured limb is also an important finding.
However, none of these warning signs has proven to be reliable. The other classic Ps of pallor, pulselessness, and paresis have very poor predictive value. In fact, pallor and pulselessness are rarely present in compartment syndrome, and by the time paresis manifests, the damage is largely irreversible. On the other hand, breakthrough pain in a patient with a previously well-functioning continuous block may be an early warning sign of ACS.
Clinical Pearl
• Pain out of proportion to the injury is an important symptom.
In general, establishment of the diagnosis based on clinical judgment alone can be difficult. Instead, diagnosis should be supported by objective measurement of compartmental pressures with a needle and arterial line transducer or other pressure-measuring device(s). Assuming that the correct compartment is identified, measurement of interstitial tissue pressures remains the only objective reference method for the diagnosis of ACS and is particularly useful in the unresponsive, obtunded, or anesthetized patient (Figures 2 and 3).
An absolute value above 30 mm Hg in the normotensive patient is consistent with compartment syndrome.
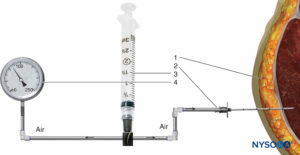
FIGURE 2. Intramuscular pressure measurement by the Whiteside technique. (1) Intramuscular needle, 18 gauge. (2) Perfusion line.(3) 20-mL syringe. (4) Mercury manometer.
This value is diminished in the hypotensive patient as the lower arterial pressure renders the limbs even more susceptible to ischemic injury. Near-infrared spectroscopy is another noninvasive method suggested for monitoring the oxygen saturation of hemoglobin and myoglobulin in the tissue at risk (Figure 4). More recently, intramuscular pH monitoring has been introduced as an additional diagnostic tool in order to accurately identify ACS.
The Upper Limb
There are several compartments of the upper extremity that, when injured, may result in compartment syndrome requiring fasciotomy in the arm, forearm, or hand. The arm has two compartments: anterior and posterior (Figure 5).
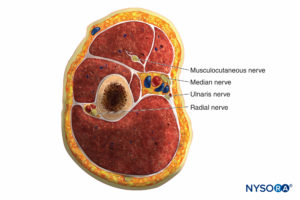
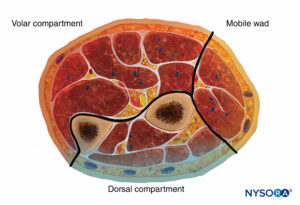
The forearm has three compartments: the volar and dorsal compartments and the compartment containing the muscles of the mobile wad. Mubarak et al. have demonstrated that these compartments are interconnected, unlike the compartments of the leg (Figures 6 and 7).
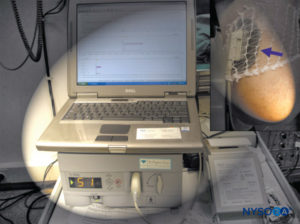
FIGURE 3. Near-infrared spectroscopy is a noninvasive method for monitoring the oxygen saturation of hemoglobin and myoglobulin.
Consequently, decompression of the volar compartment alone may decrease the pressure in the other two compartments. Regardless, dorsal compartment fasciotomy should still be performed if the dorsal compartment remains tight after volar decompression. The muscles of the volar compartment of the forearm include the digital and wrist flexors and the forearm pronators. These muscles are tested by passive extension of the digits and wrist and by supination of the forearm.
The dorsal forearm compartment contains the thumb and finger metacarpophalangeal joint extensors, the ulnar wrist extensors, and the forearm supinators and is tested by passive finger, thumb, and wrist flexion and by forearm pronation. The mobile wad includes the brachioradialis and the two radial wrist extensors and is tested by passive wrist flexion.
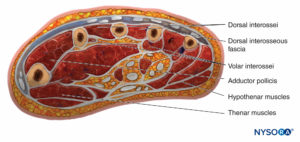
There are 10 compartments in the hand, the most prominent being the dorsal and palmar interosseous compartments, of which there are four and three, respectively (Figure 8). The other compartments are the hypothenar, thenar, and adductor. The compartment containing the adductor muscle of the thumb is often overlooked when doing fasciotomies. Studies using Renografin dye have shown no connection between the dorsal interossei and the other compartments, showing that each compartment must be decompressed separately.
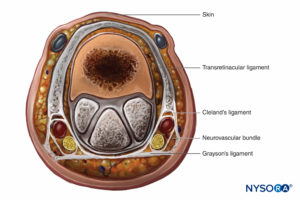
The finger is enclosed in a tight investing fascia and is compartmentalized by the fascia and the volar skin at the flexor crease. Although no muscle bellies are distal from the metacarpophalangeal joints, ischemia and engorgement can lead to tissue loss (Figure 9).
