INTRODUCTION
Ultrasonography (US) as a means to guide peripheral nerve block (PNB) was first explored by anesthesiologists at the University of Vienna in the mid-1990s. Although radiologists had made use of ultrasound technology to guide needles for biopsy, the application of this imaging modality for PNB was novel at that time. The utility of ultrasound to facilitate a range regional anesthesia techniques including brachial plexus and femoral blocks was demonstrated. A decade later, colleagues from the University of Toronto, Canada, began to embrace this technology, further demonstrating its utility and describing in detail the sonoanatomy of the brachial plexus. A number of advances in technology took place in the meantime, including smaller and more mobile ultrasound platforms, improved resolution, and needle recognition software, all cumulatively leading to increased bedside utility of ultrasound by anesthesiologists.
ADVANTAGES OF ULTRASOUND GUIDANCE
The previously used surface anatomy-based techniques, such as nerve stimulation, palpation of landmarks, fascial “clicks,” paresthesias, and transarterial approaches, did not allow for the monitoring of the disposition of the local anesthetic injectate. Ultrasound guidance, however, offers a number of important practical advantages for nerve block. Ultrasound allows visualization of the anatomy of the region of interest. This allows more informed guidance for the needle pathway to the target while avoiding structures that might be damaged by the needle. Ultrasound also allows visualization of the needle tip as it is passed through the tissues, confirming alignment with the intended path, again reducing the likelihood of inadvertent needle trauma to unintended structures. Perhaps most important, real-time ultrasound imaging permits continual visualization of local anesthetic solution delivery to ensure proper distribution, with the potential for adjustment of the needle tip position as necessary to optimize local anesthetic distribution.
Introduction of ultrasound guidance in regional anesthesia has led to refinement of many nerve block techniques, expanded use of PNB, and greater acceptance by surgical colleagues and patients.
ULTRASOUND AND SONOANATOMY
Ultrasound-guided PNB may be broken down into two fundamental aspects: imaging structures in the plane of section, including the target nerve, and guiding the needle. Understanding and recognition of three-dimensional anatomic structures on a twodimensional image requires training in the technology and sonoanatomy pattern recognition (Table 1).
TABLE 1. Optimizing sonoanatomy visualization.
| Choose appropriate transducer/frequency Understand underlying anatomic relationships Apply varying degree of pressure with transducer Align transducer with underlying nerve target Rotate transducer to fine-tune image Tilt the transducer to optimize image |
As anatomic recognition remains essential to placing blocks, even with realtime visual guidance, specialty society guidelines for training residents and fellows continue to stress the importance of anatomical dissection and gross anatomy training as an inherent component of learning ultrasound-guided regional anesthesia (UGRA). In a study conducted over a 1-month regional anesthesia rotation, residents demonstrated markedly improved recognition of relevant structures at the sites of several different PNBs, using ultrasound imaging. In an evaluation of ultrasound-guided interscalene block instruction, residents demonstrated increasing efficiency of sonoanatomy recognition as their experience over the course of the rotation increased.
More innovative methods of training have shown promise as well. Integrating an anatomic program into the software of a bedside ultrasound machine has been shown to improve scores on a written test of anatomy. After exposure to a multimedia anatomy presentation, residents and community anesthesiologists demonstrated increased knowledge of ultrasound anatomy on a posttest, although they were not able to improve scores on a practical examination of sonoanatomy on live models. However, the optimal link between anatomic knowledge and recognition of two-dimensional anatomic patterns on ultrasound has not yet been adequately explored.
Certain basic tenets of optimizing an ultrasound image are applicable to all nerve blocks. For instance, sonography requires an understanding of mechanics and ergonomics. Novices are subject to errors such as probe fatigue, reversing probe orientation, and inadequate equipment preparation. To optimize the ultrasound image, the mnemonic PART (pressure, alignment, rotation, tilting) has been recommended. Pressure is necessary to minimize the distance to the target and compress underlying subcutaneous adipose tissues. Alignment refers to placing the transducer in a position over the extremity (or trunk) at which the underlying nerve is expected to be in the field of view. Rotation allows fine-tuning of the view of the target structure. Tilting helps to bring the face of the probe into a perpendicular arrangement with the underlying target to maximize the number of returning echoes and thus provide the best image (Figure 1). In-depth discussion on optimizing ultrasound imaging is discussed in “Optimizing an Ultrasound Image“.

