Jason Choi, Liane Germond, and Alan C. Santos
INTRODUCTION
Most women experience moderate to severe pain during labor and delivery, often requiring some form of pharmacologic analgesia. The lack of proper psychological preparation combined with fear and anxiety can greatly enhance the patient’s sensitivity to pain and further add to the discomfort during labor and delivery. However, skillfully conducted obstetric analgesia, in addition to relieving pain and anxiety, may benefit the mother in many other ways. This chapter focuses on the management of obstetric patients with a primary focus on regional anesthesia techniques.
Physiologic Changes of Pregnancy
Pregnancy results in significant changes affecting most maternal organ systems (Table 1). These changes are initiated by hormones secreted by the corpus luteum and the placenta. Such changes have important implications for the anesthesiologist caring for the pregnant patient. This chapter reviews the most relevant physiologic changes of pregnancy and discusses the approach to obstetric management using regional anesthesia.
Changes in the Cardiovascular System
Oxygen consumption increases during pregnancy, as the maternal cardiovascular system is required to meet the increasing metabolic demands of a growing fetus. The end result of these changes is an increase in heart rate (15%–25%) and cardiac output (up to 50%) compared with values before pregnancy. In addition, lower vascular resistance is found in the uterine, renal, and other vascular beds. These changes result in a lower arterial blood pressure because of a decrease in peripheral resistance, which exceeds the increase in cardiac output. Decreased vascular resistance is mostly due to the secretion of estrogens, progesterone, and prostacyclin. Particularly significant increases in cardiac output occur during labor and in the immediate post-partum period owing to added blood volume from the contracted uterus.
NYSORA Tips
- Cardiovascular changes and pitfalls in advanced pregnancy include the following:
- Increase in heart rate (15%–25%) and cardiac output (up to 50%).
- Decrease in vascular resistance in the uterine, renal, and other vascular beds.
- Compression of the lower aorta in the supine position may further decrease uteroplacental perfusion and result in fetal asphyxia.
- Significant hypotension is more likely to occur in pregnant versus nonpregnant women undergoing regional anesthesia, necessitating uterine displacement or lateral pelvic tilt maneuvers, intravascular preloading, and vasopressors.
From the second trimester onward, aortocaval compression by the enlarged uterus becomes progressively more important, reaching its maximum effect at 36–38 weeks, after which it may be relieved some as the fetal head descends into the pelvis. Cardiac output may decrease when patients are in the supine position but not in the lateral decubitus position. Venous occlusion by the growing fetus causes supine hypotensive syndrome in 10% of pregnant women and manifests as maternal tachycardia, arterial hypotension, faintness, and pallor.
Compression of the lower aorta in this position may further decrease uteroplacental perfusion and result in fetal asphyxia. Uterine displacement or lateral pelvic tilt should be applied routinely during the anesthetic management of the pregnant patient. Uterine displacement is best achieved by placing the patient in the left lateral decubitus position. In this position, cardiac vagal activity will be augmented as compared to the supine position. Placing a wedge under the bony pelvis has been used to achieve uterine tilt. However, it has recently been demonstrated that uterine tilt is more effective when the mother is placed in the full left lateral decubitus position and then is turned supine onto the pelvic wedge.
Changes in the electrocardiogram are common in late pregnancy. The QRS axis may initially shift to the right during the first trimester, rotating to left axis by the third trimester as a result of the expanding uterus. A shortening of the PR and QT intervals and an increase in heart rate are also present. The QT interval shortening may have implications for women with long QT syndrome. Indeed, Seth et al. found a reduced risk (risk ratio [RR] = 0.38) of cardiac events during pregnancy in woman with prolonged QT syndrome. However, an increased risk of postpartum cardiac events in the first nine months after delivery was also found, which suggests that the QT interval becomes prolonged again in the early post-delivery period. There is also a tendency toward premature atrial contractions, sinus tachycardia, and paroxysmal supraventricular tachycardia.
TABLE 1. Summary of physiologic changes of pregnancy at term.
| Variable | Change | AmountTotal |
|---|---|---|
| Total blood volume | Increase | 25%–40% |
| Plasma volume | Increase | 40%–50% |
| Fibrinogen | Increase | 50% |
| Serum cholinesterase activity | Decrease | 20%–30% |
| Cardiac output | Increase | 30%–50% |
| Minute ventilation | Increase | 50% |
| Alveolar ventilation | Increase | 70% |
| Functional residual capacity | Decrease | 20% |
| Oxygen consumption | Increase | 20% |
| Arterial carbon dioxide tension | Decrease | 10 mm Hg |
| Arterial oxygen tension | Increase | 10 mm Hg |
| Minimum alveolar concentration | Decrease | 32%–40% |
Changes in the Respiratory System
Minute ventilation increases from the beginning of pregnancy to a maximum of 50% above normal by term. This is mostly a result of a 40% increase in tidal volume and a small increase in respiratory rate. Dead space does not change significantly during pregnancy; thus, alveolar ventilation is increased by 70% at term. After delivery, as blood progesterone levels decline, ventilation returns to normal within 1–3 weeks.
Elevation of the diaphragm occurs with an increase in the size of the uterus. Expiratory reserve volume, residual volume, and functional residual capacity (FRC) decrease by the third trimester of pregnancy. However, because there is also an increase in inspiratory reserve volume, total lung capacity remains unchanged. A decreased FRC is typically asymptomatic in healthy parturients. Those with preexisting alterations in closing volume as a result of smoking, obesity, scoliosis, or other pulmonary disease may experience early airway closure with advancing pregnancy, leading to hypoxemia. The Trendelenburg and supine positions also exacerbate the abnormal relationship between closing volume and FRC. The residual volume and FRC return to normal shortly after delivery.
Pregnant women often have difficulty with nasal breathing. Friability of the mucous membranes during pregnancy can cause severe bleeding, especially on airway instrumentation. These changes are caused by increased extracellular fluid and vascular engorgement. It may also be difficult to perform laryngoscopy in obese, short-necked parturients with enlarged breasts. Use of a short-handled laryngoscope has proved helpful.
NYSORA Tips
Airway edema may be severe in pregnant women, particularly in those with preeclampsia, those in whom the Trendelenburg position is used for prolonged periods, and those in whom tocolytic agents are used.
Metabolic Changes
Oxygen consumption increases during early pregnancy, with an overall increase of 20% by term. Regardless, increased alveolar ventilation occurring during pregnancy actually leads to a reduction in the partial pressure of carbon dioxide in arterial blood (PaCO2) to 32 mm Hg and an increase in the partial pressure of oxygen in arterial blood (PaO2) to 106 mm Hg. The plasma buffer base decreases from 47 to 42 mEq; consequently, the pH remains practically unchanged. The maternal uptake and elimination of inhalational anesthetics are enhanced because of the increased alveolar ventilation and decreased FRC. However, the decreased FRC and increased metabolic rate predispose the mother to the development of hypoxemia during periods of apnea or hypoventilation.
Changes in the Gastrointestinal System
The effects of pregnancy on the gastrointestinal system are controversial. It has been proposed that enhanced progesterone production causes decreased gastrointestinal motility and slower absorption of food. Gastric secretions are more acidic, and lower esophageal sphincter tone is decreased. However, more recent studies using radiographic, ultrasound, and dye dilution techniques have demonstrated that gastric emptying of liquid and solid materials does not decrease at any time during pregnancy.
The risk of regurgitation on induction of general anesthesia depends, in part, on the gradient between the lower esophageal sphincter and intragastric pressures. In parturients with “heartburn,” the lower esophageal sphincter tone is greatly reduced. No single routine prophylactic regimen can be recommended with certainty. The efficacy of prophylactic nonparticulate antacids is diminished by inade-quate mixing with gastric contents, improper timing of administration, and the tendency for antacids to increase gastric volume. The administration of histamine (H2)-receptor antagonists, such as cimetidine and ranitidine, requires anticipation and careful timing since their onset of action is relatively slow. In those women at greatest risk, an argument can be made for the administration of intravenous (IV) metoclopramide before elective cesarean section delivery. This dopamine antagonist hastens gastric emptying and increases resting lower esophageal sphincter tone in both nonpregnant and pregnant women. However, there have been conflicting data on its efficacy (perhaps due to timing of administration) and the frequency of side effects, such as extrapyramidal reactions and transient neurologic dysfunction.
Endocrine Changes Influencing Plasma Volume, Blood Composition, and Glucose Metabolism
Plasma volume and total blood volume begin to increase in early gestation, resulting in an increase of 40%–50% and 25%–40%, respectively, at term. These changes are due to an increased mineralocorticoid activity during pregnancy, which results in sodium retention and increased body water content. The relatively smaller increase in red blood cell volume (20%) accounts for a relative reduction in hemoglobin (to 11–12 g/L) and hematocrit (to 35%); the platelet count, however, remains unchanged. Plasma fibrinogen concentrations increase during normal pregnancy by approximately 50%, whereas clotting factor activity is variable. Coagulation factors I, VII, VIII, IX, X, and XII increase during pregnancy, whereas factor XI and XIII concentrations decrease and factor II and V concentrations remain unchanged during pregnancy.
Serum cholinesterase activity declines to a level of 20% below normal by term and reaches a nadir in the puerperium. The net effect of these changes in the serum cholinesterase is of negligible relevance to the metabolism of clinically used doses of succinylcholine or ester-type local anesthetics (2-choloroprocaine). The albumin–globulin ratio declines because of the relatively greater reduction in albumin concentration. A decrease in serum protein concentration may be clinically significant in that the free fractions of protein-bound drugs can be expected to increase.
Human placental lactogen and cortisol increase the tendency toward hyperglycemia and ketosis, which may exacerbate preexisting diabetes mellitus. The patient’s ability to handle a glucose load is decreased, and the transplacental passage of glucose may stimulate fetal secretion of insulin, leading, in turn, to neonatal hypoglycemia in the immediate postpartum period.
Altered Drug Responses in Pregnancy
Pregnancy results in a progesterone-mediated increase in neural sensitivity to local anesthetics. Lower doses of local anesthetic are needed per dermatomal segment of epidural or spinal block. This has been attributed to an increased spread of local anesthetic in the epidural and subarachnoid spaces as a result of epidural venous engorgement and enhanced sensitivity to local anesthetic block due to progesterone. The minimum alveolar concentration for inhalational agents is decreased by 8–12 weeks of gestation and may be related to an increase in progesterone levels.
NYSORA Tips
- During pregnancy, there is a progesterone-mediated increase in neural sensitivity to local anesthetics.
- Doses of local anesthetic need to be lowered per dermatomal segment of epidural or spinal block.
PLACENTAL TRANSFER OF LOCAL ANESTHETICS
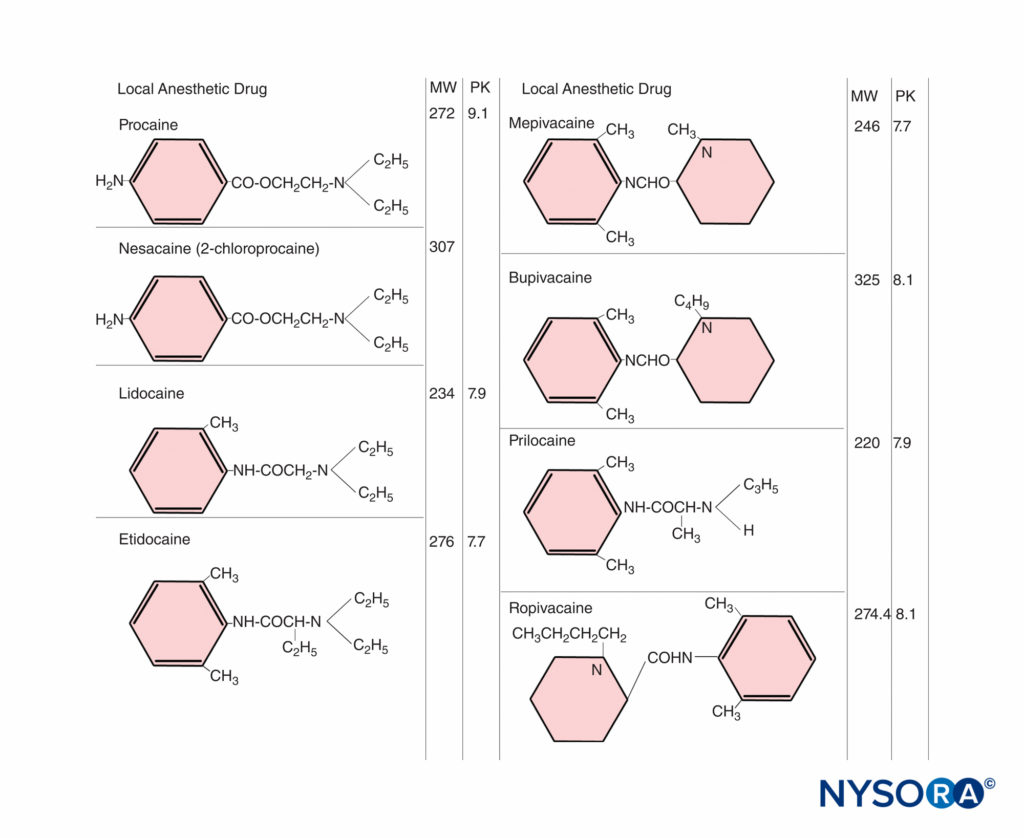
Local anesthetics readily cross the placenta by simple diffusion. Several factors influence the placental transfer of drugs, including the physicochemical characteristics of the drug itself, maternal drug concentrations in the plasma, properties of the placenta, and hemodynamic events within the fetomaternal unit.Highly lipid-soluble drugs, such as local anesthetics, cross biologic membranes more readily, and the degree of ionization is important because the nonionized moiety of a drug is more lipophilic than the ionized drug. Local anesthetics are weak bases, with a relatively low degree of ionization and considerable lipid solubility. The relative concentrations of drug existing in the nonionized and ionized forms can be estimated from the Henderson–Hasselbalch equation:
pH = pKa + log (base)/(cation)
The ratio of base to cation becomes particularly important with local anesthetics because the nonionized form penetrates tissue barriers, whereas the ionized form is pharmacologically active in blocking nerve conduction. The pKa (acid dissociation constant) is the pH at which the concentrations of free base and cation are equal. For the amide local anesthetics, the pKa values (7.7–8.1) are sufficiently close to physiologic pH so that changes in maternal or fetal biochemical status may significantly alter the proportion of ionized and nonionized drug (Figure 1). At steady state, the concentrations of nonionized local anesthetics in the fetal and maternal plasma are equal. With fetal acidosis, there is a greater tendency for drug to exist in the ionized form, which cannot diffuse back across the placenta. This causes a larger total amount of local anesthetic to accumulate in the fetal plasma and tissues. This is called ion trapping.
NYSORA Tips
The prolonged administration of highly protein-bound drugs (eg, bupivacaine) may lead to substantial fetal accumulation of the drugs.
The effects of maternal plasma protein binding on the rate and amount of local anesthetic accumulating in the fetus are inadequately understood. Animal studies have shown that the transfer rate is slower for drugs that are extensively bound to maternal plasma proteins, such as bupivacaine. However, with the prolonged administration of highly protein-bound drugs, such as bupivacaine, substantial accumulation of drug can occur in the fetus.