The Lower Limb
Thigh
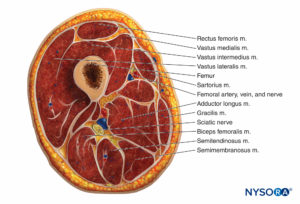
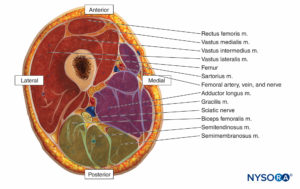
The thigh muscles are divided into three compartments invested by thick fascia: the anterior, medial, and posterior (Figures 10 and 11). Because thigh compartment syndrome is uncommon, it may go unrecognized. A history of anticoagulant use is common in patients with thigh compartment syndrome.
Signs and symptoms include a history of thigh swelling and/or hematoma and pain after a minor injury in a patient who is anticoagulated. Although rare, the thigh syndrome can also occur in patients after joint replacement surgery. The combination of minor trauma and anticoagulation produces bleeding into muscle and tissue spaces, leading to increased compartment pressure. Pain ranges from mild to severe and may be elicited only when the hip and knee are flexed and extended. Other findings of vascular occlusion—loss of pulse, pallor, paresthesias, and paralysis—are frequently absent.
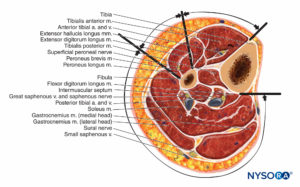
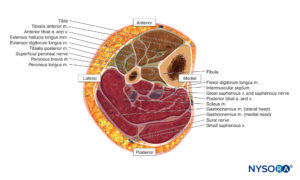
Lower Leg
The lower leg contains four compartments, each invested by inelastic fascia (Figures 12 and 13). Each compartment contains a major nerve: the deep peroneal in the anterior compartment, the superficial peroneal in the lateral compartment, the saphenous in the superficial posterior compartment, and the tibial in the deep posterior compartment. Swelling in the lateral or anterior compartment can compress both the deep and superficial peroneal nerves against the neck of the fibula.
The superficial peroneal nerve usually lies in the interval between the two peroneal muscles for a short distance and then emerges anterior to the peroneus brevis. It pierces the lateral compartment fascia at the junction of the middle and distal third of the leg.
The anatomy of the superficial and deep posterior compartments is somewhat variable, but both compartments, and especially the deep compartment, are frequently involved in compartment syndrome.
Foot
The foot has numerous rigidly bound compartments, and even mild bleeding into these spaces can elevate the pressures dramatically (Figure 14). According to Manoli and Weber, there are nine compartments in the foot. Three compartments run the entire length of the foot (medial, lateral, and superficial).
Five compartments are contained within the forefoot (adductor and four interossei). The calcaneal compartment is confined to the hind foot but communicates with the posterior compartment of the leg. This compartment contains the quadratus muscle and the lateral plantar neurovascular bundle. The clinically most relevant compartments are the medial, central, lateral, and interossei.
A wide spectrum of injuries can result in compartment syndrome of the foot, the most likely ones being crush injuries, especially those associated with multiple metatarsal fractures.
Often, the only reliable method of diagnosis is by clinical suspicion and measurement of the intracompartmental pressures.
Loss of posterior tibial or dorsalis pedis pulse is notoriously unreliable in the early diagnosis of compartment syndrome. The earliest clinical findings are muscle and nerve ischemia and pain. Although this pain might be confused with the pain of the injury itself, it may be exacerbated by gentle, passive dorsiflexion of the toes, which stretches the intrinsic muscles of the foot.
Lack of sensation is generally accepted as an important sign of nerve ischemia, but it is not reliable when compared with a two-point discrimination and light touch over the plantar aspect of the foot and toes.
Clinical Pearl
• Compartment pressure measurement is the only objective and accurate test to diagnose and monitor compartment syndrome.
Compartment pressure measurement is the only objective and accurate test to diagnose and record compartment syndrome, particularly because changes in compartment pressures can precede the clinical signs of compartment syndrome.
The central compartment can be measured by passing a needle between the metatarsal and abductor hallucis muscle at the base of the first metatarsal. The interossei compartment is measured in two positions by introducing the needle through the intermetatarsal spaces, preferably between the second and fourth web spaces to avoid punctures to the dorsalis pedis within the first intermetatarsal region.
The calcaneal or quadratus compartment is measured by inserting the needle 5 cm distal and 2 cm inferior to the medial malleolus and advancing through the abductor’s muscle.