FIGURE 1. Fine adjustment of the probe tilt is necessary to optimize echo return from the target structure and enhance image resolution (yellow arrowheads indicate sciatic nerve at the popliteal fossa).
NYSORA Tips
- To optimize the ultrasound image, the mnemonic PART has been recommended: pressure, alignment, rotation, tilting.
- Recognition and understanding of sonoanatomy requires knowledge of the underlying three-dimensional anatomy.
- Optimum visualization of the target nerve requires appropriate transducer pressure, alignment with nerve, and rotation and tilting of the probe to fine-tune the image.
OPTIMIZING NERVE AND NEEDLE IMAGING WITH ULTRASOUND-CLINICAL SCENARIOS
Nerve imaging may be performed in either short-axis (probe face perpendicular to axis of nerve) or long-axis (probe face parallel to axis of nerve) position (Figure 2).

FIGURE 2. The median nerve. A: Cross-section (target structure out of plane to ultrasound beam; yellow arrowhead). B: Longitudinal section (target structure in plane to ultrasound beam; red arrowheads).
It is frequently easier to recognize the round, often-hyperechoic, neural element with short-axis imaging, especially for a beginner. Because most nerve blocks are conducted in the extremities, this orientation results in a transducer position that is transverse, across the long axis of the arm or leg. In general, understanding the course of the nerves, based on knowledge of gross anatomy, allows one to align and rotate the transducer perpendicular to the course of the nerve subsequently adjusting the tilt as described previously to optimize the image.
Once the nerve and surrounding anatomy are identified, a needle path may be chosen so that it is imaged either in plane (needle parallel to long axis of probe) or out of plane (needle perpendicular to long axis of probe) to the ultrasound beam. While neither method has been shown superior for either block success or patient safety, the preferred approach may vary with anatomic or technical considerations. However, with in-plane imaging, it is possible to maintain an image of the entire needle, including the tip, although it can be challenging to keep the needle entirely in the viewing plane of the transducer. This method is especially beneficial during instruction, as the supervisor has continual visualization of the needle tip as it is advanced through the tissues.
During out-of-plane imaging, the observer is able to see only the cross section of the needle, which appears as a small hyperechoic dot, at any plane along its entire length, so that distinguishing the tip from the shaft is much more difficult.
Guiding the needle tip to the target while maintaining the entire needle in the plane of imaging, however, can be challenging (Table 2).
TABLE 2. Optimizing needle imaging with ultrasound.
| Utilize a shallow angle of approach, if possible “Heel” the transducer to make the face more parallel to the needle Rotate the transducer to ensure the entire needle is seen Tilt the transducer as necessary Choose an “echogenic” needle Apply needle recognition software, if available “Hydrolocation” may help ascertain needle tip location |
Appropriate adjustment of the bed height and ergonomic placement of the ultrasound so that operator’s eyes can easily and rapidly shift from the image to the field (Figure 3), where needle alignment with the long axis of the probe can be ensured, is beneficial. It is surprisingly easy for the transducer to wander away from the plane of the needle while one’s vision is fixed on the ultrasound screen. This is more likely if the operator has the probe and needle aligned perpendicular to his or her own axis of viewing, as opposed to aligning the needle and probe with the viewing axis.

FIGURE 3. Ergonomic positioning for bed height and ultrasound position.
In a study of novice medical students learning the basics of UGRA, Speer et al found that the subjects required less time to locate the target, and were better able to keep the needle visualized in plane on the ultrasound image, when eyes, needle, probe, and viewing screen were aligned. Needle guides may also permit improved imaging of the needle during approach to the target, although more work has been done in vascular access. One disadvantage of needle guides is that they restrict needle motion to one plane, which may not be always desirable.
Nerves in short axis have an appearance that is to some extent determined by their proximity to the neuraxis. Although in most areas nerves are round, they may appear fusiform, such as the musculocutaneous nerve in the proximal arm, or ovalshaped, such as the sciatic nerve in the infragluteal region. In close association with the spine, nerves and nerve roots are comprised primarily of neural tissue, with minimal connective tissue. Because neural tissue appears hypoechoic on ultrasound imaging, while the connective tissue between fascicles is hyperechoic, nerves near the neuraxis appear as dark nodules.
