For much of the past decade, fluoroscopy held sway as the favorite imaging tool of many practitioners performing interventional pain procedures. Quite recently, ultrasound has emerged as a “challenger” to this well-established modality. The growing popularity of ultrasound application in regional anesthesia and pain medicine reflects a shift in contemporary views about imaging for nerve localization and target-specific injections. For regional anesthesia, ultrasound has already made a marked impact by transforming antiquated clinical practice into a modern science. No bedside tool ever before has allowed practitioners to visualize needle advancement in real time and observe local anesthetic spread around nerve structures. For interventional pain procedures, this radiation-free, point-of-care technology will also find its unique role and utility in pain medicine and can complement some of the imaging demands not met by fluoroscopy, computed tomography, and magnetic resonance imaging. And over time, practitioners will discover new benefits of this technology, especially for dynamic assessment of musculoskeletal pain conditions and improving accuracy of needle injection for small nerves, soft tissue tendons, and joints.
1. INTRODUCTION
Interventional pain procedures are commonly performed either with image-guidance fluoroscopy, computed tomography (CT), or ultrasound (US) or without image guidance utilizing surface landmarks. Recently, three-dimensional rotational angiography (3D-RA) suites also known as flat detector computed tomography (FDCT) or cone beam CT (CBCT) and digital subtraction angiography (DSA) have been introduced as imaging adjuncts. These systems are indicative of a trend toward increased use of specialized visualization techniques. Pain medicine practice guidelines suggest that most procedures require image guidance to improve the accuracy, reproducibility (precision), safety, and diagnostic information derived from the procedure [1]. Historically, pain medicine practitioners were slow adopters of image-guidance techniques, largely because the most common parent specialty (anesthesiology) had a culture of using surface landmarks to aid the perioperative performance of various nerve blocks and vascular line placements [2]. Indeed, some pain medicine practitioners in the 1980s and early 1990s felt that studies advocating the inaccuracy of epidural steroid injections performed with surface landmarks [3] were published more for specialty access than to increase patient safety or improve outcomes.
Ultrasound has recently exploded in popularity for perioperative regional block, but utilization of other imaging modalities in the perioperative arena, e.g., fluoroscopy, has lagged behind, despite more accurate placements compared to surface landmark-driven placements [2]. Technology acquisition costs and the physician learning required to master the new technologies are significant barriers to full implementation of many advanced imaging systems. However, the increasing national focus on safety in clinical medicine may ultimately mandate the use of optimal image guidance for selected procedures. In most cases, studies are lacking to compare the various types of image guidance in terms of patient outcomes, safety, and cost value for specific procedures. This is further complicated by the fact that many procedures in pain medicine have been considered poorly validated for the conditions being treated [4–6]. Thus, it may not matter if a particular image-guidance technique improves the reliability of a given procedure, if that procedure ultimately loses favor due to poor evidence or lack of evidence. Whether high-technology imaging brings safety and/or cost savings to the performance of evidence-based pain procedures is, thus, of paramount importance. The risks of the image guidance must also be considered as part of any imaging technology that is felt to be necessary for routine use. For example, a risk/benefit ratio of CT scanning relative to an equally suitable alternative technique may force physicians to use the lesser technology in some cases. CT as a diagnostic tool has come under greater scrutiny with the recent publication of several trials depicting the meteoric rise in the annual performance of CT scans (now over 72 million per year) and the large doses of radiation received by adults and particularly children [7]. Cancer risk from CT radiation has been modeled after longitudinal studies of cancer occurrences in atomic bomb survivors [8]. Now, it seems that the risk of cancer is something that should be more actively considered when CT is utilized. Radiation risks are not trivial and likely amount to about 14,000 or more future cancer deaths as a consequence of year 2007 CT scans [7]. For those treating patients with chronic pain, one needs to merely consider how many patients with an elusive diagnosis receive advanced imaging in efforts to find the cause of that pain. Thus, repeating imaging studies with a fairly low yield may actually be harming our patients. Ultrasound guidance, the focus of this atlas, has many advocates for these same radiation safety issues [9]. The use of ultrasound, however, is limited in many obese or larger adults [10], and the cost of some advanced systems capable of rendering deeper structures with high clarity can surpass the cost for fluoroscopes in some cases. The use of imaging modalities such as 3D-RA and DSA is being advocated by others. While a FDCT suite is extremely expensive, DSA is actually a relatively inexpensive add-on to a conventional fluoroscope that may have a substantial role in the safe performance of transforaminal epidural steroid injections [11]. For example, when performing injections or other procedures in critical areas, such as the left T11 and T12, the territory of the great segmental medullary artery of Adamkiewicz, digital subtraction can demonstrate vascular uptake more clearly (Fig. 1). Ultimately, further study will be necessary to ascertain the most safe, accurate, and cost-effective practices for image-guided procedures.