The concentration gradient of free drug between the maternal and fetal blood is a significant factor. On the maternal side, the dose administered, the mode and site of administration, and the use of vasoconstrictors can influence fetal exposure. The rates of distribution, metabolism, and excretion of the drug, which may vary, are equally important. Higher doses result in higher maternal blood concentrations. The absorption rate can vary with the site of injection. For instance, an IV bolus results in the highest blood concentration. It was once believed that intrathecal administration resulted in negligible plasma concentrations of local anesthetics. However, we now know that spinal anesthesia induced with 75 mg lidocaine results in maternal plasma concentrations that are similar to those reported by others after epidural anesthesia. Furthermore, significant levels of the drug can be found in the umbilical vein at birth. Repeated administration can result in high maternal blood concentrations, depending on the dose and frequency of reinjection, in addition to the kinetic characteristics of the drug. The half-life of amide local anesthetic agents is relatively long, so that repeated injection may lead to accumulation in the maternal plasma (Figure 2). In contrast, 2-chloroprocaine, an ester local anesthetic, undergoes rapid enzymatic hydrolysis in the presence of pseudo-cholinesterase. After epidural injection, the mean half-life in the mother is approximately 3 minutes; after reinjection, 2-chloroprocaine can be detected in the maternal plasma for only 5–10 minutes, and no accumulation of this drug has occurred.
Pregnancy is associated with physiologic changes that also may influence maternal pharmacokinetics and the action of anesthetic drugs. These changes may be progressive during the course of gestation and are often difficult to predict for an individual drug. Nonetheless, the elimination half-life of bupivacaine after epidural injection has been shown to be similar in pregnant and nonpregnant women.
Fetal regional blood flow changes can also affect the amount of drug taken up by individual organs. For example, during asphyxia and acidosis, a greater proportion of the fetal cardiac output perfuses the fetal brain, heart, and placenta. Infusion of lidocaine resulted in increased drug uptake in the heart, brain, and liver of asphyxiated baboon fetuses compared with nonasphyxiated control fetuses.
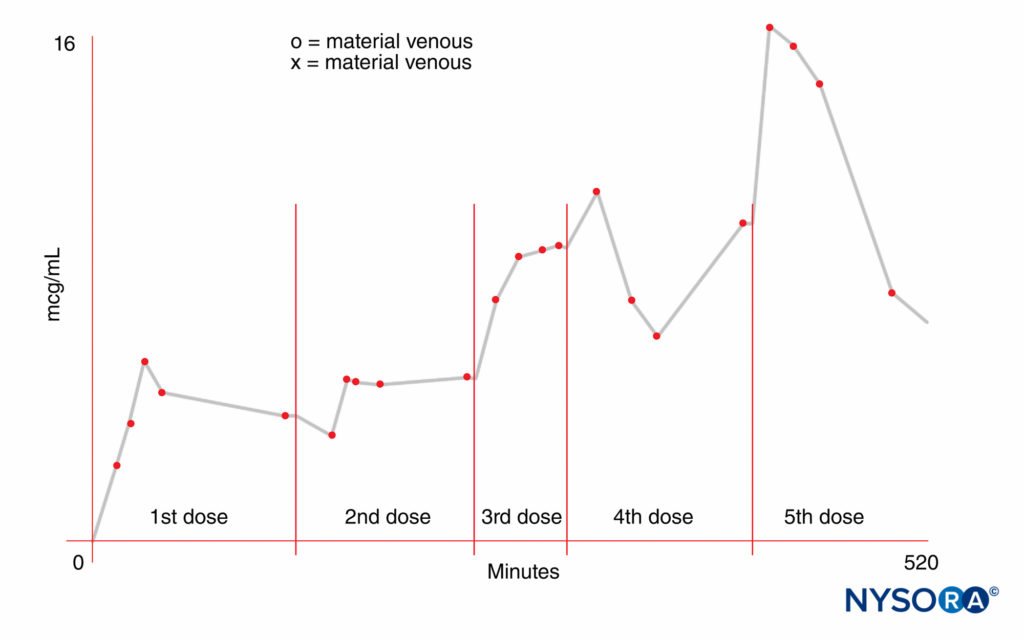
FIGURE 2. Increased maternal blood concentration of local anesthetic after repeated doses (300 mg) of mepivacaine.
Risk of Drug Exposure: Fetus Versus Newborn
The fetus can excrete local anesthetics back into the maternal circulation after the concentration gradient of the free drug across the placenta has been reversed. This may occur even if the total plasma drug concentration in the mother exceeds that in the fetus, because there is lower protein binding in fetal plasma. 2-chloroprocaine is the only drug that is metabolized in the fetal blood so quickly that even with acidosis, substantial exposure in the fetus is avoided.
Both term and preterm infants have the hepatic enzymes necessary for the biotransformation of amide local anesthetics. In a comparative study, the pharmacokinetics of lidocaine among adult ewes and fetal/neonatal lambs indicated that the metabolic clearance in the newborn was similar to, and renal clearance greater than, that in the adult. However, the half-life was longer in the newborn; this is related to a greater volume of distribution and tissue uptake, so that, at any given moment, the neonate’s liver and kidneys are exposed to a smaller fraction of lidocaine accumulated in the body. Similar results have been reported in another study involving lidocaine administration to human infants in a neonatal intensive care unit.
Neonatal depression occurs at blood concentrations of mepivacaine or lidocaine that are approximately 50% less than those producing systemic toxicity in the adult. However, infants accidentally injected in utero with mepivacaine (intended for maternal caudal anesthesia) stopped convulsing when the mepivacaine level decreased below the threshold for convulsions in the adult. The relative central nervous toxicity and cardiorespiratory toxicity of local anesthetics have been studied in sheep. The doses required to produce toxicity in the fetal and newborn lamb were greater than those required in the ewe. In the fetus, this difference was attributed to placental clearance of drug into the mother and better maintenance of blood gas tensions during convulsions, whereas in the newborn lamb, a larger volume of distribution was probably responsible for the higher doses needed to induce toxic effects.
It has been suggested that bupivacaine may be implicated as a possible cause of neonatal jaundice because of its high affinity for fetal erythrocyte membranes, resulting in a decrease in filterability and deformability rendering subjects more prone to hemolysis. However, a more recent study has failed to show demonstrable bilirubin production in newborns whose mothers were given bupivacaine for epidural anesthesia during labor and delivery.
Neurobehavioral studies have revealed subtle changes in newborn neurologic and adaptive function with regional anesthesia. In the case of most anesthetic agents, these changes are minor and transient, lasting for only 24–48 hours.
ANESTHESIA FOR LABOR & VAGINAL DELIVERY
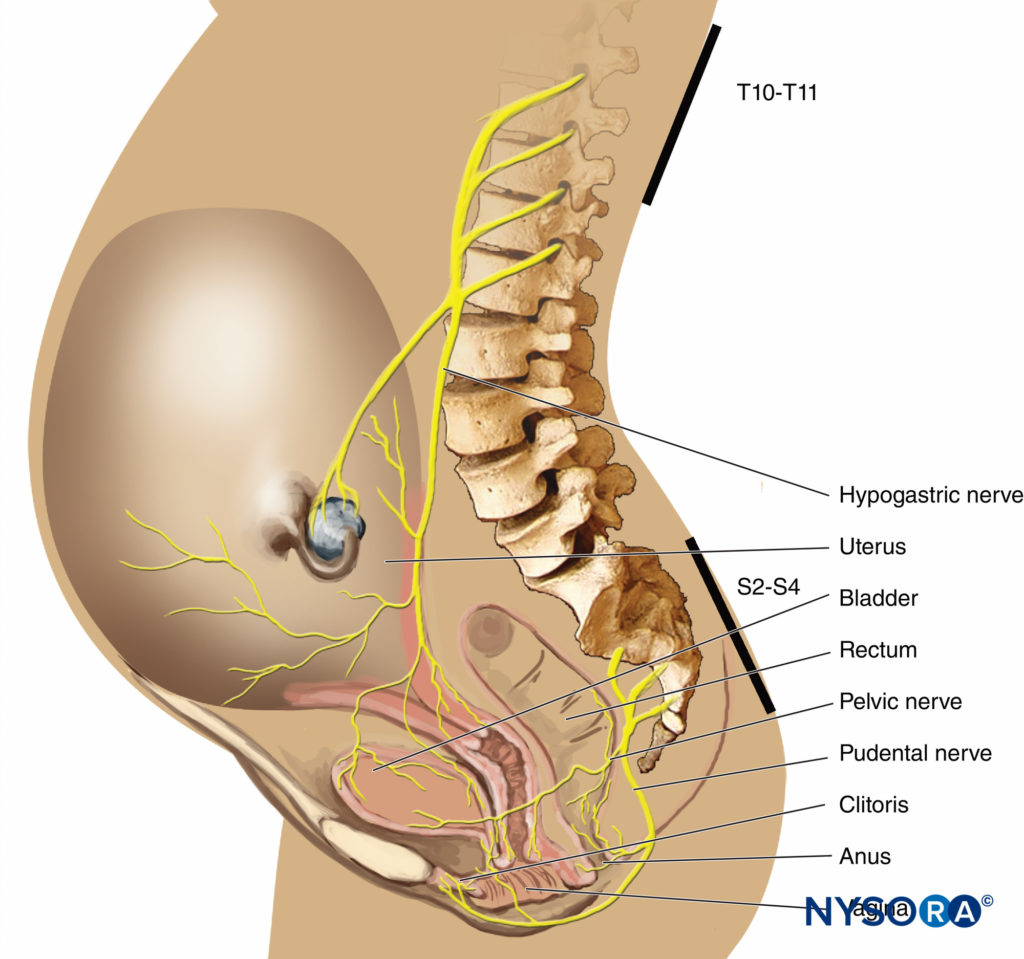
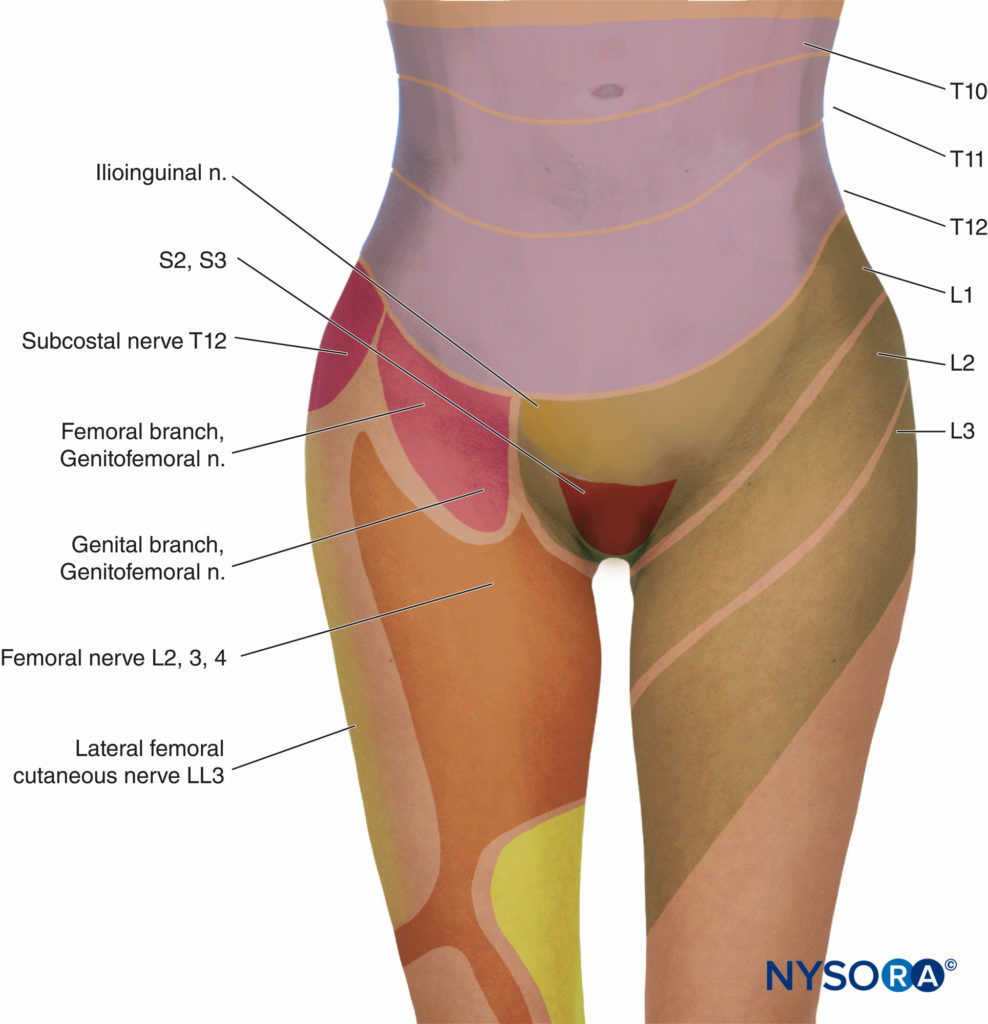
In the first stage of labor, pain is caused by uterine contractions related to dilation of the cervix and distention of the lower uterine segment. Pain impulses are carried in visceral afferent type C fibers, which accompany the sympathetic nerves. In early labor, only the lower thoracic dermatomes (T11–T12) are affected. However, with progressive cervical dilation during the transition phase, adjacent dermatomes may be involved and pain referred from T10 to L1. During the second stage, additional pain impulses due to distention of the vaginal vault and perineum are carried in the pudendal nerve, which is composed of lower sacral fibers (S2–S4).
Regional analgesia may benefit the mother in other ways beyond relieving pain and anxiety. In animal studies, pain may cause maternal hypertension and reduced uterine blood flow. Epidural analgesia blunts the increases in maternal cardiac output, heart rate, and blood pressure that occur with painful uterine contractions and “bearing-down” efforts. By reducing the maternal secretion of catecholamines, epidural analgesia may convert a previously dysfunctional labor pattern to a normal one. Regional analgesia can benefit the fetus by eliminating maternal hyperventilation with pain, which often leads to a reduced fetal arterial oxygen tension owing to a leftward shift of the maternal oxygen–hemoglobin dissociation curve.
The most frequently chosen methods for relieving the pain of parturition are psychoprophylaxis, systemic medication, and regional analgesia. Inhalational analgesia, conventional spinal analgesia, and paracervical block are less commonly used. General anesthesia is rarely necessary but may be indicated for uterine relaxation in some complicated deliveries.
Systemic Analgesia
The advantages of systemic analgesics include ease of administration and patient acceptability. However, the drug, dose, timing, and method of administration must be chosen carefully to avoid maternal and neonatal depression. Drugs used for systemic analgesia are opioids, tranquilizers, and occasionally ketamine.
Systemic Opioids
In the past, meperidine was the most commonly used systemic analgesic to ameliorate pain during the first stage of labor. It can be administered by IV injection (effective analgesia in 5–10 minutes) or intramuscularly (peak effect in 40–50 minutes). It was also commonly used for postoperative pain in the general population. But with the popularity of its administration, disturbing side effects began to emerge. One of the most serious side effects was the occurrence of seizures both from the primary drug effect and from the drug’s metabolite, normeperidine. In the pregnant patient at risk for seizures—that is, with pregnancy-induced hypertension or preeclampsia—confusing the picture by the administration of a drug known to cause seizures complicates patient care. Other side effects are nausea and vomiting, dose-related depression of ventilation, orthostatic hypotension, the potential for neonatal depression, and euphoria out of proportion to the analgesic effect, leading to misuse of the drug. Meperidine may also cause transient alterations of the fetal heart rate, such as decreased beat-to-beat variability and tachycardia. Among other factors, the risk of neonatal depression is related to the interval between the last drug injection and delivery. The placental transfer of an active metabolite, normeperidine, which has a long elimination half-life in the neonate (62 hours), has also been implicated in contributing to neonatal depression and subtle neonatal neurobehavioral dysfunction. The effects of systemically administered meperidine on the course of labor are controversial. It has been suggested that meperidine administration may prolong the latent phase of labor but shorten the cumulative length of the first stage of labor. However, a recent study showed no benefit to the administration of meperidine in order to possibly shorten the first stage of labor in women having dystocia.