As nerves course peripherally, the number of fascicles increases, although they diminish in size, while the amount of connective tissue also increases. These changes lead to an increasingly complex “honeycomb” appearance on ultrasound in short-axis viewing (Figure 4). Unfortunately, because of the technology limitations of the current ultrasound machines, the number and arrangement of fascicles within a peripheral nerve may not be accurately portrayed.

FIGURE 4. A: Proximal nerve appearance in the interscalene groove (yellow arrows indicate nerve roots) with little echogenic connective tissue. B: More distal in the supraclavicular fossa (red arrows indicate brachial plexus trunks) with “honeycomb” appearance.
While different tissues have characteristic appearances on ultrasound, nerve may not be easily be distinguished from tendon when both are viewed in short axis. However, using knowledge of anatomy, the operator can follow the course of the structure caudad-cephalad to determine the nature of the structure imaged. The tendons will eventually disappear into the muscle of origin or insert into bones. A good example is the median nerve at the wrist, where it is difficult to discern the neural structure from the many tendons in the carpal tunnel, versus at the midforearm, where the nerve is much more visually distinct, as it is situated between two layers of muscle, with no surrounding tendons (Figure 5).
An important aspect of preparing for a block is to obtain the preferred imaging plane while planning the route for needle path. The operator should make certain that no vulnerable structures are in the projected course, such as a blood vessel, the pleura, or sensitive structures such as periosteum.

FIGURE 5. A: The median nerve at the wrist among many tendons within the carpal tunnel. B: The median nerve more proximal in the forearm surrounded by muscle.
This process is referred to as a “preblock scan,” which can contribute to patient safety and block success. In addition to two-dimensional imaging, the color Doppler setting should be utilized to identify small vessels, which may readily be confused with nerve structures (particularly roots) when viewed in short axis (Figure 6).

FIGURE 6. The supraclavicular brachial plexus with surrounding vasculature. The subclavian artery is indicated by the multicolor area, with the transverse cervical artery indicated by the red area.
To maintain the view of the needle tip and shaft, several techniques can be used. The more parallel the needle is to the face of the probe, the more echoes are transmitted back to the transducer, resulting in a superior image. This can be accomplished by gently indenting the skin at the needle insertion site or by moving the insertion site further away from the probe, resulting in a less-acute angle of insertion (Figure 7). The limitation of this approach is that a longer needle may be required, and more tissue is traversed en route to the target.

FIGURE 7. Needle insertion directly beside the ultrasound probe may result in difficult visualization. Insertion at a distance from the probe permits a shallower approach, allowing for stronger echo return and better visualization of the needle (green arrow) although traversing a longer tissue path.
Another technique, referred to as heeling, involves pressing in on the edge of the transducer opposite the side of needle insertion, which results in a more parallel alignment of the probe face with the needle. In addition, the needle itself may be structurally altered to increase its echogenicity; commercially available versions of these “echogenic needles” usually have been etched on the surface of the shaft with crosshatches to create a greater degree of scatter of the ultrasound beam.
As noted, needle guides may be utilized to improve needle imaging, though at the cost of constraint of movement. Laser guidance systems have also been created to improve alignment, with some success. One novel, alternative method of targeted needle placement and local anesthetic delivery utilizes a GPS guidance system, which may be especially useful when imaging is made difficult by steep needle angles. Proprietary software for needle localization at steep angles makes use of spatial compound imaging, which combines images of different angles of insonation. This results in enhanced needle imaging with both standard and echogenic block needles. Finally, localization of the needle tip may be accomplished with “hydrolocation,” in which small volumes of either dextrose solution or local anesthetic are injected to visualize spread within tissues, which typically reveals the position of the needle tip.
NYSORA Tips
• Several different techniques are useful to maintain visualization of the needle with ultrasound imaging, including use of a shallow angle of approach, “heeling” the transducer, commercially available echogenic needles, and physical measures such as rotation and tilting of the transducer.
• In addition, hydrolocation with a small injection of fluid can be utilized to facilitate localization of the needle in difficult situations.