2. C-ARM FDCT
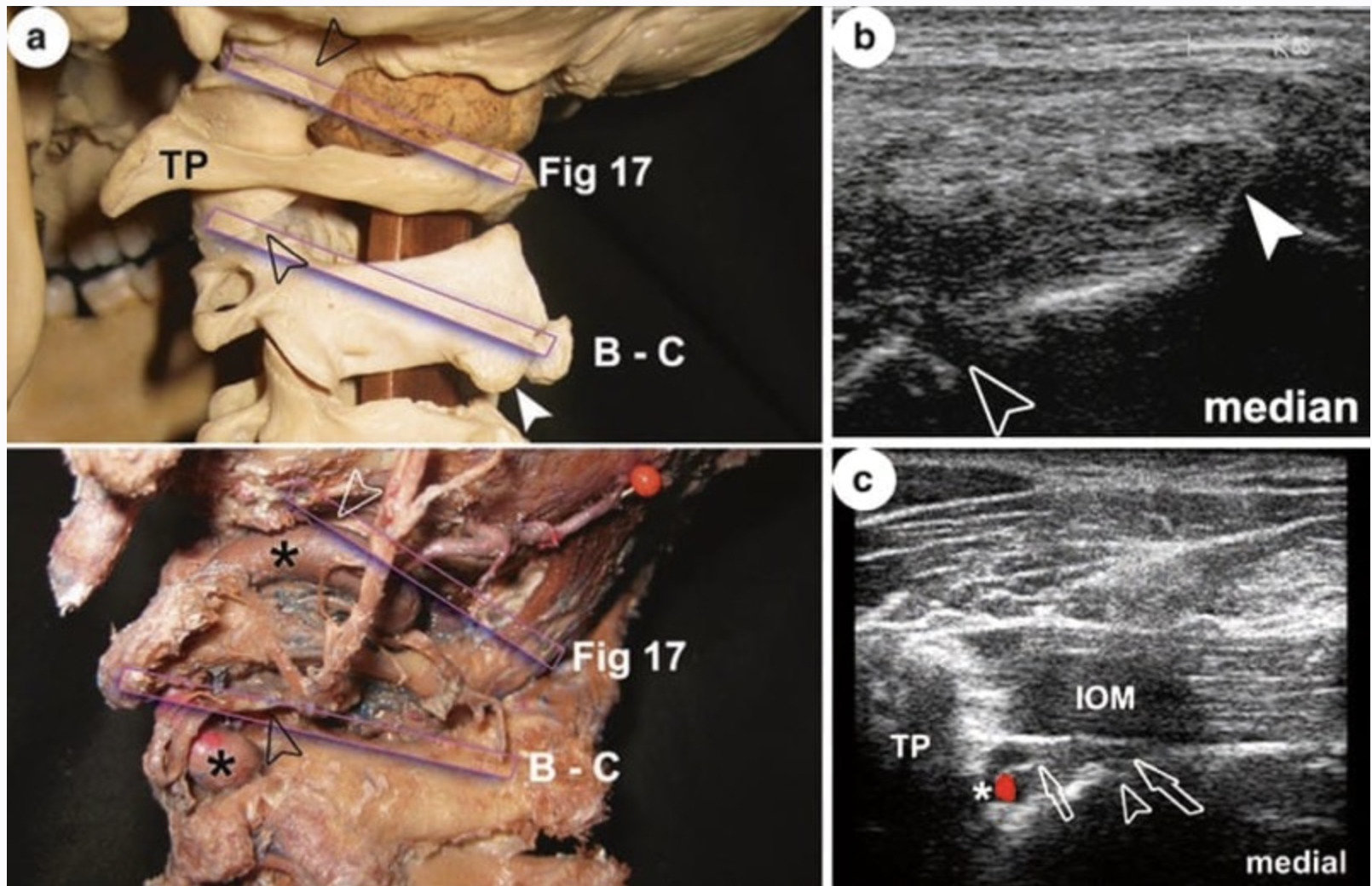
Most pain procedures require cross-sectional or 3D soft tissue imaging to accurately target structures in a complex anatomical landscape. Very few procedures are intended to target bony structures, with the exception of such procedures as vertebral and sacral augmentation, bone biopsies, and a few others. Yet, fluoroscopy remains the most popular imaging method, for primarily soft tissue targets, despite its limitations. Intradiscal procedures, vertebral augmentation, neuromodulation procedures, and deep abdominopelvic and head and neck blocks may be examples of some procedures where a limited CT scan capability (FDCT) would enhance the accuracy and safety of the procedure as compared to plain fluoroscopy. C-arm FDCT and C-arm CBCT utilize different gantries but are nearly synonymous terms for a modern 3D imaging system that can also integrate 2D data from fluoroscopy, sometimes US, and DSA in a single suite. Interventional radiologists and some pain physicians are using these advanced image-guidance systems to aid procedural performance in certain cases, with an expanding list of potential indications. FDCT is accomplished via a single rotation of the fluoroscope gantry, rendering a complete volumetric data set using a flat panel detector. These flat panel detectors have significantly better resolution than older image intensifiers. This is