Experience with the newer synthetic opioids, such as fentanyl and alfentanil, has been limited. Although they are potent, their usefulness during labor is limited by their short duration of action. However, these drugs offer an advantage when analgesia of rapid onset but short duration is necessary (eg, with forceps application). For example, a single IV injection of fentanyl, up to 1 mcg/kg, results in prompt pain relief without severe neonatal depression but for a short period of time. Alfentanil may be associated with greater neonatal depression than equivalent meperidine patient-controlled analgesia (PCA). In another study, alfentanil PCA failed to provide adequate analgesia compared to fentanyl PCA. For more prolonged analgesia, fentanyl can be administered with patient-controlled delivery devices. More commonly, fentanyl (15–25 mcg) and sufentanil (5–10 mcg) have been used with local anesthetics in an initial spinal dose with a local anesthetic during the placement of a continuous spinal–epidural for labor with excellent relief of pain.
Remifentanil is an opioid that is rapidly metabolized by serum and tissue cholinesterases, and consequently, has a short (3-minute), context-sensitive half-time. When used in bolus dosing (0.3–0.8 mcg/kg per bolus), remifentanil has been found to have an acceptable level of maternal side effects and a minimal effect on the neonate. Remifentanil crosses the placenta and appears to be either rapidly metabolized or redistributed in the neonate. In one study, Apgar and neurobehavioral scores were good in neonates whose mothers were given an IV infusion of remifentanil, 0.1 mcg/kg/min, during cesarean section delivery under epidural anesthesia. When administered by PCA, remifentanil has been found to provide better pain relief, equivalent hemodynamic stability, less sedation, and less oxygen desaturation compared with meperidine. In a recent double-blind trial, remifentanil PCA was compared to lumbar epidural for equivalent analgesia. Twenty-six percent of women in the remifentanil group reported acceptable pain scores compared to 56% of women receiving lumbar epidural analgesia. In countries outside the United States, intermittent nitrous oxide has been used for labor analgesia. When comparing remifentanil with nitrous oxide, remifentanil was found to provide better pain relief with fewer side effects. However, remifentanil can result in hypoventilation and hypoxemia, thus oxygen saturation should be routinely monitored during remifentanil IV PCA.
Opioid agonist-antagonists, such as butorphanol and nalbu-phine, have also been used for obstetric analgesia. These drugs have the proposed benefits of a lower incidence of nausea, vomiting, and dysphoria, as well as a “ceiling effect” on depression of ventilation. Intramuscular (IM) nalbuphine has been compared to meperidine in a double-blind study. Analgesia was comparable in both groups; however, nalbuphine was associated with increased maternal sedation compared to meperidine. Butorphanol is probably the most popular of the mixed agonist-antagonists; unlike meperidine, it is metabolized into inactive metabolites and has a ceiling effect on depression of ventilation in doses exceeding 2 mg. Butorphanol results in comparable maternal pain relief to meperidine and no difference in Apgar scores. However, butorphanol use was associated with fewer maternal side effects, such as nausea, vomiting, and dizziness, than meperidine. A potential disadvantage is a high incidence of maternal sedation. The recommended dose is 1–2 mg by IV or IM injection. Nalbuphine 10 mg IV or IM is an alternative to butorphanol.Naloxone, a pure opioid antagonist, should not be adminis-tered to the mother shortly before delivery to prevent neonatal ventilatory depression because it reverses maternal analgesia at a time when it is most needed. In some instances, naloxone has been reported to cause maternal pulmonary edema and even cardiac arrest. If necessary, the drug should be given directly to the newborn IM (0.1 mg/kg).
Ketamine
Ketamine is a potent analgesic. However, it may also induce unac-ceptable amnesia that may interfere with the mother’s recollection of the birth. Nonetheless, ketamine is a useful adjuvant to incomplete regional analgesia during vaginal delivery or for obstetric manipulations. In low doses (0.2–0.4 mg/kg), ketamine provides adequate analgesia without causing neonatal depression.
Regional Analgesia Techniques
Regional techniques provide excellent analgesia with minimal depressant effects in mother and fetus. The techniques most commonly used for labor anesthesia include central neuraxial (spinal, epidural, and combined spinal–epidural), paracervical, and pudendal blocks and, less frequently, lumbar sympathetic blocks. Hypotension resulting from sympathectomy is the most common complication that occurs with central neuraxial block. Therefore, maternal blood pressure must be monitored at regular intervals, typically every 2–5 minutes for approximately 15–20 minutes after the initiation of the block and at routine intervals thereafter. Regional analgesia may be contraindicated in the presence of severe coagulopathy, acute hypovolemia, or infection at the site of needle insertion. Chorioamnionitis without sepsis is not a contraindication to central neuraxial block.
Epidural Analgesia
Effective analgesia for the first stage of labor is achieved by block-ing the T10–Ll dermatomes with a low concentration of local anesthetic, often in combination with a lipid-soluble opioid. For the second stage of labor, because of pain due to vaginal distention and perineal pressure, the block should be extended to include the pudendal segments, S2–4 (Figures 3 and 4).There has been concern that the early initiation of epidural analgesia during the latent phase of labor (2–4 cm cervical dilation) may result in prolongation of the first stage of labor and a higher incidence of dystocia and cesarean section delivery, particularly in nulliparous women. Generally speaking, the first stage of labor is not prolonged by epidural analgesia, provided that aortocaval compression is avoided. Chestnut et al. demonstrated that the incidence of cesarean section delivery was no different in nulliparous women having epidural analgesia initiated during the latent phase (at 4 cm dilation) compared with women whose analgesia was initiated during the active phase. Others have shown that epidural analgesia is not associated with an increased incidence of cesarean section delivery compared with IV PCA in nulliparous women.
However, a prolongation of the second stage of labor has been reported in nulliparous women, possibly owing to a decrease in expulsive forces or malposition of the fetus.
Thus, with the use of epidural analgesia, the American College of Obstetricians and Gynecologists (ACOG) has defined an abnormally prolonged second stage of labor as longer than 3 hours in nulliparous women and longer than 2 hours in multiparous women.
A longer second stage of labor may be minimized by the use of an ultra-dilute local anesthetic solution in combination with an opioid. Long-acting amides, such as bupivacaine, ropivacaine, and levobupivacaine, are most frequently used because they produce excellent sensory analgesia while sparing motor function, particularly at the low concentrations used for epidural analgesia.
NYSORA Tips
- Analgesia during the first stage of labor is achieved by blocking the T10–Ll dermatomes with a low concentration of local anesthetic (see Figure 3).
- Analgesia for the second stage of labor and delivery requires the block of the S2–4 segments because of pain due to vaginal distention and perineal pressure.
Analgesia for the first stage of labor may be achieved with 5–10 mL of bupivacaine, ropivacaine, or levobupivacaine (0.125%–0.25%), followed by a continuous infusion (8–12 mL/h) of 0.0625% bupivacaine or levobupivacaine, or 0.1% ropivacaine. Fentanyl 1–2 mcg/mL or sufentanil 0.3–0.5 mcg/mL may be added. During the actual delivery, the perineum may be blocked with 10 mL of 0.5% bupivacaine, 1% lidocaine, or, if a rapid effect is required, 2% chloroprcaine in the semirecumbent position.There is controversy regarding the need for a test dose when using a dilute solution of local anesthetic.
Catheter aspiration alone is not always diagnostic. For that reason, some authors believe that a test dose should be administered to improve detection of an intrathecally or intravascularly placed epidural catheter. If injected into a blood vessel, 15 mcg epinephrine results in a change in heart rate of 20–30 bpm with a slight increase in blood pressure within 30 seconds of administration. The duration is approximately 30 seconds. The anesthesiologist should observe the pulse oximeter during the first minute after injection to determine whether an accidental intravascular injection has occurred. However, the tachycardia associated with an intravenous test dose of epinephrine is not a reliable indicator of intravascular injection during labor because it may be confounded coincident with a painful uterine contraction. In addition, epinephrine is not reliable in patients who have received a beta-adrenergic receptor antagonist. Other subtle signs of intravascular injection may include a feeling of apprehension or unease or palpitations. It is important to fractionate the total dose of local anesthetic and observe the patient at one-minute intervals.
Patient-controlled epidural analgesia (PCEA) is a safe and effective alternative to conventional bolus or infusion techniques. Maternal acceptance is excellent, and demands on anesthesia manpower may be reduced. Studies have shown that PCEA with a relatively low continuous epidural infusion and top-ups required fewer anesthetic interventions compared to PCEA without a basal rate epidural infusion. Initial analgesia is achieved with bolus doses of local anesthetic. Once the mother is comfortable, PCEA may then be started with a maintenance infusion (8–12 mL/h) of local anesthetic (bupivacaine, levobupivacaine, or ropivacaine 0.0625%–0.125%) with or without an opioid (fentanyl 1–2 mcg/mL or sufent-anil 0.3–0.5 mcg/mL). The machine may be programmed to administer an epidural demand bolus of 8 mL with a lockout period of 10 minutes between doses.
The caudal, rather than lumbar, approach may result in a faster onset of perineal analgesia and therefore may be preferable to the lumbar epidural approach when an imminent vaginal delivery is anticipated. However, caudal analgesia is no longer popular because of occasionally painful needle placement, a high failure rate, potential contamination at the injection site, and risks of accidental fetal injection. Before caudal injection, a digital rectal examination must be performed to exclude needle placement in the fetal presenting part. Low spinal “saddle block” has virtually eliminated the need for caudal anesthesia in modern practice.
Spinal Analgesia
A single intrathecal injection, usually of an opioid and a small dose of local anesthetic, for labor analgesia has the benefits of a reliable and rapid onset of analgesia for the first stage of labor. However, repeated intrathecal injections may be required for a long labor, thus increasing the risk of postdural puncture headache. In addition, motor block may be uncomfortable for some women and may prolong the second stage of labor. This technique is most useful for multiparous parturients who are rapidly progressing in labor and require analgesia or anesthesia of short duration before complete cervical dilation and anticipated vaginal delivery or in settings in which continuous epidural analgesia is not possible.
Microcatheters were introduced for continuous spinal anesthesia in the 1980s. They were subsequently withdrawn when found to be associated with neurologic deficits, possibly related to maldistribution of local anesthetic in the cauda equina region. Fortunately, in a recent multi-institutional study, no cases of neurologic symptoms occurred after the use of 28-gauge microcatheters for continuous spinal analgesia in laboring women. Spinal anesthesia is also a safe and effective alternative to general anesthesia for instrumental delivery.
Combined Spinal–Epidural Analgesia
Combined spinal–epidural (CSE) analgesia is an ideal analgesic technique for use during labor. It combines the rapid, reliable onset of profound analgesia resulting from spinal injection with the flexibility and longer duration of epidural techniques. In a recent meta-analysis, the onset of analgesia for CSE was significantly faster than with an epidural technique (2–5 minutes vs. 10–15 minutes).
Technique
After identifying the epidural space using a conventional (or specialized) epidural needle, a longer (127-mm), pencil-point spinal needle is advanced into the subarachnoid space through the epidural needle. After intrathecal injection, the spinal needle is removed, and an epidural catheter inserted. Intrathecal injection of fentanyl 10–25 mcg or sufentanil 2.5–5 mcg, alone or in combination with up to 1 mL of isobaric bupivacaine 0.25%, produces profound analgesia lasting for 60–120 minutes with minimal motor block.
It should be noted that the incidence of pruritus is greater with intrathecal opioid administration than with epidural opioid administration. An opioid alone may provide sufficient relief for the early latent phase, but the addition of bupivacaine is almost always necessary for satisfactory analgesia during advanced labor. Other adjuvants have also been used. The addition of adjuvants, such as clonidine and neostigmine, has been disappointing. An epidural infusion of bupivacaine 0.03%–0.0625% with an opioid may be started within 10 minutes of spinal injection. Alternatively, the epidural component may be activated when necessary. Women with hemodynamic stability and preserved motor function who do not require continuous fetal monitoring may ambulate with assistance. Before ambulation, women should be observed for 30 minutes after intrathecal or epidural drug administration to assess maternal and fetal well-being. A recent study indicated that the early administration of CSE analgesia to nulliparous women did not increase the cesarean section delivery rate.
NYSORA Tips
Intrathecal injection of fentanyl 10–25 mcg or sufent-anil 5–10 mcg alone or, more commonly, with 1 mL isobaric bupivacaine 0.25% produces profound analge-sia lasting for 90–120 minutes with minimal motor block.
The most common side effects of intrathecal opioids are pruritus, nausea, vomiting, and urinary retention. Rostral spread resulting in delayed respiratory depression is rare with fentanyl and sufentanil and usually occurs within 30 minutes of injection. Transient nonreassuring fetal heart rate patterns may occur because of uterine hyperstimulation, presumably as a result of a rapid decrease in maternal catecholamines resulting in the unopposed effects of oxytocin.
A preliminary study by O’Gorman et al. suggests that fetal bradycardia may occur in the absence of uterine hyperstimulation or hypotension and is unrelated to utero-placental insufficiency. The incidence of fetal heart rate abnormalities may be greater in multiparous woman with a rapidly progressing, painful labor. Most studies have demonstrated that the incidence of emergency cesarean section delivery is no greater with CSE analgesia than after conventional epidural analgesia. Postdural puncture headache is always a risk after intrathecal injection. However, the incidence of headache is no greater with CSE analgesia compared with standard epidural analgesia.
Unintentional intrathecal catheter placement through the dural puncture site is also rare after use of a 27-gauge spinal needle for CSE analgesia. The potential exists for epidurally administered drug to leak intrathecally through the dural puncture, particularly if large volumes of drug are rapidly injected. In fact, epidural drug requirements are approximately 30% less with CSE analgesia than with standard lumbar epidural techniques for cesarean section delivery. Some clinicians do not advocate the CSE analgesia technique for labor because of the concern for an “unproven” epidural catheter that may need to be used emergently for cesarean section delivery. The patient may have a partial block insufficient for surgery with an epidural that may or may not work. An algorithm for patient management in the event of an incomplete epidural is presented in Figure 5.
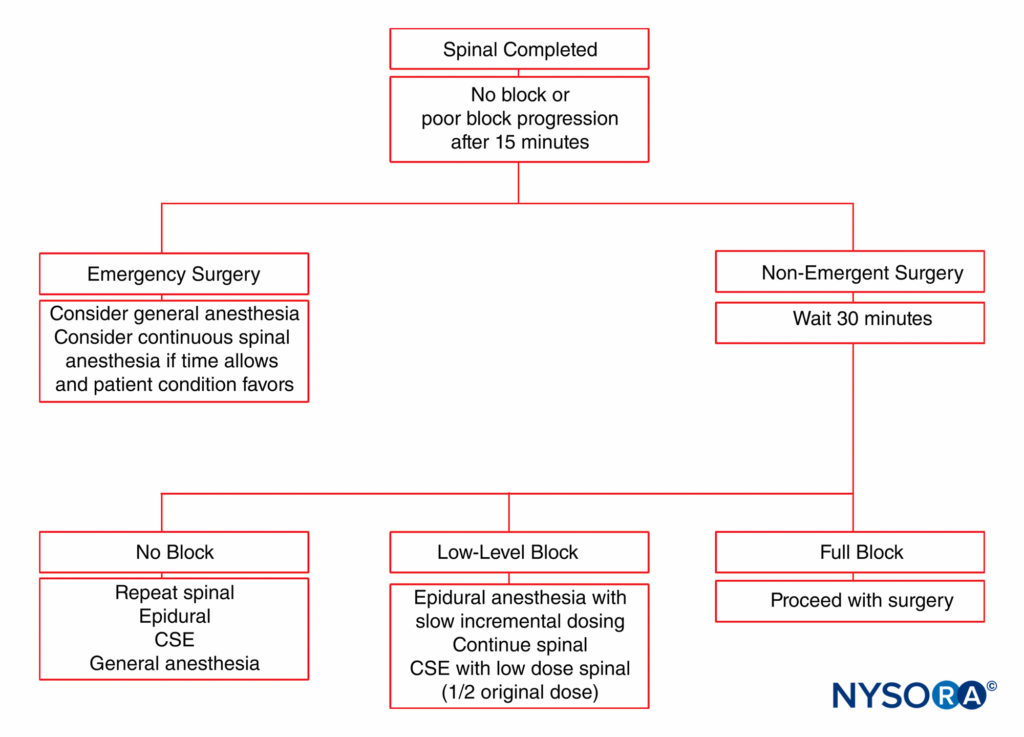
FIGURE 5. Management algorithm for an obstetric patient with inadequate neuraxial anesthesia. CSE, combined spinal–epidural.