• The needle should be advanced with continuous visualization to avoid injury to anatomic structures.
• A preblock scan, including use of the color Doppler function, helps plan the course of the needle.
• Passage of the needle tip through fascial planes that abut a nerve should be conducted in a tangential fashion to avoid impaling the nerve when the fascia “releases” the needle.
SAFE NEEDLE GUIDANCE WITH ULTRASOUND
In advancing the needle tip toward the targeted nerve with in-plane imaging, one should be cautious and deliberate, attempting to maintain the needle in plane at all times (Table 3). The in-plane needle tip is characterized by a double-echo return generated from the beveled surface.
TABLE 3. Safety tips during ultrasound-guided nerve blocks.
| Perform a “preblock scan” to ascertain anatomy Utilize the color Doppler setting to identify blood vessels Do not advance needle if tip is not localized “Hydrodissection” can be utilized to delineate anatomy When pushing through fascia toward a nerve, approach tangentially Pass through fascia slowly, awaiting a “pop” or sudden release Reoptimize image of needle tip after passing through fascia When in doubt about needle-nerve interface, gently move needle to ascertain that the nerve does not move with it (indicating that tip is embedded within epineurium) |
Ultrasound is reflected from both the superficial and the deep walls of the needle, resulting in a step appearance that can be distinguished from the single return of the needle shaft. A subtle sliding motion of the ultrasound probe can aid in confirming the location of the tip as the beam walks up and down the needle shaft.
Commonly, fascial planes will be encountered that resist advance of the needle. These tough layers of connective tissue may be seen to “tent” as the tip pushes against them, suddenly giving way and snapping back to their original position. This abrupt change may have two consequences: First, the needle may advance quickly and inadvertently beyond the intent of the operator (unless this is anticipated); second, the needle may move out of plane. At this point, the forward motion of the needle should be stopped until the in-plane image is once again optimized. It is common for such fascial planes to lie just superficial or adjacent to the nerve target, as at the interscalene groove, the axillary neurovascular bundle, or the femoral nerve. This motion may actually result in the needle thrusting forward and encountering the nerve if the sudden give of the fascial plane is not anticipated. For this reason, it is recommended to approach nerves tangentially, projecting the advance of the needle so that its tip will lie adjacent to the nerve, but not aiming for its center.
The resistance encountered by these tough facial planes may also inadvertently redirect a needle when approached at a shallow angle. Temporarily steepening the needle angle may permit an easier and more controlled passage. Unfortunately, ultrasound guidance does not always produce clear images that allow one to distinguish the nerve tissue from surrounding tissue. In such situations, as the needle is advanced, “hydrodissection” (deliberate injection of fluid into tissue planes) can be utilized to separate structures, allowing better clarity in imaging, with either dextrose or local anesthetic solution.
In addition, the behavior of tissues can be observed as the needle is advanced to help localize the needle tip in relation to neural tissue.
While it was once held that contacting a nerve with a needle tip would likely result in paresthesia, and, indeed, this was considered an appropriate nerve localization technique, we now know that paresthesia is not consistently elicited with needlenerve contact. This emphasizes the need to accurately localize the needle tip with ultrasound imaging as well as using additional monitoring during PNBs to detect hazardous needle-nerve relationships, such as nerve stimulation and injection pressure monitoring.
NYSORA Tips
- Deposition of local anesthetic solution should be optimized by taking advantage of fascial planes or sheaths that can contain or channel the drug around the nerve
and longitudinally along its course. - For nerves without such local fascial containment, the solution should be injected in a circumferential manner to hasten onset of the block.
USE OF PERIPHERAL NERVE STIMULATION WITH ULTRASOUND
The peripheral nerve stimulator (PNS) has been a standard tool in nerve localization during PNBs for several decades, with a high degree of success and low complication rate. However, the widespread adoption of ultrasound imaging has called into question its ongoing role in PNB. Over a decade ago, Perlas et al evaluated the sensitivity of upper extremity peripheral nerves to peripheral nerve stimulation during ultrasound imaging of needle to nerve contact. The authors reported that, despite visualizing the needle tip indenting the surface of the nerve, with the stimulator set to deliver a current of 0.5 mA or less, no motor stimulation occurred over 25% of the time.