in contrast to conventional CT which uses multiple detectors and requires several rotations of the gantry, with the patient being moved into the CT scanner [12]. With FDCT, the patient is stationary through the imaging cycle. CT images do take approximately 5–20 s to be acquired; thus this is not a true real-time CT fluoroscopy procedure. Images from FDCT scanning have lower resolution due to scattered radiation, but in many cases the lower resolution images are more than adequate for the intended procedure. However, during the 200° gantry rotation of a FDCT system, experiments have shown that radiation doses are less than that for a single helical CT [12]. Carefully limiting the field of scanning will decrease radiation dose to the patient and improve image contrast. CBCT units may have significant application for intraoperative minimally invasive surgical applications. Surgeons using CBCT for minimally invasive spine procedures tended to want to utilize the higher technology of the CBCT in their cases in an escalating fashion with increasing exposure to the new technology [13].
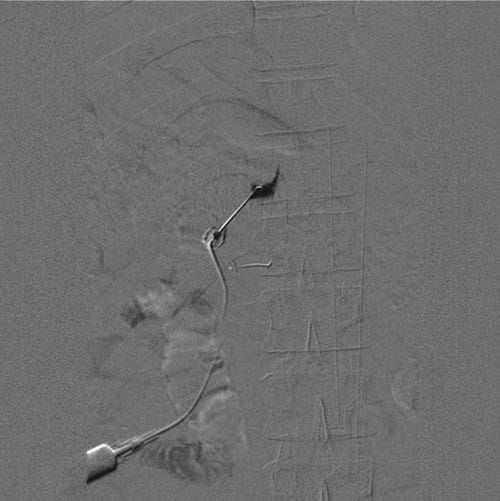
Fig.1 A digital subtraction image of a thoracic dorsal root ganglion contrast injection at T11 prior to pulsed radiofrequency. Note that the contrast spreads medial to the pedicle. Below, a second needle has been placed at the pedicle of T12 just inferior to the sagittal bisector.