Paracervical Block
As recently as 2001, only 2%–3% of parturients in the United States received paracervical block during labor. Although paracervical block effectively relieves pain during the first stage of labor, it is now rarely used in the United States because of its association with a high incidence of fetal bradycardia, particularly with the use of bupivacaine. This may be related to uterine artery constriction or increased uterine tone.
The use of levobupivacaine compared to racemic bupivacaine has been demonstrated to result in fewer fetal bradycardias. Paracervical block is a useful technique to provide analgesia for uterine curettage. The technique is very simple and involves a submucosal injection of local anesthetic at the vaginal fornix near the neural fibers innervating the uterus (Figure 6).
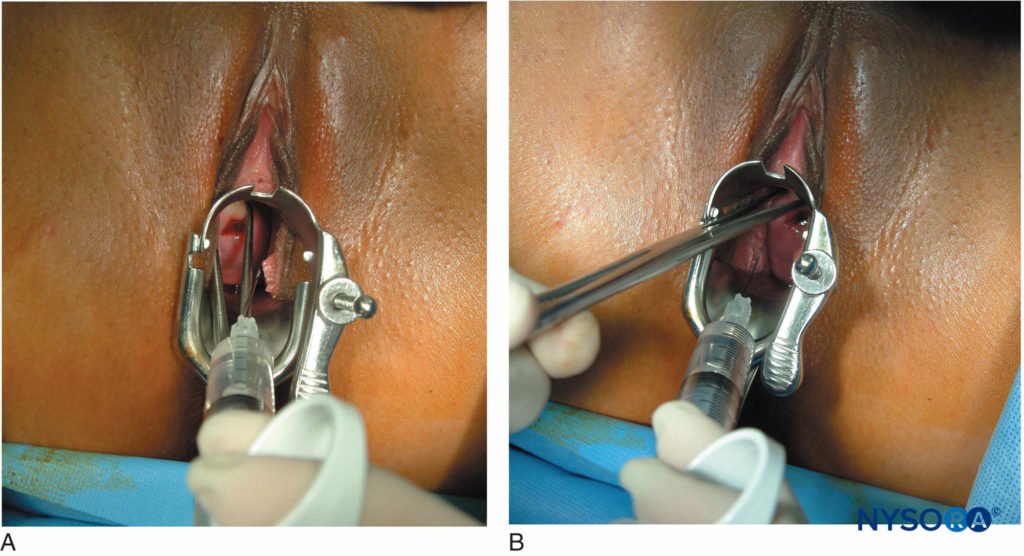
FIGURE 6. (A) and (B): Paracervical block for uterine curettage. The technique involves a submucosal injection of local anesthetic at the vaginal fornix, near the neural fibers innervating the uterus.
Paravertebral Lumbar Sympathetic Block
Paravertebral lumbar sympathetic block is a reasonable alternative to central neuraxial block. Lumbar sympathetic block effectively interrupts the painful transmission of cervical and uterine impulses during the first stage of labor. Leighton et al. showed that women who received lumbar sympathetic blocks had a more rapid rate of cervical dilatation during the first two hours of analgesia and a shorter second stage of labor compared to epidural analgesia. However, there was no difference in the rate of dilatation during the active phase of labor. Although there is less risk of fetal bradycardia with lumbar sympathetic block compared with paracervical block, technical difficulties associated with the performance of the block and risks of intravascular injection have hampered its routine use. Hypotension may also occur with lumbar sympathetic blocks.
Pudendal Nerve Block
The pudendal nerves are derived from the lower sacral nerve roots (S2–4) and supply the vaginal vault, perineum, rectum, and sections of the bladder. The nerves are easily blocked transvaginally where they loop around the ischial spines.
A recent study demonstrated that a pudendal nerve block does not provide reliable analgesia for the second stage of labor, probably related to the upper vagina being innervated by lumbar, rather than sacral, fibers. However, the block is useful for episiotomy and repair. There may also be post-partum benefits of pudendal nerve block. For instance, a unilateral nerve stimulator–guided pudendal nerve block with ropivacaine was associated with decreased pain and less need for supplemental analgesia during the first 48 hours after the performance of mediolateral episiotomy at vaginal delivery.
ANESTHESIA FOR CESAREAN SECTION DELIVERY
The most common indications for cesarean section delivery include failure to progress, nonreassuring fetal status, cephalopelvic disproportion, malpresentation, prematurity, and prior uterine surgery involving the corpus. The choice of anesthesia should depend on the urgency of the procedure in addition to the condition of the mother and fetus. After a comprehensive discussion of the risks and benefits of all anesthesia options, the mother’s desires should be considered. Before the initiation of any anesthetic technique, resuscitation equipment for mother and neonate should be made available (Table 2).
TABLE 2. Resuscitation equipment in the delivery room.
| Radiant warmer |
| Suction with manometer and suction trap |
| Suction catheters |
| Wall oxygen with flow meter |
| Resuscitation bag-mask positive pressure ventilation device (≤ 750 mL) |
| Infant face masks |
| Infant oropharyngeal airway |
| Endotracheal tubes: 2.5, 3.0, 3.5, and 4.0 mm |
| Endotracheal tube stylets |
| Laryngoscope(s) and blade(s) |
| Sterile umbilical artery catheterization tray |
| Needles, syringes, three-way stopcocks |
| Medications and solutions: • 1:10,000 epinephrine • Naloxone hydrochloride • Sodium bicarbonate • Volume expanders |
Advantages of Regional Anesthesia in the Obstetric Patient
Cesarean delivery accounts for more than 30% of all births and is the most common surgical procedure performed in the United States, with more than 1 million performed each year. A 1992 survey of obstetric anesthesia practices in the United States demonstrated that most patients undergoing cesarean section delivery do so under spinal or epidural anesthesia.
Regional techniques have several advantages: They reduce the risk of gastric aspiration, avoid the use of depressant anesthetic drugs, and allow the mother to remain awake during delivery. Operative blood loss may also be reduced with regional compared with general anesthesia. Generally speaking, with regional techniques, the duration of antepartum anesthesia does not affect neonatal outcome, provided that there is no protracted aortocaval compression or hypotension. The risk of hypotension may be greater than during vaginal delivery because the sensory block must extend to at least the T4 dermatome. Studies have shown preloading with crystalloid does not reliably prevent neuraxial anesthesia–induced hypotension. In fact, recent studies indicate that intravenous co-loading at the time of intrathecal injection is as effective as prehydrating before neuroblock. If hypotension occurs despite these measures, left uterine displacement should be increased, the rate of IV infusion augmented, and IV ephedrine 5–15 mg (or phenylephrine 25–50 mcg) administered incrementally. The greatest success in preventing hypotension has been found with a continuous low-dose infusion of phenylephrine until delivery.
Spinal Anesthesia
Subarachnoid block is probably the most commonly adminis-tered regional anesthetic for cesarean section delivery because of its speed of onset and reliability. It has also become an alternative to general anesthesia for emergency cesarean section.
Hyperbaric solutions of lidocaine 5%, tetracaine 1.0%, or bupivacaine 0.75% have been used. However, bupivacaine has now become the most widely used drug for spinal anesthesia for cesarean delivery. Using 0.75% hyperbaric bupivacaine, Norris has shown that it is not necessary to adjust the dose of drug based on the patient’s height. In addition, the patient’s age, weight, and vertebral column length do not affect the resulting neuraxial block. Recent studies using spinal ropivacaine have shown less hypotension and faster recovery but a slower onset compared to bupivacaine. However, it has been questioned whether ropivacaine produces spinal anesthesia of similar quality to that of bupivacaine. Hemodynamic monitoring during cesarean section should be similar to that used for other surgical procedures, with the exception that blood pressure should be monitored at a minimum of every 3 minutes before the birth of the baby. Before delivery, oxygen should be routinely administered to optimize fetal oxygenation. Reports of transient neurologic syndrome and/or cauda equina syndrome have been associated with lidocaine in doses greater than 60 mg, whether in a 5% or 2% preparation. This has led some clinicians to avoid the use of lidocaine for intrathecal administration (see “Systemic Toxicity of Local Anesthetics” below). Table 3 lists local anesthetics and the dosages commonly used for cesarean section delivery with subarachnoid block.
TABLE 3. Local anesthetics commonly used for cesarean section delivery with subarachnoid block.
| Dosage per Height of Patient (cm) | Bupivacaine 0.75% in 8.25% Dextrose (mg) | Bupivacaine 0.5% (Isobaric) (mg) |
|---|---|---|
| 150–160 cm | 8 | 8 |
| 160–180 | 10 | 10–12.5 |
| > 180 cm | 12 | 12.5–15 |
| Onset of action | 2–4 min | 5–10 min |
Despite an adequate dermatomal level, women may experience varying degrees of visceral discomfort, particularly during exteriorization of the uterus and traction on abdominal viscera. Improved perioperative analgesia can be provided by the addition of fentanyl 20 mcg or preservative-free morphine 0.1 mg to the local anesthetic solution. Preservative-free morphine produces significant analgesia in doses ranging from 0.1 to 0.25 mg. Higher doses of spinal morphine result in greater pruritus. Delayed respiratory depres-sion can occur with spinal morphine but is extremely rare and more often associated with comorbid conditions such as obesity. The respiratory depression is due to the rostral spread of subarachnoid morphine. In a retrospective study of 1915 parturients receiving spinal morphine 0.15 mg for cesarean delivery, five patients (0.26%) experienced bradypnea, and one patient required naloxone. In addition, spinal clonidine, in doses of 60 to 150 mcg, improves intraoperative analgesia and decreases shivering in women undergoing cesarean delivery. However, hypotension and sedation have been reported with spinal clonidine and may limit its routine use. Nausea and vomiting may be alleviated by the administration of ondansetron or metoclopramide. Maternal sedation should be avoided if possible. If the initial block is not adequate, concern exists regarding a repeat spinal injection and the potential for inadvertent high spinal anesthetic. See Figure 5 for a range of options available in situations in which spinal anesthesia fails to prove adequate for surgery.
NYSORA Tips
- Even with an adequate dermatomal level for surgery, women undergoing cesarean section may experience discomfort, particularly during exteriorization of the uterus and traction on abdominal viscera.
- Perioperative analgesia may be enhanced by the addition of fentanyl 20 mcg or preservative-free morphine 0.1 mg to the local anesthetic solution.
Lumbar Epidural Anesthesia
Epidural anesthesia has a slower onset of action and a larger drug requirement to establish an adequate sensory block compared with spinal anesthesia. The advantages are a perceived reduced risk of postdural puncture headache and the ability to titrate the local anesthetic through the epidural catheter. However, correct placement of the epidural catheter and avoidance of inadvertent intrathecal or intravascular injection are essential.
Aspiration of the epidural catheter for blood or cerebrospinal fluid is not 100% reliable for detecting catheter misplacement. For this reason, a “test dose” is often used to rule out inadvertent intravascular or intrathecal catheter placement. A small dose of local anesthetic, lidocaine 45 mg or bupivacaine 5 mg, produces a readily identifiable sensory and motor block if injected intrathecally. However, a recent study has suggested that ropivacaine 15 mg was not a useful intrathecal test dose because a slow onset of motor block may preclude the timely diagnosis of intrathecal injection. The addition of epinephrine 15 mcg with careful continuous heart rate and blood pressure monitoring may herald intravascular injection with a transient increase in heart rate and blood pressure. However, an epinephrine test dose is not reliable because false-positive results do occur in the form of tachycardia related to painful uterine contractions. In addition, epinephrine may potentially reduce uteroplacental perfusion in some patients. Electrocardiography and the application of a peak-to-peak heart rate criterion may improve detection (10 beats over maximum heart rate preceding epinephrine injection). Rapid injection of 1 mL of air with simultaneous precordial Doppler monitoring appears to be a reliable indicator of intravascular catheter placement. Most important, a negative test, although reassuring, does not eliminate the need for the fractional administration of local anesthetic.
NYSORA Tips
- Aspiration of the epidural catheter for blood or cerebro-spinal fluid is not absolutely reliable for detecting cath-eter misplacement.
- A “test dose” is often used to rule out inadvertent intra-vascular or intrathecal catheter placement.
- A small dose of local anesthetic, lidocaine 45 mg or bupivacaine 5 mg, produces a readily identifiable sen-sory and motor block if injected intrathecally.
- The addition of epinephrine 15 mcg with careful hemodynamic monitoring may signal intravascular injection when followed by a transient increase in heart rate and blood pressure.
- However, the use of an epinephrine test dose is controversial because false-positive results do occur in the pres-ence of uterine contractions.
Local Anesthetic Choices
The most commonly used local anesthetic agents are 2-chloro-procaine 3%, bupivacaine 0.5%, and lidocaine 2% with epinephrine 1:200,000. Adequate anesthesia can be usually achieved with 15–25 mL of local anesthetic given in divided doses. The patient should be monitored as with spinal anesthesia. Because of its extremely high rate of metabolism in maternal and fetal plasma, 2-chloroprocaine provides a rapid-onset, reliable block with minimal risk of systemic toxicity. It is the local anesthetic of choice in the presence of fetal acidosis and when a preexisting epidural block is to be rapidly extended for an urgent cesarean section delivery. Neurologic deficits after massive inadvertent intrathecal administration of the drug have occurred with the formulation containing a relatively high concentration of sodium bisulfite at a low pH.
In a new formulation of 2-chloroprocaine (Nesacaine-MPF), ethylenediaminetetraacetic acid (EDTA) has been substituted for sodium bisulfite. However, severe spasmodic back pain has been described after epidural injection of large volumes of Nesacaine-MPF in surgical patients, but not in parturients. This has been attributed to an EDTA-induced leaching of calcium from paravertebral muscles. The most recent formulation of 2-chloroprocaine contains no additives and is packaged in an amber vial to prevent oxidation.
Bupivacaine 0.5% provides profound anesthesia of slower onset for cesarean section delivery but of longer duration of action. Considerable attention has been focused on the drug because it was reported that unintentional intravascular injection could result not only in convulsions but also in almost simultaneous cardiac arrest, with patients often refractory to resuscitation. The greater cardiotoxicity of bupivacaine (and etidocaine) compared with other amide local anesthetics has been well established.When using potent long-acting amide local anesthetics, fractioning the induction dose is critical. Lidocaine has an onset and duration intermediate to those of 2-chloroprocaine and bupivacaine. The need to include epinephrine in the local anesthetic solution to ensure adequate lumbosacral anesthesia limits the use of lidocaine in women with maternal hypertension and uteroplacental insufficiency.