Acute Compartment Syndrome and Regional Anesthesia
There is a significant medicolegal aspect of ACS and its outcome in clinical practice; in 50% of all cases and claims related to ACS, data show that decisions are ruled in favor of the plaintiff (the patient). Several case reports and case series suggest that regional anesthesia may have delayed diagnosis of ACS. In contrast, there are also a number of cases and reviews suggesting that regional anesthesia may not mask timely diagnosis and, in fact, may even facilitate detection of ACS. Consequently, the use of a regional anesthesia technique in the face of risk factors for ACS remains controversial.
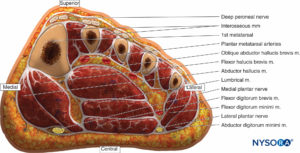
FIGURE 14. Coronal section of the foot through the base of the metatarsals depicting the medial, central, lateral, and interosseous compartments.
In recent literature, “best practice rules” have been suggested to reduce the risk of missing a compartment syndrome in children undergoing surgery with perioperative regional anesthesia; these rules may also be applicable to adults. It should be noted that although these recommendations appear succinct, they are largely theoretical considerations to help guide the clinical decision making; they have not been put to test in clinical practice.
Clinical Pearl
When regional analgesia is planned to treat intractable pain in patients with risk of compartment syndrome:
• Reduce the concentration of local anesthetics (0.1% to 0.25% bupivacaine, levobupivacaine, or ropivacaine) as lower concentrations are less likely to mask ischemic pain.
• For continuous infusions of bupivacaine, levobupivacaine, or ropivacaine, concentrations should be limited to 0.1%.
• In high-risk surgeries for compartment syndrome (eg, tibial compartment surgery), restricting both volume and concentration is advisable.
• Patients should have careful follow-up by acute pain services to allow for early detection of potential signs and symptoms (h).
• If ACS is clinically suspected, compartment pressure measurement without delay is mandatory.
TREATMENT OF COMPARTMENT SYNDROME
Emergency fasciotomy remains the definitive treatment for a diagnosis of compartment syndrome because of its well-documented, limb-saving results. It is universally accepted as being the best chance for complete recovery and for prevention of further tissue necrosis. Treatment is based primarily on the clinical picture together with corroborative compartmental pressure measurements (Figure 15). The surgeon should proceed emergently with a decompression fasciotomy when clinically indicated because the exact pressure at which fasciotomy should be performed remains controversial. Most studies have shown that fasciotomy is indicated when the compartment pressure reaches 30 mm Hg. Fasciotomy is also recommended when the compartmental pressure is within 30 mm Hg of the patient’s diastolic pressure.
After a complete fasciotomy, there is rarely a need for additional releases. The fasciotomy incisions are always left open with wound closure delayed for a minimum of 5 days. The patient is followed up clinically unless anesthetized or obtunded, in which case regular compartment pressure measurements should be made.
Clinical Pearl
• Emergency fasciotomy remains the definitive treatment for compartment syndrome.
• Its limb-saving results make it universally accepted as the best chance for complete recovery and for prevention of further tissue necrosis.
• Fasciotomy is indicated when compartment pressure reaches 30 mm Hg.