Many creative interventionalists are adapting the FDCT capability to new procedures, such as diskography, without the need for a postprocedural standard CT (Figs. 2 and 3). In diskography, it is usual and customary to perform contrast injections into the presumed diseased disk as well as a control disk. A postprocedural delayed CT image to better quantitate annular tears and contrast leak into the spinal canal is considered standard. CBCT technology may allow these CT images to be performed in the same suite, saving time and expense. This “single-suite” concept for specific blocks can also save on radiation exposure for both the patient and the physicians.
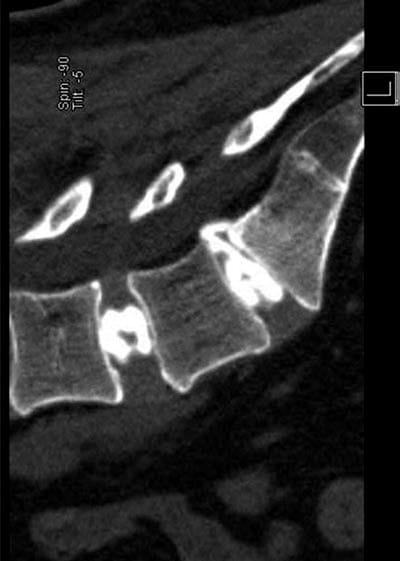
Fig. 2 A sagittal CT view of a two-level diskogram. Note an annular tear at L5/S1 with epidural extravasation.
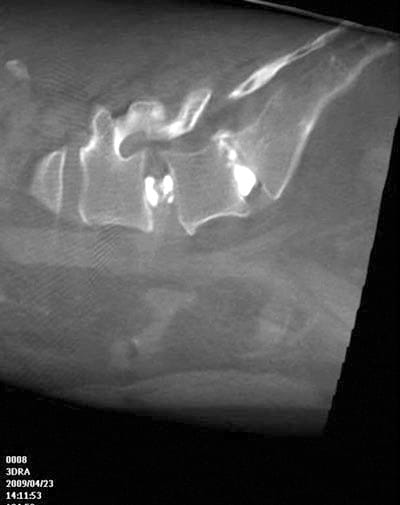
Fig. 3 Compare similar FDCT/3D-RA sagittal diskogram in the same patient as above. The epidural extravasation is seen again.
Deep plexus blocks such as celiac or superior hypogastric plexus blocks may benefit from the ability to better quantitate the spread of injected contrast in multiple planes. Potentially, factors such as local tumor burden or lymphadenopathy that limit the spread of the contrast and neurolytic solution may be noted earlier with these advanced imaging techniques. For example, Goldschneider et al. [14] performed celiac plexus blocks in children utilizing 3D-RA to show the benefits of examining contrast spread in three dimensions. Similarly, superior hypogastric blocks (Fig. 4a–c) have added detail when a 3D image is rendered. In another recent report [15], Knight et al. performed vertebroplasty in a patient with a retropulsed bone fragment in the spinal canal, normally at least a relative contraindication. The authors utilized FDCT technology to visualize these areas during the injection of the polymethyl methacrylate cement and avoid spinal cord injury [15]. Neuromodulation, particularly spinal cord stimulation, may be more easily targeted in some cases with FDCT technology. The anterior or lateral movement of the electrodes could more easily be seen, eliminating the need for multiple repositionings of the electrode and needle in the epidural space. The utilization of FDCT/ CBCT/3D-RA technology to better treat patients seems to be limited only by one’s imagination.