Prolonged postoperative pain relief can be provided by the epidural administration of an opioid, such as morphine 4 mg, or the use of PCEA. Delayed respiratory depression may occur with the use of morphine; hence, the patient must be monitored carefully in the postoperative period. Recently, a lipid-encapsulated preparation of morphine (DepoDur) has been approved for post–cesarean section delivery analgesia. It can be used only epidurally, can last up to 48 hours, and the patient must be monitored for delayed respiratory depression. A potential limitation in obstetrics is that once the drug is administered, additional local anesthetic cannot be injected epidurally for a period of up to one hour since the local anes-thetic may cause an uncontrolled release of morphine from the lipid. Carvalho et al. evaluated the epidural administration of 5, 10, and 15 mg of extended-release morphine for post–cesarean section analgesia and demonstrated that the 10 mg and 15 mg doses provided good analgesia for up to 48 hours after surgery. No significant side effects were observed. Another study showed lower pain scores and fewer supplemental analgesia requirements for patients receiving extended-release morphine compared to preservative-free morphine. No differences in nausea, pruritus, or sedation scores were observed. In addition, the bolus administration of epidural fentanyl (50–100 mcg) has been found to result in activity at both spinal and supraspinal sites of action and to improve the quality of anesthesia.
ANESTHETIC COMPLICATIONS
Maternal Mortality
A study of anesthesia-related deaths in the United States between 1979 and 1990 showed that the case fatality rate with general anesthesia was 16.7 times greater than that with regional anesthesia. Most anesthesia-related deaths were a result of cardiac arrest due to hypoxemia when difficulties securing the airway were encountered. Pregnancy-induced anatomical and physiological changes, such as reduced FRC, increased oxygen consumption, and oropharyngeal edema may expose the patient to serious risks of desaturation during periods of apnea and hypoventilation.
Pulmonary Aspiration
The risk of the inhalation of gastric contents is increased in pregnant women, particularly if difficulty is encountered estab-lishing an airway or if airway reflexes are obtunded. Measures to decrease the risks of aspiration include comprehensive airway evaluation, prophylactic administration of nonparticulate antacids, and the preferred use of regional anesthesia. If aspiration occurs, management includes immediate treatment of hypoxemia with continuous positive airway pressure (CPAP) and possible rigid bronchoscopy. Recent studies do not support the administration of corticosteroids or lung lavage with saline and bicarbonate to neutralize acidity. Prophylactic antibiotics are not recommended because gastric contents are sterile.
Hypotension
Regional anesthesia may be associated with hypotension, which is related to the degree and rapidity of local anesthetic–induced sympatholysis. Thus, greater hemodynamic stability may be observed with epidural anesthesia, where gradual titration of local anesthetic allows for better control of the block level as well as for adequate time for vasopressor administration in anticipation of blood pressure reduction.
The risk of hypotension is lower in laboring women compared with nonlaboring women. Maternal prehydration with 15 mL/kg of lactated Ringer’s solution before the initiation of regional anesthesia and the avoidance of aortocaval compression may decrease the incidence of hypotension. It has been demonstrated that for effective prevention of hypotension, the blood volume increase from preloading must be sufficient to result in a significant increase in cardiac output. This was possible only with the administration of hetastarch 0.5–1 L. Nonetheless, controversy exists regarding the efficacy of volume loading in the prevention of hypotension. A recent study using a prophylactic phenylephrine infusion combined with a rapid crystalloid co-loading given at the time of intrathecal injection markedly reduced the incidence of spinal anesthesia–induced hypotension. If hypotension does occur despite prehydration, therapeutic measures should include increasing the displacement of the uterus, rapid infusion of IV fluids, titration of IV ephedrine (5–10 mg), and oxygen administration. In the presence of maternal tachycardia, phenylephrine 25–50 mcg may be substituted for ephedrine in women with normal uteroplacental function. Continued vigilance and active management of hypotension can prevent serious sequelae in both mother and neonate.
High Spinal Anesthesia
High, or total, spinal anesthesia is a rare complication of intrathe-cal injection in modern-day practice. It occurs with an excessive cephalad spread of local anesthetic in the subarachnoid space. Unintentional intrathecal administration of epidural medication as a result of dural puncture or catheter migration may also result in this complication. Left uterine displacement and continued fluid and vasopressor administration may be necessary to achieve hemodynamic stability. The reverse Trendelenburg position does not prevent cephalad spread and may cause cardiovascular collapse because of venous pooling related to sympathectomy. Rapid control of the airway is essential, and endotracheal intubation may be necessary to ensure oxygenation without aspiration.
NYSORA Tips
- Obstetric patients often complain of difficulty breathing during cesarean section delivery under neuraxial anesthesia.
- Although most common reasons are inability to feel “breathing” as the abdominal and thoracic segments are anesthetized (including the stretch receptors), practitioners must rule out an impending “high spinal” anesthetic by repetitive examinations.
- The following maneuvers are useful to rule out the pos-sibility of high neuraxial anesthesia:
– The ability of the patient to phonate
– The ability of the patient to squeeze the practitioner’s hand (indicates that the block level is below the level of the brachial plexus (C6–T1)
Systemic Toxicity of Local Anesthetics
Unintended intravascular injection or drug accumulation after repeated epidural injection can result in high serum levels of local anesthetic. Rapid absorption of local anesthetic from highly vascular sites of injection may also occur after paracervical and pudendal blocks. Resuscitation equipment should always be available when any major nerve block is undertaken. IV access, airway equipment, emergency drugs, and suction equipment should be immediately accessible. To avoid systemic toxicity of local anesthetic agents, strict adherence to recommended dosages and avoidance of unintentional intravascular injection are essential.
Despite these precautions, life-threatening convulsions and, more rarely, cardiovascular collapse may occur. Seizure activity has been treated with IV thiopental 25–50 mg or diazepam 5–10 mg. In current clinical practice, propofol 20–50 mg or midazolam 2–4 mg is more commonly used.The airway should be evaluated and oxygenation main-tained. If cardiovascular collapse does occur, the Advanced Cardiac Life Support (ACLS) algorithm should be followed. In a 2006 case report, lipid emulsion was used to treat refractory cardiac arrest resulting from bupivacaine toxicity. The mechanism of action is unclear but may result from the greater affinity of bupivacaine for the lipid or because the lipid provides a substrate for a bupivacaine-poisoned mitochondrial energy system. Further study is required to deter-mine the efficacy of this treatment. However, it would seem prudent that treatment of a pregnant woman intoxicated with bupivacaine should include the administration of lipid emulsion early on in the resuscitation. The current recommended protocol for lipid rescue (see http://www.lipidrescue.org) involves a 20% lipid emulsion: a 1.5 mL/kg initial bolus, followed by 0.25 mL/kg/min for 30–60 minutes. The early administration of lipid has also been shown to prevent progression to cardiac arrest when bupivacaine was injected intravascularly.
Whenever there is maternal cardiac arrest, regardless of cause, the fetus should be delivered early on, usually within 5 minutes, if attempts at resuscitation are unsuccessful in relieving aortocaval compression and ensuring the efficiency of cardiac massage.
Postdural Puncture Headache
Pregnant women have a higher risk of developing a postdural puncture headache (PDPH) should an inadvertent dural puncture occur. In a recent meta-analysis, the risk of PDPH was 52.1% (95% confidence interval [CI], 51.4–52.8%) after an accidental dural puncture with an epidural needle. The reduced epidural pressure increases the risk of cerebrospinal fluid leakage through the dural opening. Russell et al. reported that the placement of an intrathecal catheter after an accidental dural puncture did not lower the incidence of headache or the need for blood patch compared to repeating an epidural. The incidence of headache was higher with the use of a 16-gauge compared to an 18-gauge epidural needle. The pathophysiology and management of postdural puncture headache are discussed in greater detail in Postdural Puncture Headache.
Neurologic Complications
Neurologic sequelae of central neuraxial block, although rare, have been reported. Pressure exerted by a needle or catheter on spinal nerve roots produces immediate pain and necessitates repositioning. Infections such as epidural abscess and meningitis are very rare and may be a manifestation of systemic sepsis. In recent years, several cases of epidural abscess have been reported after epidural catheterization in obstetric patients.
Potential risk factors identified from these cases are entry-point infections from usual causative organisms (eg, Staphylococcus aureus), possible systemic sources of infection, poor aseptic technique, and prolonged catheterization. Epidural hematoma can also occur, usually in association with coagulation defects. However, epidural hematoma may also occur spontaneously, unrelated to instrumentation. The pathogenesis may be due to a weakened epidural vascular architecture. Nerve root irritation may have a protracted recovery, lasting weeks or months. Peripheral nerve injury as a result of instrumentation, lithotomy position, or compression by the fetal head may occur even in the absence of neuraxial technique.
REGIONAL ANESTHESIA IN COMPLICATED PREGNANCY
Pregnancy and parturition are considered high risk when accompanied by conditions unfavorable to the well-being of the mother or fetus, or both. Maternal problems may be related to the pregnancy; that is, preeclampsia-eclampsia, hypertensive disorders of pregnancy, or antepartum hemorrhage resulting from placenta previa or abruptio placentae. Diabetes mellitus; cardiac, chronic renal, and neurologic problems; sickle cell disease; asthma; obesity; and drug abuse are not related to pregnancy but often are affected by it. Prematurity (gestation of less than 37 weeks), postmaturity (gestation of 42 weeks or longer), intrauterine growth retardation, and multiple gestation are fetal conditions associated with risk. During labor and delivery, fetal malpresentation (eg, breech, transverse lie), placental abruption, compression of the umbilical cord (eg, prolapse, nuchal cord), precipitous labor, or intrauterine infection (eg, prolonged rupture of membranes) may increase the risk to the mother or fetus. In general, the anesthetic management of the high-risk parturient is based on the same maternal and fetal considerations as for the management of healthy mothers and fetuses. However, there is less room for error because many of these functions may be compromised before the induction of anesthesia.
Preeclampsia-EclampsiaPathophysiology and Signs and Symptoms
Hypertensive disorders occur in approximately 7% of all pregnancies and are a major cause of maternal mortality. The most recent diagnostic criterion for preeclampsia is referred to as “proteinaceous increase in blood pressure.” The presence or absence of edema is no longer considered one of the required criteria. Rather than a specific blood pressure elevation, a blood pressure that is consistently 15% above baseline is now considered diagnostic. The added appearance of convulsions is diagnostic for eclampsia. Preeclampsia-eclampsia is a disease unique to humans, occurring predominantly in young nulliparous women. Symptoms usually appear after the twentieth week of gestation, occasionally earlier with a hydatidiform mole. Delivery of the infant and placenta is the only effective treatment; as a result, preeclampsia is a leading cause of iatrogenic preterm delivery in developed countries.
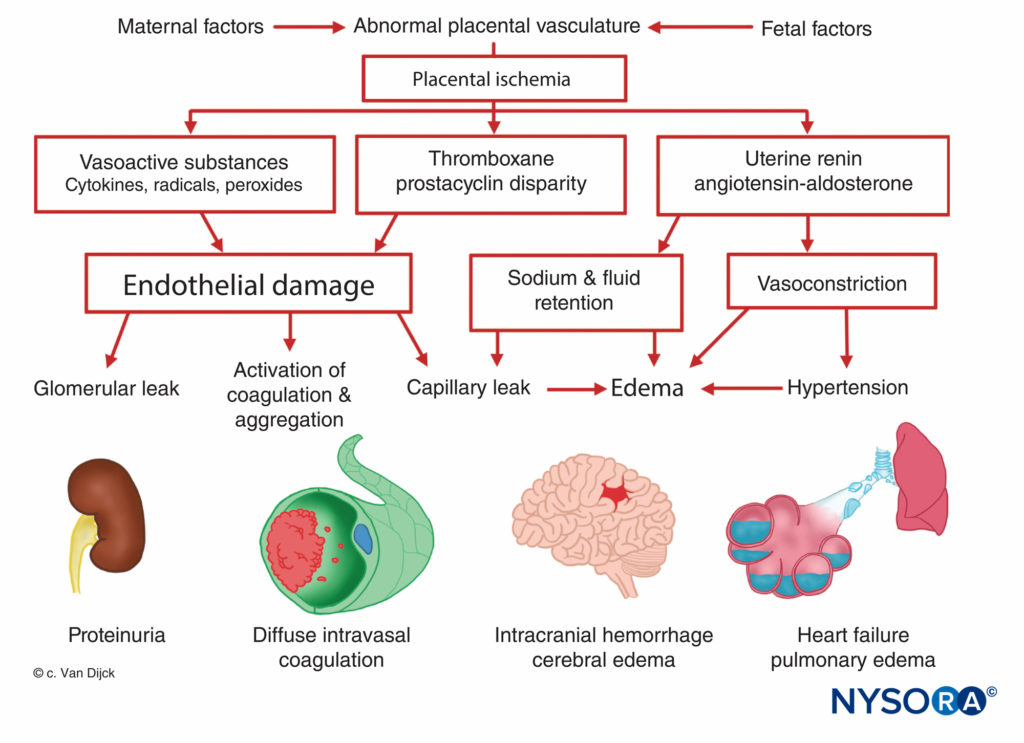
The origin of preeclampsia-eclampsia is unknown, but all patients manifest placental ischemia. Decreased placental per-fusion occurs in early pregnancy in women destined to become preeclamptic, and there is a failure of the normal trophoblastic invasion. In normal pregnancy, the diameter of spiral arteries increase approximately four-fold to create flaccid tubes that provide a low-resistance pathway to the intervillous space. This angiogenesis is a result of the trophoblast invasion into the decidual and myometrial segments of the spiral arteries. However, in preeclamptic women, the myometrium is not invaded. This causes superficial placental implantation, resulting in decreased placental perfusion and ischemia related to stiff, muscular spiral arteries. Placental ischemia results in a release of uterine renin, an increase in angiotensin activity, and a widespread arteriolar vasoconstriction causing hypertension, tissue hypoxia, and endothelial damage (Figure 7). The fixation of platelets at sites of endothelial damage results in coagulopathies, occasionally in disseminated intravascular coagulation. Enhanced angioten-sinmediated aldosterone secretion leads to increased sodium reabsorption and edema. Proteinuria, a sign of preeclampsia, is also attributed to placental ischemia, which leads to local tissue degeneration and a release of thromboplastin with subsequent deposition of fibrin in constricted glomerular vessels. As a result, an increased permeability to albumin and other plasma proteins occurs. Furthermore, there is a decreased production of prostaglandin E, a potent vasodilator secreted in the trophoblast, which normally balances the hypertensive effects of the rennin–angiotensin system.
Many of the symptoms associated with preeclampsia, including placental ischemia, systemic vasoconstriction, and increased platelet aggregation, may result from an imbalance between the placental production of prostacyclin and thromboxane. During normal pregnancy, the placenta produces equal amounts of the two, but in a preeclamptic pregnancy, there is seven times more thromboxane than prostacyclin.
According to the latest theory, endothelial cell injury is central to the development of preeclampsia. This injury occurs as a result of reduced placental perfusion, leading to a production and release of substances (possibly lipid peroxidases) causing endothelial cell injury. Abnormal endothelial cell function contributes to an increase in peripheral resistance and other abnormalities noted in preeclampsia through a release of fibronectin, endothelin, and other substances.
In rodent models, two placental antiangiogenic proteins have been identified and likely play a role in the pathogenesis of preeclampsia. Soluble fms-like tyrosine kinase-1 (sFlt-1) is upregulated in the placenta of preeclamptic women. The elevated sFlt-1 protein levels antagonize and reduce vascular end-thelial growth factor (VEGF) and placental growth factor (PlGF). Levine et al. demonstrated that increased sFlt-1 levels and reduced PlGF levels predicted the subsequent development of preeclampsia. Another antiangiogenic protein, soluble endoglin (sEng), is elevated in cases of HELLP syndrome (which consists of hemolysis, elevated liver enzymes, and low platelet count).