• After a complete fasciotomy is performed, additional release is rarely needed.
SUMMARY
Prolonged surgery, especially in patients undergoing procedures in the Trendelenburg or lateral decubitus positions, poses a risk of compartment syndrome. The Trendelenburg position requires
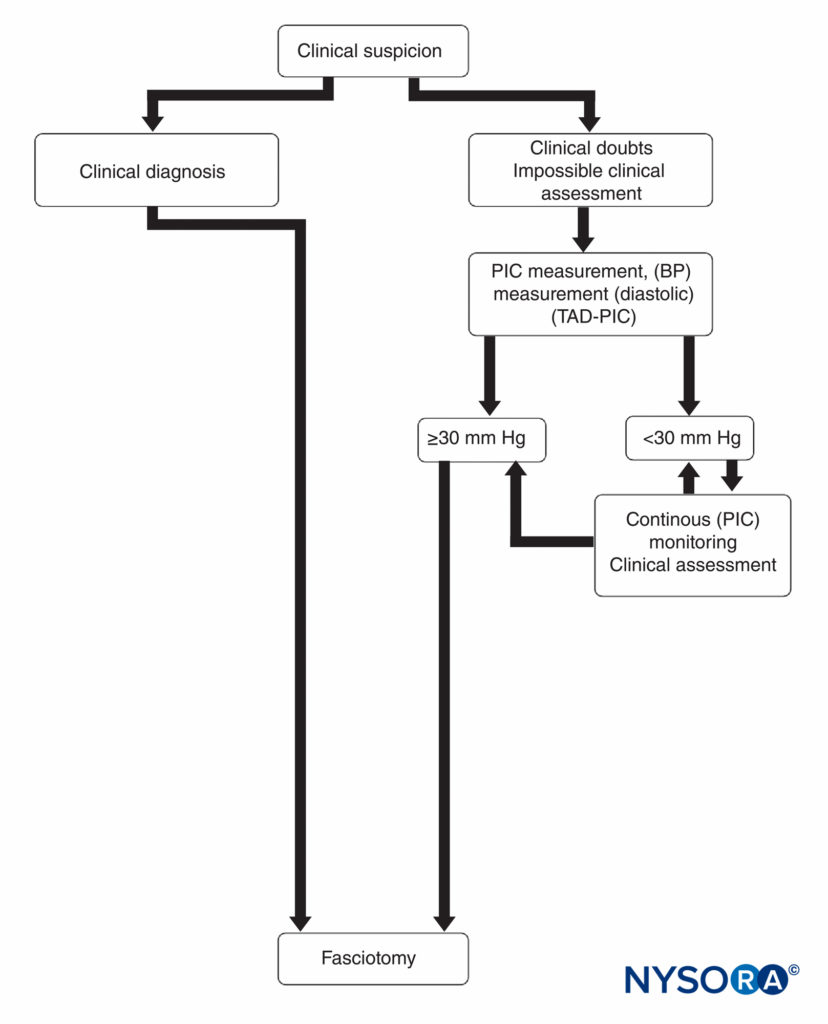
FIGURE 15. Diagnosis and management of compartment syndrome. BP, blood pressure; PIC, pressure within the compartment; TAD-PIC, diastolic blood pressure.
that the legs are strapped at a higher level than the heart. This can be avoided by repositioning and redraping the legs, or, if this is not possible, the head-down tilt position should be reversed every 2 hours so that reperfusion of the lower limbs can occur. In the lateral decubitus position, the down arm and the down leg must be well padded to avoid excessive compression.
On recent analysis of risk factors, age appeared to be a strong predictor of developing ACS (P < 0.001), with the highest prevalence between 12–19 years and 20–29 years. Occupation and implant type were the only other factors that remained significant after adjusting for age.
Patients on anticoagulation medication tend to have a high risk of thigh compartment syndrome, even with relatively minor trauma or surgical interventions. This clinical scenario must be approached with a high index of suspicion.
In conclusion, the use of regional anesthesia in patients with risk of compartment syndrome is controversial. Therefore, regional blocks should be performed in consultation with the patient and with the surgical team. When deemed beneficial to patient care, regional anesthesia can be used when indicated to alleviate severe pain; however, astute management, compartment tissue monitoring, and perhaps lower concentrations and volumes of local anesthetics should be considered.
Continue reading: Complications and Prevention of Neurologic Injury with Peripheral Nerve Blocks
REFERENCES
- Matsen F, Winquist R, Krugmire R: Diagnosis and management of compartment syndromes. J Bone Joint Surg 1980;62A:286.
- Schwartz J, Brumback R, Lakatos R: Acute compartment syndrome of the thigh: A spectrum of injury. J Bone Joint Surg 1989;71:392–400.
- von Volkmann R: Die ischamischen Kontakturen. Zentralbl Chir 1881; 8:801.
- Jepson P: Ischemic contracture, experimental study. Ann Surg 1926; 68A:820.
- Matsen F: Compartmental syndrome. A unified concept. Clin Orthop 1975;113:8–14.
- Botte M, Santi M, Prestianni C, Abrams R: Ischemic contracture of the foot and ankle: Principles of management and prevention. Orthopedics 1996;19:235–244.
- Ziv I, Mosheiff R, Zeligowski A, et al: Crush injuries of the foot with compartment syndrome: Immediate one-stage management. Foot Ankle 1989;9:185–189.
- Whitesides T, Harada H, Morimoto K: The response of skeletal muscle to temporary ischemia: An experimental study. J Bone Joint Surg 1971;53A: 1027–1028.
- McQueen M, Gaston P, Court-Brown C: Acute compartment syndrome: Who is at risk? J Bone Joint Surg 2000;82B:200–203.
- Ulmer T: The clinical diagnosis of compartment syndrome of the lower
leg: Are clinical findings predictive of the disorder? J Orthop Trauma
2002;16:572–577. - Shuler FD, Dietz MJ: Physician’s ability to manually detect isolated
elevations in leg intracompartmental pressure. J Bone Joint Surg Am
2010; 92:361-367. - McQueen MM. Acute compartment syndrome. In: Bucholz RW, Court-Brown CM, Heckman JD, Tornetta P 3rd (eds): Rockwood and Green’s Fractures in Adults, 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2010:689–708.