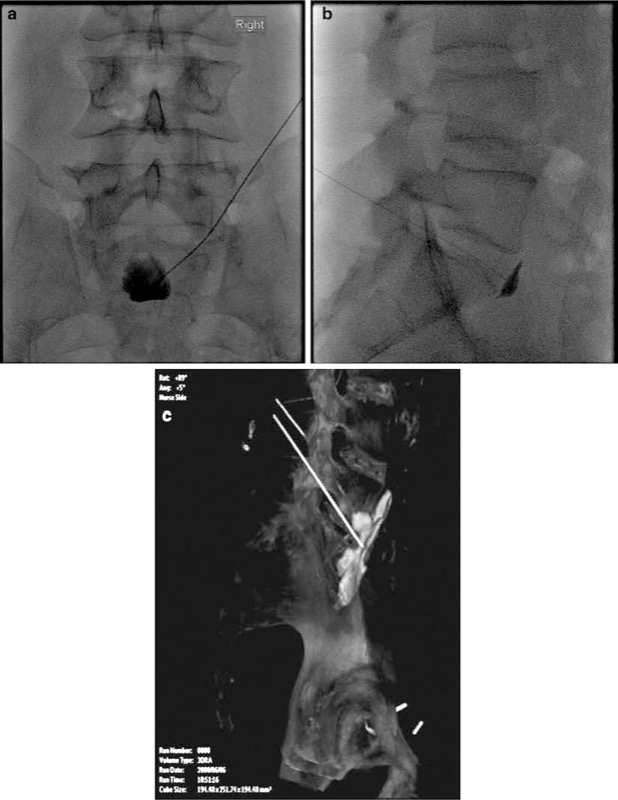
Fig.4 (a) AP view of fluoroscopic superior hypogastric plexus block, (b) lateral view of superior hypogastric plexus block, and (c) 3D-RA view of contrast in three dimensions.
3. ULTRASOUND
Ultrasound has become extremely popular in acute pain block procedures, and chronic pain practitioners are slowly adopting ultrasound as both a diagnostic and image-guided block aid. Chronic pain procedures may include nerve blocks (such as the brachial or lumbar plexus) commonly performed in an acute perioperative nerve block suite but also may require an image-guided injection of more distal branches of the plexus or at less common locations (proximal to sites of trauma or entrapment or neuroma formation). block of various small sensory or mixed nerves, such as the ilioinguinal [16, 17], lateral femoral cutaneous [18], suprascapular [19], pudendal [20], intercostal [21], and various other sites, has been performed. In addition, many spinal procedures including epidurals, selective spinal nerve blocks [22, 23], facet joint, medial branch blocks, and third occipital nerve blocks [24, 25], as well as sympathetic blocks (stellate ganglion) [26] may be performed. Finally, a broad array of possible applications for peripheral neuromodulation electrode placement may be possible with ultrasound guidance [27].
4. INTRA-ARTICULAR INJECTIONS
Intra-articular injections of medications (primarily corticosteroids) are extremely common procedures performed by physicians from primary care disciplines as well as specialists. While few would dispute that these procedures are easy to do and very accurate, whether image guidance can improve the outcome of intra-articular procedures was not specifically known. A recent study of intra-articular injections suggests that these may be one area where the use of image guidance is useful [28]. The study of 148 painful joints (shoulder, knee, ankle, wrist, hip) compared the use of US guidance to a surface landmark-based injection. The authors found that the use of US led to a 43% decrease in procedural pain, a 25.6% increase in the rate of responders, and a 62% decrease in the nonresponder rate. Sonography also increased the rate of detection of effusion by 200% as compared to the use of surface landmarks. None would dispute that the use of image guidance would add to the cost of the actual procedures. However, health-care economics studies would be required to ascertain whether the improved outcomes would lead to better health-care value viewed through a long-term perspective.