NYSORA Tips
Preeclampsia is classified as severe if it is associated with any of the following:
- Systolic blood pressure consistently more than 15% above baseline
- Diastolic blood pressure consistently more than 15% above baseline
- Proteinuria of 5 g/24 h
- Oliguria of 400 mL/24 h
- Cerebrovisual disturbances
- Pulmonary edema or cyanosis
- Epigastric pain
- Intrauterine growth retardation
In severe preeclampsia-eclampsia, all major organ systems are affected because of widespread vasospasm. Global cerebral blood flow is not diminished, but focal hypoperfusion cannot be ruled out. Postmortem examination has revealed hemorrhagic necrosis in the proximity of thrombosed precapillaries, suggesting intense vasoconstriction. Edema and small foci of degeneration have been attributed to hypoxia. Petechial hemorrhages are common after the onset of convulsions. Symptoms related to the above changes include headache, vertigo, cortical blindness, hyperreflexia, and convulsions. Cerebral hemorrhage and edema are the leading causes of death in preeclampsia-eclampsia, which together account for approximately 50% of deaths. Heart failure may occur in severe cases as a result of peripheral vasoconstriction and increased blood viscosity from hemoconcentration. Decreased blood supply to the liver may lead to periportal necrosis of variable extent and severity. Subcapsular hemorrhages account for the epigastric pain encountered in severe cases.In the kidneys, there is swelling of glomerular endothelial cells and deposition of fibrin, leading to a constriction of the capillary lumina. Renal blood flow and glomerular filtration rate decrease, resulting in reduced uric acid clearance and, in severe cases, increase in creatinine.
Although preeclampsia is accompanied by exaggerated retention of water and sodium, the shift of fluid and proteins from the intravascular into the extravascular compartment may result in hypovolemia, hypoproteinemia, and hemoconcentration, which may be further aggravated by proteinuria. The risk of uteroplacental hypoperfusion and poor fetal outcome correlates with the degree of maternal plasma and protein depletion.
Platelet adherence at sites of endothelial damage may result in consumption coagulopathy, which develops in approximately 20% of patients with preeclampsia. Mild thrombocytopenia, with a platelet count of 100,000–150,000/mm, is the most common finding. The prolongation of prothrombin and partial thromboplastin times indicates a consumption of procoagulants. Bleeding time, prolonged in approximately 25% of patients with normal platelet counts, is no longer considered a reliable test of clotting. The HELLP syndrome is a particular form of severe preeclampsia characterized by hemolysis, elevated liver enzymes, and low platelets.
The goals of the management of the patient with preeclamp-sia-eclampsia are to prevent or control convulsions, improve organ perfusion, normalize blood pressure, and correct clotting abnormalities. The mainstay of anticonvulsant therapy in the United States is magnesium sulfate. Its efficacy in preventing seizures has been well substantiated, but its mechanism of action remains controversial. The patient usually receives a loading dose of 4 g in a 20% solution, administered over 5 minutes, followed by a continuous infusion of 1–2 g/h.
Antihypertensive therapy in preeclampsia is used to lessen the risk of cerebral hemorrhage in the mother while maintaining, or even improving, tissue perfusion. There is no evidence to suggest that antihypertensive therapy delays disease progression or improves perinatal outcome. Plasma volume expansion combined with vasodilation fulfills these goals. Hydralazine is the most commonly used vasodilator because it increases uteroplacental and renal blood flows. However, side effects include tachycardia, palpitations, headache, and neonatal thrombocytopenia. Nitroprusside is used during laryngoscopy and intubation to prevent dangerous elevations in blood pressure. Trimethaphan, a ganglion blocking agent, is useful in hypertensive emergencies when cerebral edema and increased intracranial pressure are a concern because it does not cause vasodilation in the brain. Other agents that have been used to control maternal blood pressure include α-methyldopa, nitroglycerine, and, now more frequently, labetalol.
Consumption coagulopathy may require infusion of fresh whole blood, platelet concentrates, fresh frozen plasma, and cryoprecipitate. Delivery is indicated in refractory cases or if the pregnancy is close to term. In severe cases, aggressive management should continue for at least 24–48 hours after delivery.
Anesthesia Management
There are very few contraindications for epidural anesthesia in labor and delivery. In the presence of severe clotting abnormalities or severe plasma volume deficit, the risk–benefit ratio favors other forms of anesthesia. In volume-depleted patients positioned with left uterine displacement, epidural anesthesia does not cause an unacceptable reduction in blood pressure and leads to a significant improvement in placental perfusion. With the use of radioactive xenon, it was shown that the intervillous blood flow increased by approximately 75% after the induction of epidural analgesia (10 mL bupivacaine 0.25%). The total maternal body clearance of amide local anesthetics is prolonged in preeclampsia, and repeated administration of these drugs can lead to higher blood concentrations than in normotensive patients.
For cesarean section delivery, the sensory level of regional anesthesia must extend to T3–4, making adequate fluid therapy and left uterine displacement even more vital.
Epidural anesthesia has been preferred to spinal anesthesia in preeclamptic women because of its slower onset of action and controllability. In the past, the rapid onset of sympathectomy related to spinal anesthesia was associated with hypoten-sion, particularly in volume-depleted patients. However, in two recent studies, the incidence of hypotension, perioperative fluid and ephedrine administration, and neonatal conditions were found to be similar in preeclamptic women who received either epidural or spinal anesthesia for cesarean delivery. Aya et al. conducted a prospective cohort study that showed the risk of significant spinal anesthesia–induced hypotension was significantly lower in preeclamptic women compared to normotensive pregnant women. There is an increased sensitivity to vasopressors in preeclampsia; therefore, lower doses of ephedrine and phenylephrine are usually required to correct hypotension.
Antepartum Hemorrhage
Antepartum hemorrhage occurs most commonly in association with placenta previa (abnormal placental implantation on the lower uterine segment and partial to total occlusion of the internal cervical os) and abruptio placentae. Placenta previa occurs in 0.11% of all pregnancies, resulting in up to a 0.9% incidence of maternal and a 17–26% incidence of perinatal mortality. It may be associated with abnormal fetal presenta-tion, such as transverse lie or breech. Placenta previa should be suspected whenever a patient presents with painless, bright red vaginal bleeding, usually after the seventh month of pregnancy. The diagnosis is confirmed by ultrasonography. Unless the lowest placental edge is more than 2 cm from the internal cervical os, an abdominal delivery is usually required. If the bleeding is not profuse and the fetus is immature, obstetric management is conservative to prolong the pregnancy. In severe cases or if the fetus is mature at the onset of symptoms, prompt delivery is indicated, usually by cesarean section. An emergency hysterectomy may be required because of severe hemorrhage, even after the delivery of the placenta, because of uterine atony. In patients who have undergone prior uterine surgery, particularly prior cesarean delivery, the risk of severe hemorrhage is even greater, owing to a higher incidence of placenta acreta (penetration of the myometrium by placental villi).
Abruptio placentae occurs in 0.2–2.4% of pregnant women, usually in the final 10 weeks of gestation and in association with hypertensive diseases. Complications include Couvelaire uterus (which occurs when extravasated blood dissects between the myometrial fibers), renal failure, disseminated intravascular coagulation, and anterior pituitary necrosis (ie, Sheehan syndrome). The maternal mortality is high (1.8–11.0%), and the perinatal mortality rate is even higher (in excess of 50%). The diagnosis of abruptio placentae is based on the presence of uterine tenderness, hypertonus, and vaginal bleeding of dark, clotted blood. Bleeding may be concealed if the placental margins have remained attached to the uterine wall. Changes in maternal blood pressure and pulse rate, indicative of hypovolemia, may occur if the blood loss is severe. Fetal movements may increase during acute hypoxia and decrease if hypoxia is gradual. Fetal bradycardia and death may ensue.
Anesthesia Management
The establishment of invasive monitoring (arterial line, central venous catheter) and blood volume replacement via a 14- or 16-gauge catheter is usually required. If clotting abnormalities exist, blood components and fresh frozen plasma, cryoprecipitate, and platelet concentrates may be required. The choice of anesthetic for a woman with placental abruption depends on maternal and fetal condition and how urgently the procedure needs to be performed. General anesthesia is indicated in the presence of uncontrolled hemorrhage and coagulation abnormalities.
Epidural anesthesia may be used, particularly if there is a functioning epidural in place during labor at the time of abruption and there is no hemodynamic instability. Vincent et al. observed that epidural anesthesia significantly worsened maternal hypotension, uterine blood flow, and fetal PaO2 and pH during untreated hemorrhage in gravid ewes. However, this was uncorrected hypotension, which, with intravascular fluid replacement hemodynamics, returned to normal even with epidural anesthesia.
Preterm Delivery
Preterm labor and delivery present a significant challenge to the anesthesiologist because both the mother and the infant may be at risk. The definition of prematurity was altered to distinguish between the preterm infant, born before the thirty-seventh week of gestation, and the small-for-gestational-age infant, who may be born at term but whose weight is more than 2 standard deviations below the mean. Although preterm delivery occurs in 8–10% of all births, it accounts for approximately 80% of early neonatal deaths. Severe complications, such as respiratory distress syndrome, intracranial hemorrhage, hypoglycemia, hypocalcemia, and hyperbilirubinemia, are prone to develop in preterm infants.Obstetricians frequently try to inhibit preterm labor to obtain time for fetal lung maturity. Delaying delivery by even 24–48 hours may be beneficial if glucocorticoids are administered to the mother to enhance fetal lung maturity. Various agents have been used to suppress uterine activity (tocolysis), such as ethanol, magnesium sulfate, prostaglandin inhibitors, β-sympathomimetics, and calcium channel blockers. β-adrenergic drugs, such as ritodrine and terbutaline, are the most commonly used tocolytics. Their predominant effect is β2 receptor stimulation, which results in myometrial inhibition, vasodilation, and bronchodilation. Numerous maternal complications, including hypotension, hypokalemia, hyperglycemia, myocardial ischemia, pulmonary edema, and death, have been reported as a result of these tocolytics.
Anesthesia Management
Complications may occur because of interactions with anes-thetic drugs and techniques. With the use of regional anesthesia, peripheral vasodilation caused by β-adrenergic stimulation increases the risk of hemodynamic instability in the presence of preexisting tachycardia, hypotension, and hypokalemia. The premature infant is known to be more vulnerable than the term newborn to the effects of drugs used in obstetric analgesia and anesthesia. However, there have been few systemic studies to determine the maternal and fetal pharmacokinetics and dynamics of drugs throughout gestation.
There are several postulated causes of enhanced drug sensi-tivity in the preterm newborn: less protein available for drug binding; higher levels of bilirubin, which may compete with the drug for protein binding; greater drug access to the central nervous system because of a poorly developed blood–brain barrier; greater total body water and lower fat content; and a decreased ability to metabolize and excrete drugs. However, most drugs used in anesthesia exhibit low to moderate degrees of binding in the fetal serum: approximately 50% for bupivacaine, 25% for lidocaine, 52% for meperidine, and 75% for thiopental.In selecting the anesthetic drugs and techniques for delivering a preterm infant, concerns regarding drug effects on the newborn are far less important than preventing asphyxia and trauma to the fetus. For vaginal delivery, well-conducted epidural anesthesia is advantageous in providing good perineal relaxation. Before induction of epidural block, the anesthesiologist should ascertain that the fetus is neither hypoxic nor acidotic. Asphyxia results in a redistribution of fetal cardiac output, which increases oxygen delivery to vital organs such as the brain, heart, and adrenals. Regardless, these changes in the preterm fetus may be better preserved with bupivacaine or chloroprocaine than with lidocaine. Preterm infants with breech presentation are usually delivered by cesarean section. Regional anesthesia can be success-fully used, with nitroglycerin available for uterine relaxation if needed.
NYSORA Tips
- When delivering a preterm infant, concerns about drug effects on the newborn are far less important than pre-venting asphyxia and trauma to the fetus.
- Before inducing epidural block, it should be ascer-tained that the fetus is neither hypoxic nor acidotic.
Regional analgesia during labor and vaginal delivery has become the preferred technique of pain relief in selected high-risk patients because it prevents obtundation of the mother and depression of the fetus and reduces many of the potential adverse physiologic effects of labor, such as increased oxygen consumption and hemodynamic alterations. For cesarean section delivery, regional anesthesia has emerged as a safe and effective technique in high-risk parturients, partly because of the added ability to provide prolonged postoperative analgesia.
NONOBSTETRIC SURGERY IN THE PREGNANT WOMAN
Approximately 1.6–2.2% of pregnant women undergo surgery for reasons unrelated to parturition. Apart from trauma, the most common emergencies are abdominal, intracranial aneu-rysms, cardiac valvular disease, and pheochromocytoma.
When the necessity for surgery arises, anesthetic considerations are related to the alterations in maternal physiologic condition with advancing pregnancy, the teratogenicity of anesthetic drugs, the indirect effects of anesthesia on uteroplacental blood flow, and the potential for abortion or premature delivery. The risks must be balanced to provide the most favorable outcome for mother and child. Five major studies have attempted to relate surgery and anesthesia during human pregnancy to fetal outcome as determined by anomalies, premature labor, or intrauterine death. Although these studies failed to correlate surgery and anesthetic exposure with congenital anomalies, all studies demonstrated an increased incidence of fetal death, particularly after operations performed in the first trimester. No particular anesthetic agent or technique was implicated. The condition that necessitated surgery was the most relevant factor, with fetal mortality greatest following pelvic surgery or procedures performed for obstetric indications; that is, cervical incompetence.
The cytotoxicity of anesthetic agents is closely associated with biodegradation, which, in turn, is influenced by oxygenation and hepatic blood flow. Thus, the complications associated with anesthesia—maternal hypoxia, hypotension, vasopressor administration, hypercarbia, hypocarbia, and electrolyte disturbances—may be greater factors in teratogenesis than the use of the agents themselves.
The experimental evidence on exposure to specific drugs and agents is discussed briefly, with the understanding that it is difficult to extrapolate laboratory data to the clinical situation in humans. Very large numbers of patients must be exposed to a suspected teratogen before its safety can be ascertained. Complicating factors include the frequency of maternal exposure to a multiplicity of drugs; the difficulty in separating the effects of the underlying disease process and surgical treatment from those of the drug administered; differing degrees of risk with stage of gestation; and the variety, rather than the consistency, of anomalies that appear in association with one agent. With regard to regional anesthetic agents, local anesthetics have not been shown to be teratogenic in animals or humans.
Caution has been exercised with sedatives before block placement because of several reports describing a specific rela-tionship between diazepam and oral clefts; however, other stud-ies have not confirmed this. A prospective study of 854 women who ingested diazepam during the first trimester did not demonstrate a higher risk of cleft palate or cleft lip. Currently, diazepam is not a proven teratogen.
Recently, there has been concern relating to adverse neuro-cognitive effects in children exposed to anesthetics during fetal life. Indeed, neonatal rats exposed to in utero general anesthesia had a greater degree of neuroadaptive deficits than a control group. Similarly, infants born under general anesthesia for cesarean delivery have also been found to display mild impairment of cognition compared to regional anesthesia. The difficulty with this study is that it was retrospective in nature, and the potential for patient selection bias existed. For instance, the use of general anesthesia is reserved for emergency situations, such as nonreassurring fetal status or placental abruption, which, in our opinion, would affect neurocognition more than the anesthetic. Most worrisome is that infants born via vaginal delivery had similar profiles as children born by cesarean delivery with general anesthesia. Nonetheless, it would seem prudent to avoid general anesthesia in preference for regional techniques during pregnancy because of the maternal and fetal considerations previously discussed. There are few data to guide medical decision making, but altering the type of surgical technique (eg, converting from laparoscopic to open appendectomy) should be entertained in order to facilitate the use of regional anesthesia. As maternal pain and apprehension may result in decreased uterine blood flow and deterioration of the fetus (similar to infusions of epinephrine or norepinephrine), early intervention to relieve postoperative pain with regional techniques (ie, peripheral nerve block or epidural infusion) should be considered.