Several studies, with a variety of different blocks, have been performed to assess the utility of this localization tool in association with UGRA. Whether for supraclavicular block, for axillary block, or for femoral block authors have shown that addition of the nerve stimulator as a nerve localization tool during ultrasound-guided PNB was not contributory to success.
Moreover, Robards et al found that absence of a motor response to PNS between 0.2 and 0.5 mA during popliteal block did not always exclude placement of the needle within the nerve, and that stimulation might actually lead to unnecessary manipulation of the needle into the nerve.
However, the stimulator may be useful as an adjunct to UGRA for reasons other than ensuring block efficacy. Because it has been well established that a threshold of nerve stimulation lower than 0.2 mA indicates high likelihood of needle tip placement within the nerve, the stimulator may be employed during UGRA as a safety monitor. The nerve stimulator is particularly necessary during US-guided block of the deep nerves, or when the ultrasound image is less precise than desired. In this setting, an evoked motor response could warn against intrafascicular injection of local anesthetic.
Moreover, in some circumstances, it may be desirable to identify different nerves with more precision, as during axillary block, for which the PNS serves to delineate the nerves by their specific motor response to electrical stimulation. There may be, in some anatomic locations, neural structures that can be challenging to identify by visualization alone, whether they are the target of block or one simply wishes to avoid them with the needle; in these cases, a PNS may be invaluable to provide this identification.
Finally, there are nerves that do not lend themselves readily to ultrasound visualization, primarily because of depth or osseous interference with ultrasound transmission. The most common example of this is the posterior approach to the lumbar plexus, in which ultrasound can be used to identify local osseous structures to guide the block, but for which the PNS remains a valuable tool for guidance of the needle tip into proximity with the nerves of the plexus.
Taken overall, a plethora of data indicate that routine use of a nerve stimulator during ultrasound-guided nerve blocks yields clinically relevant safety information that can influence the clinical decision making and positively affect patient safety.
However, the primary purpose of the suggested routine use of nerve stimulation with UGRA is for safety monitoring, rather nerve localization (Figure 8). In this capacity, the nerve stimulator can be simply set at 0.5 mA (0.1 ms), 2 Hz, without changing the current intensity throughout the procedure.
While the motor response is not sought, occurrence of the motor response should necessitate cessation of the needle advancement and slight withdrawal of the needle as a motor response at this current delivery setting almost always indicates needle-nerve contact or intraneural needle placement.
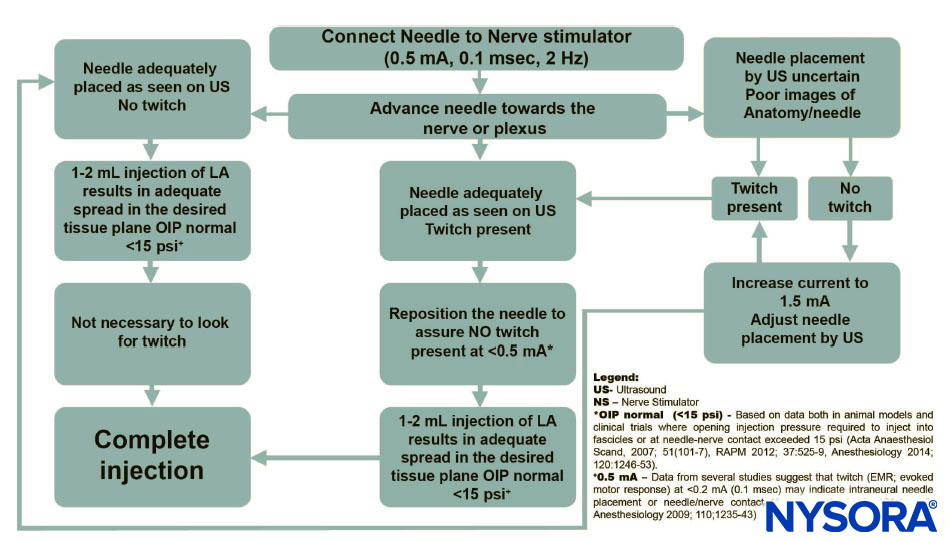
FIGURE 8. Algorithm: The primary purpose of the suggested routine use of nerve stimulation with UGRA is for the purpose of safety monitoring, rather than nerve localization.