5. TRIGGER POINT AND MUSCULAR INJECTIONS
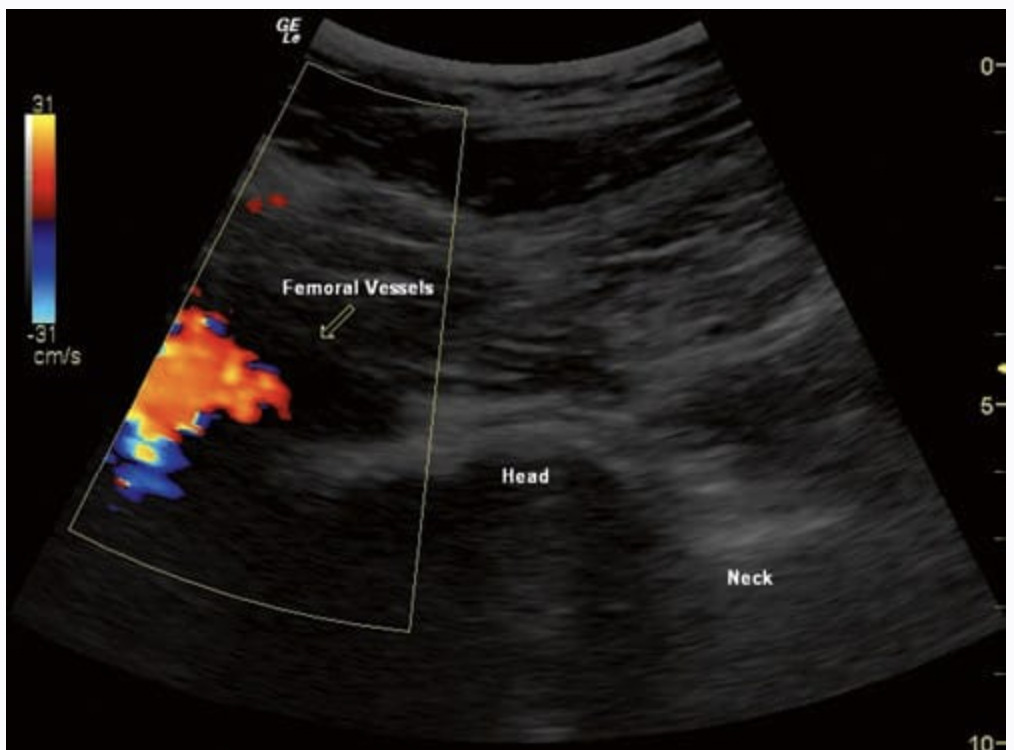
The performance of most deep muscular and trigger point injections has been relegated to a trivial office-based procedure, generating little enthusiasm from the interventional pain community. Image guidance (fluoroscopy) for these soft tissue structures was not helpful, and many physicians considered the performance of the procedures to be “the art of medicine.” However, the addition of ultrasound may be changing the way one views these procedures. Certainly, it is easy to see how a target such as a piriformis muscle could be identified more accurately using US. It is likely that fluoroscopic techniques may actually mistake the gluteal or quadratus femoris muscles on occasion. In addition, the anatomic variability and proximity of neurovascular structures, including the sciatic nerve, make visualization important. US also allow the use of a diagnostic exam (hip rotation) to aid in the proper identification of the muscle (Fig. 5). Studies to date suggest that the piriformis muscle is easily injected using this modality [29]. Other muscular targets such as trigger points have been targeted using US guidance [30]. Pneumothorax is an all too frequent complication of thoracic area trigger points. In the 2004 ASA Closed Claims Project, 59 pneumothorax claims were filed. Of this 59, fully half (23 intercostal blocks and 1 costochondral injection) would likely have been preventable under US guidance. Additionally, 15 of the cases were trigger point muscular injections which would likely be preventable as well. Together, at least 2/3 of the pneumothorax claims (and likely even more) could be prevented with better imaging [31].
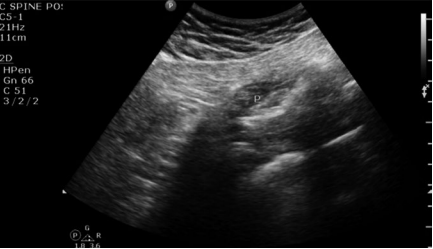
Fig. 5 A dynamic exam is depicted wherein the piriformis muscle (P) is contracted
Whether the use of US or another imaging technique is justified in all cases by the avoidance of complications may depend on a more accurate depiction of the true incidence of complications and better outcome data. Certainly, it may be true that positive responses could be more accurately replicated in some cases.