NYSORA Tips
Local anesthetics have not been shown to be teratogenic in animals or humans.
SUMMARY
Pregnancy results in a number of significant physiologic changes that require adjustment in anesthesia and analgesia techniques for the safe and effective management of the preg-nant patient. It is prudent to delay surgery, when possible, until after the birth of the infant. Only emergency surgery should be considered during the first trimester.Regional techniques have become the most accepted for pain relief during labor and vaginal delivery. Likewise, neuraxial tech-niques are now the most frequently used techniques to administer anesthetics for cesarean section delivery. Advances in regional anes-thesia and its widespread routine use have resulted in significantly enhanced maternal safety compared with that of general anesthesia.
REFERENCES
- Melzack R, Taenzer P, Feldman P, Kinch RA: Labour is still painful after prepared childbirth training. Can Med Assoc J 1981;125:357.
- Goodman RP, Killom AP, Brash AR, Branch RA: Prostacyclin production during pregnancy: comparison of production during normal pregnancy and pregnancy complicated by hypertension. Am J Obstet Gynecol 1982; 142:817.
- Kerr MG, Scott DB, Samuel E: Studies of the inferior vena cava in late pregnancy. BMJ 1964;1:532.
- Howard BK, Goodson JH, Mengert WE: Supine hypotensive syndrome in late pregnancy. Obstet Gynecol 1953;1:371.
- Kuo CD, Chen GY, Yang MJ, Tsai YS: The effect of postion on autonomic nervous activity in late pregnancy. Anaesthesia 1997;52:1161–1165.
- Kundra P, Velraj J, Amirthalingam U, et al: Effect of positioning from supine and left lateral positions to left lateral tilt on maternal blood flow velocities and waveforms in full-term parturients. Anaesthesia 2012;67: 889–893.
- Carruth JE, Mivis SB, Brogan DR, Wenger NK: The electrocardiogram in normal pregnancy. Am Heart J 1981;102:1075–1078.
- Seth R, Moss AJ, McNitt S, et al: Long QT syndrome and pregnancy. J Am Coll Cardiol 2007;49:1092–1098.
- Prowse CM, Gaensler EA: Respiratory and acid-base changes during pregnancy. Anesthesiology 1965;26:381.
- Moya F, Smith BE: Uptake, distribution and placental transport of drugs and anesthetics. Anesthesiology 1965;26:465.
- Archer GW, Marx GF: Arterial oxygenation during apnoea in parturient women. Br J Anaesth 1974;46:358.
- Whitehead EM, Smith M, Dean Y, O’Sullivan G: An evaluation of gastric emptying times in pregnancy and the puerperium. Anaesthesia 1993;48:53–57.
- La Salvia LA, Steffen EA: Delayed gastric empthing time in labor. Am J Obstet Gynecol 1950;59:1075–1081.
- Wong CA, Loffredi M, Ganchiff JN, et al: Gastric emptying of water in term pregnancy. Anesthesiology 2002;96:1395–1400.
- Davison JS, Davison MC, Hay DM: Gastric emptying time in late pregnancy and labour. J Obstet Gynaecol Br Commonw 1970;77: 37–41.
- Brock-Utne JG, Dow TGB, Dimopoulos GE, et al: Gastric and lower oesophageal sphincter (LOS) pressures in early pregnancy. Br J Anaesth 1981;53:381.
- Wyner J, Cohen SE: Gastric volume in early pregnancy: Effect of metoclopramide. Anesthesiology 1982;57:209.
- Cohen SE, Woods WA, Wyner J: Antiemetic efficacy of droperidol and metoclopramide. Anesthesiology 1984;60:67.
- Scheller MS, Sears KL: Post-operative neurologic dysfunction associated with preoperative administration of metoclopramide. Anesth Analg 1987;66:274.
- Lund CJ, Donovan JC: Blood volume during pregnancy. Am J Obstet Gynecol 1967;98:393.
- Pritchard J, MacDonald P: Maternal adaptation to pregnancy. In Williams JW, Pritchard J, MacDonald P (eds), Williams Obstetrics, 16th ed. New York: Appleton-Century-Crofts, 1980, p. 236.
- Gerbasi FR, Buttoms S, Farag A, Mammen E: Increased intravascular coagulation associated with pregnancy. Obstet Gynecol 1990;75:385–389.
- Wildsmith JAW: Serum pseudocholinesterase, pregnancy and suxamethonium. Anaesthesia 1972;27:90.
- Coryell MN, Beach EF, Robinson AR, et al: Metabolism of women during the reproductive cycle: XVII. Changes in electrophoretic patterns of plasma proteins throughout the cycle and following delivery. J Clin Invest 1950;29:1559.
- Datta S, Kitzmiller JL, Naulty JS, et al: Acid-base status of diabetic mothers and their infants following spinal anesthesia for cesarean section. Anesth Analg 1982;61:662.
- Datta S, Lambert DH, Gregus J, et al: Differential sensitivities of mammalian nerve fibers during pregnancy. Anesth Analg 1983;62:1070.
- Gin T, Chan MTV: Decreased minimum alveolar concentration of isoflurane in pregnant humans. Anesthesiology 1994;81:829.
- Brown WU, Bell GC, Alper MH: Acidosis, local anesthetics and the newborn. Obstet Gynecol 1976;48:27.
- Hamshaw-Thomas A, Rogerson N, Reynolds F: Transfer of bupivacaine, lignocaine and pethidine across the rabbit placenta: Influence of maternal protein binding and fetal flow. Placenta 1984;5:61.
- Kennedy RL, Miller RP, Bell JU, et al: Uptake and distribution of bupivacaine in fetal lambs. Anesthesiology 1986;65:247.
- Kuhnert PM, Kuhnert BR, Stitts JM, Gross TL: The use of a selected ion monitoring technique to study the disposition of bupivacaine in mother, fetus and neonate following epidural anesthesia for cesarean section. Anesthesiology 1981;55:611.
- Kuhnert BR, Philipson EH, Pimental R, et al: Lidocaine disposition in mother, fetus, and neonate after spinal anesthesia. Anesth Analg 1986;65:139.
- Morishima HO, Daniel SS, Finster M, et al: Transmission of mepivacaine hydrochloride (Carbocaine) across the human placenta. Anesthesiology 1966;27:147.
- Kuhnert BR, Kuhnert PM, Prochaska AL, Gross TL: Plasma levels of 2-chloroprocaine in obstetric patients and their neonates after epidural anesthesia. Anesthesiology 1980;53:21.
- Pihlajamaki K, Kanto J, Lindberg R, et al: Extradural administration of bupivacaine: pharmacokinetics and metabolism in pregnant and non-pregnant women. Br J Anaesth 1990;64:556.
- Morishima HO, Covino BG: Toxicity and distribution of lidocaine in nonasphyxiated and asphyxiated baboon fetuses. Anesthesiology 1981;54:182.
- Morishima HO, Finster M, Pedersen H, et al: Pharmacokinetics of lidocaine in fetal and neonatal lambs and adult sheep. Anesthesiology 1979;50:431.
- Mihaly GW, Moore RG, Thomas J, et al: The pharmacokinetics and metabolism of the anilide local anaesthetics in neonates. Eur J Clin Pharmacol 1978;13:143.
- Finster M, Poppers PJ, Sinclair JC, et al: Accidental intoxication of the fetus with local anesthetic drug during caudal anesthesia. Am J Obstet Gynecol 1965;92:922.
- Morishima HO, Pedersen H, Finster M, et al: Toxicity of lidocaine in adult, newborn and fetal sheep. Anesthesiology 1981;55:57.
- Gale R, Ferguson JE II, Stevenson D: Effect of epidural analgesia with bupivacaine hydrochloride on neonatal bilirubin production. Obstet Gynecol 1987;70:692.
- Brockhurst NJ, Littleford JA, Halpern SH: The neurological and adaptive capacity score: a systematic review of its use in obstetric anesthesia research. Anesthesiology 2000;92:237.
- Morishima HO, Yeh M-N, James LS: Reduced uterine blood flow and fetal hypoxemia with acute maternal stress: experimental observation in the pregnant baboon. Am J Obstet Gynecol 1979;134:270.
- Ueland K, Hansen JM: Maternal cardiovascular dynamics: Ill. Labor and delivery under local and caudal analgesia. Am J Obstet Gynecol 1969; 103:8.
- Moir DD, Willocks J: Management of incoordinate uterine action under continuous epidural analgesia. BMJ 1967;2:396.
- Miller FC, Petrie RH, Arce JJ, et al: Hyperventilation during labor. Am J Obstet Gynecol 1974;120:489.
- Beaule PE, Smith MI, Nguyen VN: Meperidine-induced seizure after revision hip arthroplasty. J Arthroplasty 2005;19:516–519.
- Hagmeyer KO, Mauro LS, Mauro VF: Meperidine-related seizures associated with patient-controlled analgesia pumps. Ann Pharmacother 1993;27:29–32.
- Kaiko RF, Grabinski PY, Heidrick G, et al: Central nervous system excitatory effects of meperidine in cancer patients. Ann Neurol 1983;13: 180–185.
- Kuhnert BR, Linn PL, Kennard MJ, Kuhnert PM: Effect of low doses of meperidine on neonatal behavior. Anesth Analg 1985;64:335.
- Sosa CG, Balagueer E, Alonso JG, et al: Meperidine for dystocia during the first stage of labor: a randomized controlled trial. Am J Obstet Gynecol 2004;191:1212–1218.
- Eisele JH, Wright R, Rogge P: Newborn and maternal fentanyl levels at cesarean section. Anesth Analg 1982;61:179.
- Shannon KT, Ramanathan S: Systemic medication for labor analgesia. Obstet Pain Manage 1995;2:1–6.
- Morley-Foster PK, Reid DW, Vandeberghe H: A comparison of patient-controlled analgesia fentanyl and alfentanil for labour analgesia. Can J Anaesth 2000;47:113–119.
- Muir HA, Breen T, Campbell DC, et al: Is intravenous PCA fentanyl an effective method for providing labor analgesia? Anesthesiology 1999; (Suppl):A28.
- Vercauteren M, Bettens K, Van Springel G, et al: Intrathecal labor analgesia: Can we use the same mixture as is used epidurally? Int J Obstet Anesth 1997;6:242–246.
- Breen TW, Giesinger Cm, Halpern SH: Comparison of epidural lidocaine and fentanyl to intrathecal sufentanil for analgesia in early labor. Int J Obstet Anesth 1999;8:226–230.
- Kapila A, Glass PS, Jacobs JR, et al: Measured context-sensitive half-times of remifentanil and alfentanil. Anesthesiology 1995;83:968–975.
- Evron S, Glezerman M, Sadan O, et al: Remifentanil: a novel systemic analgesic for labor pain. Anesth Analg 2005;100:233–238.
- Kan RE, Hughes SC, Rosen M, et al: Intravenous remifentanil: placental transfer, maternal and neonatal effects. Anesthesiology 1998;88:1467.
- Thurlow JA, Laxton CH, Dick A, et al: Remifentanil by patient-controlled analgesia compared with intramuscular meperidine for pain relief in labor. Br J Anaesth 2002;88:374–378.
- Volmanen P, Sarvela J, Akural EI, et al: Intravenous remifentanil vs. epidural levobupivacaine with fentanyl for pain relief in early labour: a randomized, controlled, double-blinded study. Acta Anaesthesiol Scand 2008;52:249–255.
- Volmanen P, Akural E, Raudaskoski T, et al: Comparison of remifentanil and nitrous oxide in labour analgesia. Acta Anaesthesiol Scand 2005;49: 453–458.
- Maduska AL, Hajghassemali M: A double blind comparison of butorphanol and meperidine in labor: Maternal pain relief and effect on newborn. Can Anaesth Soc J 1978;25:398.
- Wilson CM, McClean E, Moore J, Dundee JW: A double-blind comparison of intramuscular pethidine and nalbuphine in labour. Anaesthesia 1986;41:1207–1213.
- Hodgkinson R, Huff RW, Hayashi RH, Husain FJ: Double-blind comparison of maternal analgesia and newonatal neurobehaviour following intravenous butorphanol and meperidine. J Int Med Res 1979;7:224–230.
- Thorp JA, Hu DH, Albin RM, et al: The effect of intrapartum epidural analgesia on nulliparous labor: A randomized, controlled, prospective trial. Am J Obstet Gynecol 1993;169:851.
- Sharma SK, Sidawi JE, Ramin SM, et al: Cesarean delivery: A randomized trial of epidural versus patient controlled meperidine analgesia during labor. Anesthesiology 1997;87:487.
- Halpern SH, Leighton BL, Ohlsson A, et al: Effect of epidural vs parenteral opioid analgesia in the progress of labor: A meta-analysis. JAMA 1998;280:2105.
- Ramin SM, Gambling DR, Lucas MJ, et al: Randomized trial of epidural versus intravenous analgesia in labor. Obstet Gynecol 1995;86:783.
- Chestnut DH, Vincent RD, McGrath JM, et al: Does early administration of epidural analgesia affect obstetric outcome in nulliparous women who are receiving intravenous oxytocin? Anesthesiology 1994;80:1193.
- Chestnut DH, McGrath JM, Vincent RD, et al: Does early administration of epidural analgesia affect obstetric outcome in nulliparous women who are in spontaneous labor? Anesthesiology 1994;80:1201.
- American College of Obstetrics and Gynecology: Obstetric forceps. AGOG Committee on Obstetrics Maternal and Fetal Medicine, Committee Opinion, 1989.
- Chestnut DH, Laszewski LJ, Pollack RL, et al: Continuous epidural infusion of 0.0625% bupivacaine-0.0002% fentanyl during the second stage of labor. Anesthesiology 1990;72:613.
- Birnbach DJ, Chestnut DH: The epidural test dose in obstetric practice: Has it outlived its usefulness? Anesth Analg 1999;88:971.
- Norris MC, Ferrenbach D, Dalman H, et al: Does epinephrine improve the diagnostic accuracy of aspiration during labor epidural analgesia? Anesth Analg 1999;88:1073.
- Guinard JP, Mulroy MF, Carpenter RL, Knopes KD. Test doses: optimal epinephrine content with and without acute beta-adrenergic block. Anesthesiology 1990;73:386–392.
- Visconti C, Eisenach JC: Patient-controlled epidural analgesia during labor. Obstet Gynecol 1991;77:348.
- Halpern S: Recent advances in patient-controlled epidural analgesia for labour. Curr Opin Anaesthesiol 2005;18:247–251.
- Minty RG, Kelly L, Minty A, Hammett DC: Single-dose intrathecal analgesia to control labour pain: is it a useful alternative to epidural analgesia? Can Fam Physician 2007;53:437–442.
- Rigler ML, Drasner K, Krejcie TC, et al: Cauda equina syndrome after continuous spinal anesthesia. Anesth Analg 1991;72:275.
- Arkoosh VA, Palmer CM, Van Maren GA, et al: Continuous intrathecal labor analgesia: safety and efficacy. Anesthesiology 1998;(Suppl):A8.
- Simons SW, Cyna AM, Dennis AT, Hughes D: Combined spinal-epidural versus epidural analgesia in labour. Cochrane Database Syst Rev 2007;3:CD003401.