OPTIMIZING THE DELIVERY OF LOCAL ANESTHETIC NEAR THE TARGET NERVE
After accurate needle placement near the target nerve, and after ascertaining that aspiration is negative for intravascular needle placement, injection of the local anesthetic is made in the tissue plane that contains the nerve(s) to be anesthetized (Table 4).
TABLE 4. Optimizing local anesthetic deposition.
| Inject local anesthetic solution in small aliquots Observe for pain or high pressure during injection Make certain that spread of fluid is observed at needle tip during injection Aspirate between injections Be aware of intervening fascial planes that may sequester or channel the solution Avoid deposition of local anesthetic into muscle For solitary nerves in extremities, seek to create a “donut” or “halo” around nerve For nerves within a fascial enclosure, seek to “fill” the fascial confines with solutiona |
Brull et al evaluated the long-held notion that the local anesthetic solution should be directed in a circumferential manner around the visible nerve, with change of needle position if necessary, in comparison to simply allowing the solution to accumulate along one aspect of the nerve with one needle position.
They found that the resultant block set up 33% more rapidly with the former than with the latter. While the creation of a “donut” or “halo” around the nerve may be suggested as a general recommendation, some nerves, by virtue of their anatomical situation, may not require such deliberate circumferential placement. This is typically dictated by the location and configuration of overlying or surrounding fascial planes, such as in the interscalene groove and at the femoral triangle. Optimal delivery of local anesthetic around each nerve is described in subsequent specific UGRA sections.
Real-time imaging of local anesthetic injection permits assessment of correct disposition of the fluid. The injection phase should be carried out with small aliquots of local anesthetic (3–5 mL), with a short period allowed to elapse between each, to allow evidence of any symptoms of local anesthetic systemic toxicity (LAST) to be manifest before continuing to administer the drug, as recommended by the American Society of Regional Anesthesia and Pain Medicine (ASRA) guidelines.
In addition, delivery of each aliquot should be preceded by aspiration and should progress with attention to opening injection pressures or complaints of pain or paresthesia in the distribution of the target nerve.
While ultrasound has been shown to produce a lower likelihood of intravascular needle placement, intravascular injection with LAST may still occur. It is thus imperative to be aware of the location of vessels, which have a distending pressure so low that ordinary pressure at the body surface with a transducer obliterates their lumen entirely. Therefore, it is helpful to screen for the presence of vessels using color Doppler during the pre block scan. However, small vessels may be missed, and Doppler function deteriorates at greater depths.
Thus, it is imperative to observe the ultrasound image throughout the injection for evidence of tissue spread by the local anesthetic solution at the tip of the needle. Failure to visualize such spread suggests that the tip of the needle either is out of plane or is in the lumen of a vessel.
Errant needle placement has been described both into vessels and into nerves. Moayeri et al in a cadaver-based study, have shown that ultrasound imaging is sensitive to injection into the peripheral nerve, with as little as 0.5 mL causing visible evidence of nerve distension. Such visualization allows immediate withdrawal of the needle, which may reduce the chance for nerve injury, compared to injection of a large volume of local anesthetic.
CONCLUSION
Ultrasonography has revolutionized the field of regional anesthesia. The effective application of this technology requires understanding of two-dimensional anatomy, optimal imaging of the nerves and anatomical structures, accurate real-time needle guidance, and precise local anesthetic delivery. The combination of these elements ensures that the most benefit can be derived from this powerful imaging modality, ensuring high nerve block success and improved patient safety, particularly with regard to LAST.
Clinical updates
Scholten et al. (Anaesthesia, 2017) review emerging technologies to improve needle tip identification during ultrasound-guided anaesthetic procedures, showing that adjuncts such as needle guides, electromagnetic or magnetic tracking, and image-based algorithms can improve first-pass success and reduce procedure time, particularly for novice operators and out-of-plane approaches. However, most supporting data come from phantom or cadaver studies, with limited evidence of reduced complications or improved patient outcomes in clinical practice. The authors highlight education and training as the clearest current benefit, and call for clinically focused trials to determine which technologies meaningfully enhance safety and efficacy.
- Read more about the study HERE.