6. ZYGAPOPHYSEAL AND MEDIAL BRANCH BLOCKS
One of the better studies of ultrasound guidance in pain medicine evaluated third occipital nerve block procedures and peaked interest in US for many in the pain medicine community [24]. The third occipital nerve had been suggested as a therapeutic target for conditions, including high-cervical spondylosis and cervicogenic headaches, and as a predictor of success for radiofrequency ablative procedures. In that study, the accuracy of US guidance compared to that of fluoroscopy was good, with 23 of 28 needles demonstrating accurate radiographic positioning [24]. Fluoroscopic procedures targeting the third occipital nerve around the C2/C3 zygapophyseal joint have been performed utilizing three sequential needle placements. These fluoroscopy-guided placements have been very accurate but suffer from the inability to actually see the targeted nerve. Whether US is superior in some way to standard fluoroscopy remains to be tested.
7. EPIDURAL BLOCKS
Epidural techniques including interlaminar, caudal, and selective spinal root blocks have been studied in limited fashion utilizing ultrasound guidance. Fluoroscopy techniques are extremely easy and generally use small amounts of radiation; thus the advocates for US will need to perform comparative studies to demonstrate any particular advantages. Caudal procedures are perhaps most promising in this regard.
Caution should be exercised until mechanisms of ischemic injury during transforaminal epidural procedures are better understood. Lack of a contrast control in US in spite of “extraforaminal” vascular structure visibility is the most significant drawback. Even CT scanning is not foolproof for cervical transforaminal corticosteroid injections [11, 22, 23].
8. SYMPATHETIC BLOCKS
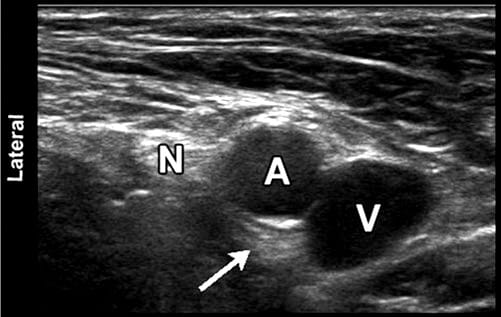
Sympathetic blocks have been studied in limited fashion with ultrasound guidance. Stellate ganglion block (SGB) was performed at C6 anterior to Chassaignac’s tubercle based on surface landmarks for years prior to modern fluoroscopy techniques which have become the standard of care in most regions. A recent analysis of 27 previously reported cases of retropharyngeal hematoma after SGB emphasized the potential for delayed bleeding and hematoma formation [32]. Although image-guided techniques were not described in this review, aspiration of blood was negative in all but four cases requiring needle redirection. One of the earliest papers examining US guidance was by Kapral et al. [26]. In this study, the nonultrasound group had three hematomas. The authors theorized that the vertebral artery might be more likely to be involved in left-sided injections. They and other researchers have raised the possibility of other arteries at risk, specifically, the ascending cervical branch off the inferior thyroid artery, which commonly passes over the C6 anterior tubercle [33]. No head-to-head comparison studies of ultrasound vs. CT or fluoroscopy for SGB have yet been performed. The advantages of ultrasound would seem to be avoidance of vascular or soft tissue injuries. The advantages of fluoroscopy or CT would appear to be ease of interpreting contrast spread patterns and better representation of 3D anatomy in the case of CT.
9. COMBINED US AND CT/FLUOROSCOPY
The use of combinations of these imaging modalities has had limited study to date but may have some indications as time and experience accumulate. For example, peripheral nerve stimulation may be best accomplished with US and FDCT or US and fluoroscopy [27]. It is possible that combined imaging techniques of US-fluoroscopy, CT-fluoroscopy, and US/ CT and other combined techniques may become normalized in particularly complicated procedures.