- Campbell DC, Camann WR, Datta S: The addition of bupivacaine to intrathecal sufentanil for labor analgesia. Anesth Analg 1995;81:305.
- Labbene I, Gharsallah H, Abderrahaman A, et al: Effects of 15 mcg intrathecal clonidine added to bupivacaine and sufentanil for labor analgesia. Tunis Med 2011;89:853–859.
- Collis RE, Davies DWL, Aveling W: Randomized comparison of combined spinal epidural and standard epidural analgesia in labour. Lancet 1995;345:1413.
- McLeod A, Fernando R, Page F, et al: An assessment of maternal balance and gait using computerized posturography. Anesthesiology 1999; (Suppl):A8.
- Wong CA, Scavone BM, Peaceman AM, et al: The risk of cesarean delivery with neuraxial analgesia given early versus late labor. N Engl J Med 2005;352:655.
- Cohen SE, Cherry CM, Holbrook RH, et al: Intrathecal sufentanil for labor analgesia: sensory changes, side-effects and fetal heart rate changes. Anesth Analg 1993;77:1155.
- Clarke VT, Smiley RM, Finster M: Uterine hyperactivity after intrathecal injection of fentanyl for analgesia during labor: A cause of fetal bradycardia? Anesthesiology 1994;81:1083.
- O’Gorman DA, Birnbach DJ, Kuczkowski KM, et al: Use of umbilical flow velocimetry in the assessment of the pathogenesis of fetal bradycardia following combined spinal epidural analgesia in parturients. Anesthesiology 2000;(Suppl):A2.
- Riley ET, Vogel TM, EI-Sayed YY, et al: Patient selection bias contributes to an increased incidence of fetal bradycardia after combined spinal epidural analgesia for labor. Anesthesiology 1999;91:A1054.
- Nielson PE, Erickson R, Abouleish E, et al: Fetal heart rate changes after intrathecal sufentanil or epidural bupivacaine for labor analgesia: incidence and clinical significance. Anesth Analg 1996;83:742.
- Albright GA, Forester RM: Does combined epidural analgesia with subarachnoid sufentanil increase the incidence of emergency cesarean section? Reg Anesth 1997;22:400.
- Norris MC, Grieco WM, Borkowski M, et al: Complications of labor analgesia: epidural versus combined spinal epidural techniques. Anesth Analg 1995;79:529.
- Leighton BL, Arkoosh VA, Huffnagle S, et al: The dermatomal spread of epidural bupivacaine with and without prior intrathecal sufentanil. Anesth Analg 1996;83:526.
- Bucklin BA, Hawkins JL, Anderson JR, Ullrich FA: Obstetric anesthesia workforce survey: twenty-year update. Anesthesiology 2005;103:645–653.
- Baxi LV, Petrie RH, James LS: Human fetal oxygenation following paracervical block. Am J Obstet Gynecol 1979;135:1109.
- Palomaki O, Huhtala H, Kirkinen P: A comparative study of the safety of 0.25% levobupivacaine and 0.25% racemic bupivacaine for paracervical block in the first stage of labor. Acta Obstet Gynecol Scand 2005;84: 956–961.
- Leighton BL, Halpern SH, Wilson DB: Lumbar sympathetic blocks speed early and second stage induced labor in nulliparous women. Anesthesiology 1999;90:1039–1046.
- Pace MC, Aurilio C, Bulletti C, et al: Subarachnoid analgesia in advanced labor: a comparison of subarachnoid analgesia and pudendal block in advanced labor. Analgesic quality and obstetric outcome. Ann NY Acad Sci 2004;1034:356–363.
- Aissaoui Y, Bruyere R, Mustapha H, et al: A randomized controlled trial of pudendal nerve block for pain relief after episiotomy. Anesth Analg 2008;107:625–629.
- Berghella V, Baxter JK, Chauhan SP: Evidence-based surgery for cesarean delivery. Am J Obstet Gynecol 2005;193:1607–1617.
- Hawkins JL, Gibbs CP, Orleans M, et al: Obstetric anesthesia workforce survey 1992 vs 1981. Anesthesiology 1994;81:A1128.
- Shnider SM, Levinson G: Anesthesia for cesarean section. In Shnider SM, Levinson G (eds): Anesthesia for Obstetrics, 2nd ed. Baltimore: Williams & Wilkins, 1987, p. 159.
- Dyer RA, Farina Z, Joubert IA, et al: Crystalloid preload versus rapid crystalloid administration after induction of spinal anesthesia for elective caesarean section. Anaesth Intensive Care 2004;32:35–35-7.
- Marx GF, Luykx WM, Cohen S: Fetal-neonatal status following cesarean section for fetal distress. Br J Anaesth 1984;56:1009.
- Norris MC: Height, weight and the spread of subarachnoid hyperbaric bupivacaine in the term parturient. Anesth Analg 1988;67:555.
- Hartwell BL, Aglio LS, Hauch MA, et al: Vertebral column length and spread of hyperbaric subarachnoid bupivacaine in the term parturient. Reg Anesth 1991;16:17–19.
- Ogun CO, Kirgiz EN, Duman A, et al: Comparison of intrathecal isobaric bupivacaine-morphine and ropivacaine-morphine for Caesarean delivery. Br J Anaesth 2003;90:659–664.
- Hunt GO, Naulty S, Bader AM, et al: Perioperative analgesia with subarachnoid fentanyl-bupivacaine for cesarean delivery. Anesthesiology 1989;71:535.
- Palmer CM, Emerson S, Volgoropolous D, et al: Dose-response relationship of intrathecal morphine for postcesarean analgesia. Anesthesiology 1999;90:437–444.
- Kato R, Shimamoto H, Terui K, et al: Delayed respiratory depression associated with 0.15 mg intrathecal morphine for cesarean section. A review of 1915 cases. J Anesth 2008;22:112–116.
- Roelants F: The use of neuraxial adjuvant drugs (neostigmine, clonidine) in obstetrics. Curr Opin Anaesthesiol 2006;19:233–237.
- Ngan Kee WD, Khaw KS, Lee BB, et al: The limitations of ropivacaine with epinephrine as an epidural test dose in parturients. Anesth Analg 2001;92:1529–1531.
- Leighton BL, Norris MC, Sosis M, et al: Limitations of epinephrine as a marker of intravascular injection in laboring women. Anesthesiology 1987;66:688.
- Gissen AJ, Datta S, Lambert D: The chloroprocaine controversy: is chloroprocaine neurotoxic? Reg Anaesth 1984;9:135.
- Hynson JM, Sessler DI, Glosten B: Back pain in volunteers after epidural anesthesia with chloroprocaine. Anesth Analg 1991;72:253.
- Albright GA: Cardiac arrest following regional anesthesia with etidocaine or bupivacaine. Anesthesiology 1979;51:285.
- Carvalho B, Riley E, Cohen SE, et al: Single-dose, sustained-release epidural morphine in the management of postoperative pain after elective cesarean delivery: results of a multicenter randomized controlled study. Anesth Analg 2005;100:1150–1158.
- Carvalho B, Roland LM, Chu FL, et al: Single-dose, extended-release epidural morphine (DepoDur) compared to conventional epidural morphine for post-cesarean pain. Anesth Analg 2007;105:176–183.
- Ginsar Y, Riley ET, Anst MS: The site of action of epidural fentanyl in humans: the difference between infusion and bolus administration. Anesth Analg 2003;97:1428–1438.
- Marik PE: Aspiration pneumonitis and aspiration pneumonia. N Engl J Med 2001;344:665–671.
- Brizgys RV, Dailey PA, Shnider SM, et al: The incidence and neonatal effects of maternal hypotension during epidural anesthesia for cesarean section. Anesthesiology 1987;67:782.
- Ueyama H, He YL, Tanigami H, et al: Effects of crystalloid and colloid preload or blood volume in the parturient undergoing spinal anesthesia for elective cesarean section. Anesthesiology 1999;91:1571.
- Rout CC, Roche DA: Spinal hypotension associated with cesarean section: will preload ever work? Anesthesiology 1999;91:1565.
- Ngan Kee WD, Kaw KS, Ng FF: Prevention of hypotension during spinal anesthesia for cesarean delivery: an effective technique using combination of phenylephrine infusion and crystalloid cohydration. Anesthesiology 2005;103:744–750.
- Ramanathan S, Grant GJ: Vasopressor therapy for hypotension due to epidural anaesthesia. Acta Anaesthesiol Scand 1988;32:559.
- Rosenblatt MA, Abel M, Fischer GW, et al: Successful use of a 20% lipid emulsion to resuscitate a patient after a presumed bupivacaine-related cardiac arrest. Anesthesiology 2006;105:217–218.
- Kasten GW, Martin ST: Resuscitation from bupivacaine-induced cardiovascular toxicity during partial inferior vena cava occlusion. Anesth Analg 1986;65:341.
- Choi PT, Galinski SE, Takeuchi L, et al: PDPH is a common complication of neuraxial block in parturients: a meta-analysis of obstetrical studies. Can J Anaesth 2003;50:460–469.
- Russell IF: A prospective controlled study of continuous spinal analgesia versus repeat epidural analgesia after accidental dural puncture in labour. Int J Obstet Anesth 2012;21:7–16.
- Maynard SE, Min J-Y, Merchan J, et al: Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111: 649–658.
- Tada S, Yasue A, Nishizawa H, Sekiya T, Hirota Y, Udagawa Y: Spontaneous spinal epidural hematoma during pregnancy: three case reports. J Obstet Gynaecol Res 2011;37:1734–1738.
- Bodurka D: What’s new in gynecology and obstetrics. J Am Coll Surg 2005;201:265–274.
- Basso O, Rasmussen S, Weinberg CR, et al: Trends in fetal and infant survival following preeclampsia. JAMA 2006;296:1357–1362.
- Luttun A, Carmeliet P: Soluble VEGF receptor Flt1: the elusive preeclampsia factor discovered? J Clin Invest 2003;111:60–62.
- Keogh RJ, Harris LK, Freeman A, et al: Fetal-derived trophoblast use the apoptotic cytokine tumor necrosis factor-alpha-related apoptosis-inducing ligand to induce smooth muscle cell death. Circ Res 2007;100:834–841.
- Walsh S: Preeclampsia: An imbalance in placental prostacyclin and thromboxane production. Am J Obstet Gynecol 1985;152:335.
- Roberts J, Taylor R, Musci T, et al: Preeclampsia: An endothelial cell disorder. Am J Obstet Gynecol 1989;152:1200.
- Venkatesha S, Toporsian M, Lam C, et al: Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 2006;12:642.
- Levine RJ, Karumanchi SA: Circulating angiogenic factors in preeclampsia. Clin Obstet Gynecol 2005;48:372–386.
- Chesley L: Plasma and red cell volumes during pregnancy. Am J Obstet Gynecol 1972:112:440.
- Rodgers R, Levin J: A critical reappraisal of the bleeding time. Semin Thromb Hemost 1990:16:1–20.
- Abalos E, Duley L, Steyn DW, Henderson-Smart DJ: Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev 207;1:CD002252.
- Groenendijk R, Trimbos M, Wallenburg H: Hemodynamic measurements in preeclampsia: preliminary observations. Am J Obstet Gynecol 1984; 150:232.
- Aya AG, Vialles N, Tanoubi I, et al: Spinal anesthesia-induced hypotension: A risk comparison between patients with severe preeclampsia and healthy women undergoing preterm cesarean delivery. Anesth Analg 2005;101: 869–875.
- Cotton D, Gonik B, Dorman K, Harris R: Cardiovascular alterations in severe pregnancy-induced hypertension: Relationship of central venous pressure to pulmonary capillary wedge pressure. Am J Obstet Gynecol 1985;151:762.
- Hogg B, Hauth J, Caritis S, et al: Safety of labor epidural anesthesia for women with severe hypertensive disease. Am J Obstet Gynecol 1999;181: 1099.
- Newsome L, Bramwell R, Curling P: Hemodynamic effects of lumbar epidural anesthesia. Anesth Analg 1986;65:31.
- Jouppila P, Jouppila R, Hollmen A, Koivula A: Lumbar epidural analgesia to improve intervillous blood flow during labor in severe preeclampsia. Obstet Gynecol 1982;52:158.
- Ramanathan J, Botorff M, Jeter J, et al: The pharmacokinetics and maternal and neonatal effects of epidural lidocaine in preeclampsia. Anesth Analg 1986;65:120.
- Wallace D, Leveno KJ, Cunningham F, et al: Randomized comparison of general and regional anesthesia for cesarean delivery in pregnancies complicated by severe preeclampsia. Obstet Gynecol 1995;86:193.
- Hood D, Curry R: Spinal versus epidural anesthesia for cesarean section in severely preeclamptic patients: a retrospective survey. Anesthesiology 1999;90:1276.
- Oyelese Y, Smulian JC: Placenta previa, placenta accrete, and vasa previa. Obstet Gynecol 2006;107:927–941.
- Chestnut DH, Dewan D, Redick L, et al: Anesthetic management for obstetric hysterectomy: a multi-institutional study. Anesthesiology 1989;70:607.
- Vincent RD Jr, Chestnut DH, Sipes SL, et al: Epidural anesthesia worsens uterine blood flow and fetal oxygenation during hemorrhage in gravid ewes. Anesthesiology 1992;76:799–806.
- Santos A, Tun E, Bobby P, et al: The effects of bupivacaine, l-nitro-l-arginine-methyl-ester and phenylephrine on cardiovascular adaptations to asphyxia in the preterm fetal lamb. Anesth Analg 1997;84:1299.
- Morishima HO, Pedersen H, Santos AS, et al: Adverse effects of maternally administered lidocaine on the asphyxiated preterm fetal lamb. Anesthesiology 1989;71:110.
- Shnider SM, Webster G: Maternal and fetal hazards of surgery during pregnancy. Am J Obstet Gynecol 1965;92:891.
- Brodsky J, Cohen E, Brown BJ, et al: Surgery during pregnancy and fetal outcome. Am J Obstet Gynecol 1980;138:1165.
- Smith B: Fetal prognosis after anesthesia during gestation. Anesth Analg 1963;42:521.
- Duncan P, Pope W, Cohen M, Greer N: Fetal risk of anesthesia and surgery during pregnancy. Anesthesiology 1986;64:790.
- Heinonen O, Slone O, Shapiro S: Birth defects and drugs in pregnancy. In Birth Defects and Drugs in Pregnancy. Littleton, MA: Publishing Sciences Group, 1977, p. 516.
- Grabowski C, Paar J: The teratogenic effects of graded doses of hypoxia on the chick embryo. Am J Anat 1958;103:313.
- Saxen I, Saxen L: Association between maternal intake of diazepam and oral clefts. Lancet 1975;2:498.
- Safra M, Oakley G: Association between cleft lip with or without cleft palate and prenatal exposure to diazepam. Lancet 1975;2:478.
- Shiono PH, Mills JL: Oral clefts and diazepam use during pregnancy. N Engl J Med 1984;311:919–920.
- Shepard TH: Catalog of Teratogenic Agents, 7th ed. Baltimore: Johns Hopkins University Press, 1992.
- Palanisamy A, Baxter MG, Keel PK, Xie Z, Crosby G, Culley DJ: Rats exposed to isoflurane in utero during early gestation are behaviorally abnormal as adults. Anesthesiology 2011;114:521–528.
- Sprung J, Fleich RP, Wilder RT, et al: Anesthesia for cesarean delivery and learning disability in a population based birth cohort. Anesthesiology 2009;111:302–310.
- Adamsons K, Mueller-Heubach E, Myers R: Production of fetal asphyxia in the rhesus monkey by administration of catecholamines to the mother. Am J Obstet Gynecol 1971;109:148.