- Tighe PJ, Elliott CE, Lucas SD, et al: Noninvasive tissue oxygen saturation determined by near-infrared spectroscopy following peripheral nerve block. Acta Anesth Scand 2011;55:1239–1246.
- Elliott KG: Intramuscular pH as a novel diagnostic tool for acute
compartment syndrome: A prospective clinical study [dissertation].
Aberdeen, Scotland: University of Aberdeen; 2007. uk.bl.ethos.485671. - Gelberman R, Zakaib G, Mubarak S, et al: Decompression of the forearm
compartments. Clin Orthop 1978;134:225–229. - Allen M, Steingold R, Kotecha M: The importance of volar compartment
in crush injuries in the forearm. Injury 1985;16:173–175. - Choyce A, Chan V, Middleton W, et al: What is the relationship between
paresthesia and nerve stimulation for axillary brachial plexus block? Reg
Anesth Pain Med 2001;26:100–104. - An H, Simpson M, Gale S, Jackson W: Acute anterior compartment syndrome in the thigh: A case report and review of the literature. J Orthop Trauma 1987;1:180–183.
- Manoli A II, Weber T: Fasciotomy of the foot: An anatomical study with
special reference to release of the calcaneal compartment. Foot Ankle
1990;10(6):267–275. - Sarraffian S: Anatomy of the Foot and Ankle. Philadelphia: J. B. Lippincott, 1983.
- Myerson M: Experimental decompression of the fascial compartment of the foot: The basis for fasciotomy in an acute compartment syndromes. Foot Ankle 1988;8:308–314.
- Dunwoody J, Reichert CC, Brown KL: Compartment syndrome associated with bupivacaine and fentanyl epidural analgesia in pediatric orthopaedics. J Pediatr Orthop 1997;17:285–328.
- Hyder N, Kessler S, Jennings A, et al: Compartment syndrome in tibial shaft fracture missed because of local nerve block. J Bone Joint Surg
1996;78-B:499–500. - Tang W, Chiu K: Silent compartment syndrome complicating total knee arthroplasty. J Arthroplasty 2000;15:241–243.
- Walker BJ, Noonan KJ, Bosenberg AT: Evolving compartment syndrome
not masked by a continuous nerve block. Reg Anesth Pain Med
2012;3:393–397. - Mar GJ, Barrington MJ, McGuirk BR: Acute compartment syndrome of the lower limb and the effect of postoperative analgesia on diagnosis. Br J Anaesth 2009;102:3–11.
- Cometa MA, Esch AT, Boezaart AP: Did continuous femoral and sciatic nerve block obscure the diagnosis or delay the treatment of acute lower leg compartment syndrome? A case report. Pain Med 2011;12:823–828.
- Kucera TJ, Boezaart AP: Regional anesthesia does not consistently block ischemic pain: Two further cases and review of the literature. Pain Med
2014;15:316–319. - Rauf J, Iohom G, O’Donnell B: Acute compartment syndrome and regional anaesthesia—a case report. Rom J Anaesth Int Care 2015;22:
51–54. - Sermeus L, Boeckx S, Camerlynck HP, et al: Postsurgical compartment syndrome of the forearm in a child. Acta Anaesthesiol Belg 2015;66: 29–32.
- Aguirre JA, Gresch D, Ropovici A, et al: Case scenario: Compartment syndrome of the forearm in patient with infraclavicular catheter. Anesthesiology 2013;118:1198–1205.
- Ivani G, Suresh S, Ecoffey C, et al: The European Society of Regional Anaesthesia and Pain Therapy and the American Society of Regional Anesthesia and Pain Medicine Joint Committee Practice Advisory on Controversial Topics in Pediatric Regional Anesthesia. Reg Anesth Pain Med 2015;40:526–532.
- Mubarak S, Owen C: Compartment syndrome and its relationship to the crush syndrome: A spectrum of disease—a review of 11 cases of prolonged limb compression. Clin Orthop 1975;113:81– 89.
- Whitesides T, Haney T, Morimoto K: Tissue pressure measurements as a determinant for the need of fasciotomy. Clin Orthop 1975;113:43–51.
- Staudt JM, Smeulders MJ, van der Horst CM: Normal compartment pressures of the lower leg in children. J Bone Joint Surg Br 2008;90: 215–219.
- McQueen MM, Duckworth AD, Aitken SA, et al: Predictors of compartment syndrome after tibial fracture. J Orthop Trauma 2015;29: 451–455.