10. CONCLUSION
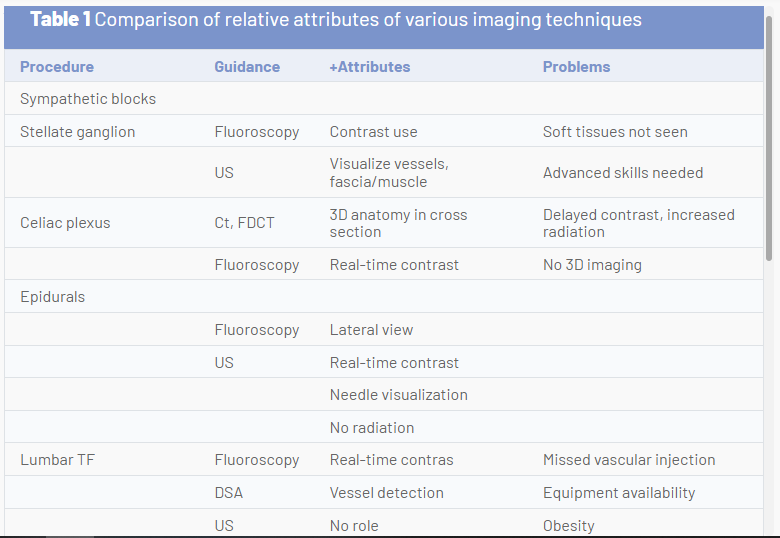
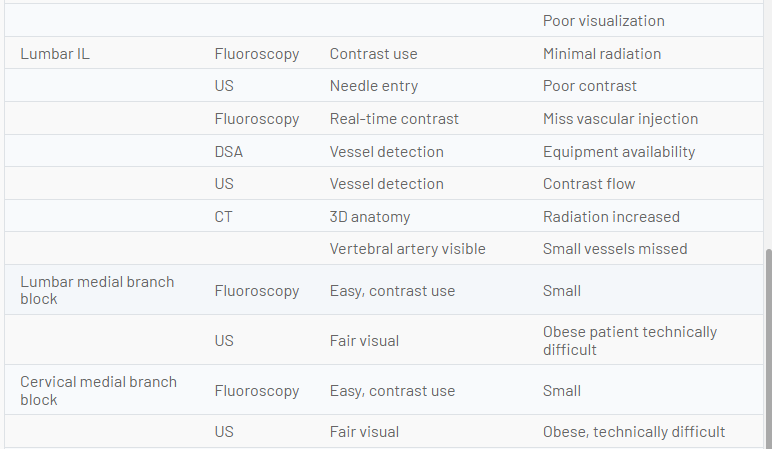
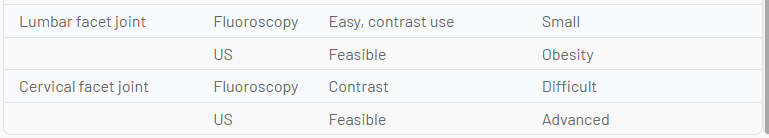
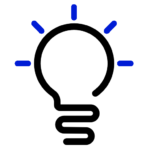
The future of image guidance for pain medicine interventions must balance risk to the patient and clinician from ionizing radiation, risks of procedural complications, outcomes, and relative value. Although ultrasound imaging is feasible in many instances, best practice may favor fluoroscopy or CT in some cases. Ultrasound appears to have advantages for musculoskeletal diagnosis and therapy for some joint and soft tissue conditions, procedures where the peritoneum or pleura may be punctured, deep muscle injections, most peripheral nerve procedures, possibly SGB, possibly caudal epidurals, and perhaps equivalency for sacroiliac joint and some medial branch blocks. Other uses will require ongoing comparison to other image-guidance techniques. The following table compares the relative attributes of various imaging techniques and points out areas where one image-guidance modality may have unique advantages relative to another (Table 1).