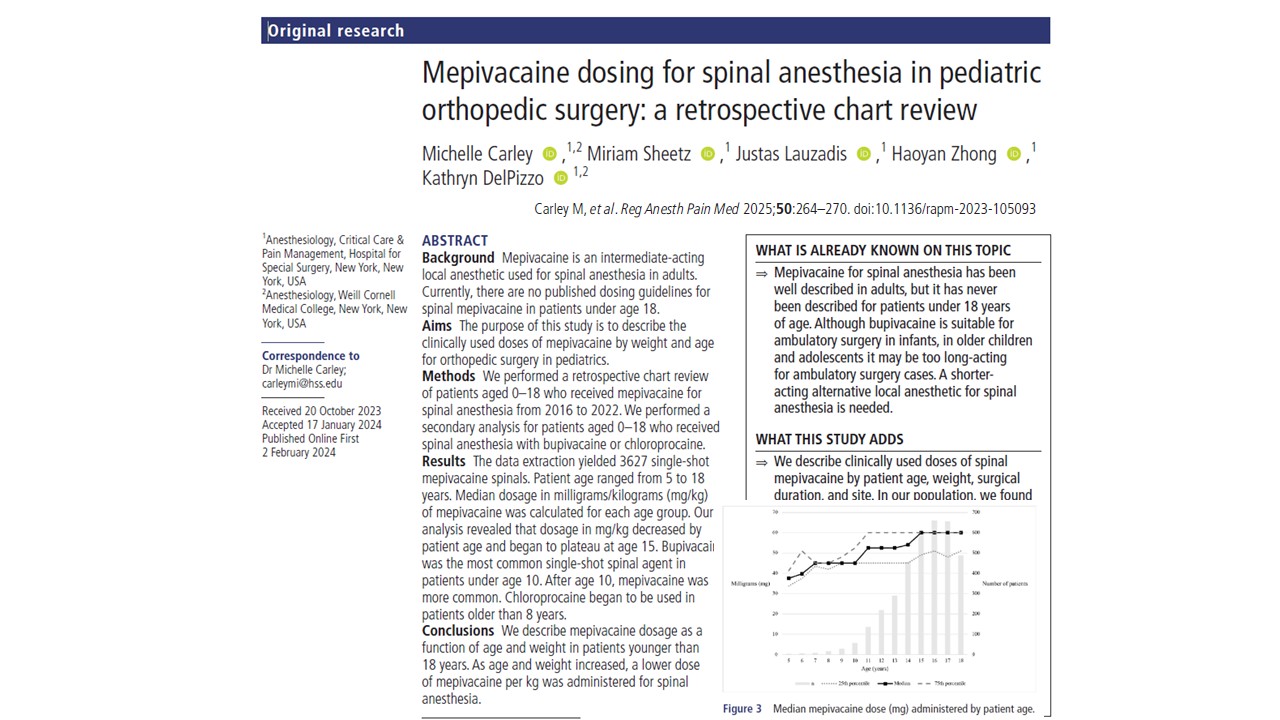
The study conducted by Carley et al., published in Regional Anesthesia & Pain Medicine (2025), offers the first comprehensive evaluation of mepivacaine dosing for spinal anesthesia in pediatric orthopedic surgery. This retrospective review of over 3,200 cases represents a significant step forward in tailoring anesthetic approaches to the unique physiological needs of children and adolescents.
Spinal anesthesia is an established technique in adult practice and increasingly in pediatric care. However, until now, no guidelines existed for using mepivacaine—a widely used intermediate-acting local anesthetic—for pediatric spinal anesthesia, especially in patients aged 5–18 years. This study fills that knowledge gap with age- and weight-specific dosing insights that can refine anesthetic care in ambulatory pediatric settings.
Why Mepivacaine?
Traditionally, bupivacaine has been the go-to agent for pediatric spinal anesthesia due to its long duration and established safety profile in infants. Yet, its prolonged effect can be a limitation in ambulatory surgery, especially for older children and adolescents.
Key advantages of mepivacaine:
- Intermediate duration of action: Reduces the risk of delayed ambulation and urinary retention.
- Faster recovery: Facilitates earlier discharge, improving throughput in surgical centers.
- Safer discharge profile: Offers sufficient duration for most pediatric orthopedic procedures without prolonged sedation or motor blockade.
Despite these benefits and decades of safe adult use, mepivacaine has been underutilized in pediatrics due to a lack of dosing guidance. Carley et al.’s study directly addresses this gap.
Study overview
- Study design: Retrospective chart review.
- Setting: Hospital for Special Surgery (HSS), New York City.
- Study period: January 2016 – May 2022.
- Population: Pediatric patients (ages 5–18) undergoing lower extremity orthopedic surgery.
- Total cases analyzed: 3,267 single-shot spinal anesthetics using 1.5% preservative-free mepivacaine.
- Inclusion criteria:
- Use of single-agent spinal mepivacaine (1.5%) without adjuncts.
- Documented patient demographics and surgical details.
- Lower extremity orthopedic procedures only.
Age-based dosing patterns: What the data show
One of the study’s most important findings is the inverse relationship between mepivacaine dose (mg/kg) and patient age.
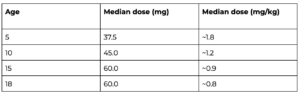
- Younger children required higher doses per kilogram, reflecting faster local anesthetic clearance.
- Dosing plateaued around age 15, suggesting a stabilization of pharmacodynamic needs in late adolescence.
- Total volume (in mL) increased with age, but weight-adjusted dosing decreased—a finding aligned with existing trends in bupivacaine and ropivacaine dosing literature.
Procedure-specific adjustments
The study also found that mepivacaine dosing varied slightly by surgical site and procedure duration:
- Hip surgeries typically receive more volume than knee or foot/ankle procedures.
- Longer surgeries correlated with higher total doses, yet all within safe limits (below 5–6 mg/kg).
This suggests that while a general age-based dose framework exists, nuanced dose adjustments are often guided by procedural demands and anticipated anesthesia duration.
Anesthesia and surgical metrics
Procedural statistics:
- Mean surgical duration: 112 minutes (IQR: 81–143).
- PACU stay: Median 207 minutes.
- Time from surgery end to OR exit: 7 minutes.
Importantly, these metrics show that mepivacaine provided sufficient coverage for procedures often exceeding the traditionally cited 60–90 minute spinal window in children.
Safety profile and conversion rates
The most striking safety outcome was the low rate of conversion to general anesthesia:
- Airway placement occurred in only 1.3% of cases, encompassing supraglottic and endotracheal devices.
- These conversions were not always due to spinal failure—some were for sedation or airway support needs.
No significant complications or overdoses were recorded, and all observed doses remained well under the FDA-recommended 5–6 mg/kg ceiling for single-shot caudal injections.
Comparative agent preferences by age
The researchers also evaluated how anesthetic agent selection varied with age:
- <10 years: Bupivacaine predominated.
- 10–14 years: Mepivacaine became more common.
- >14 years: Mepivacaine remained dominant, with increasing chloroprocaine use.
This age-dependent trend supports clinical decision-making that balances procedure duration, anticipated recovery time, and patient physiology.
Limitations
Despite the study’s strengths in size and clarity, several limitations exist:
- Retrospective design: Limits control over confounding variables and patient selection bias.
- Resolution time not directly measured: Recovery inferred from PACU time, which is influenced by multiple non-anesthetic factors.
- Institution-specific data: HSS has a unique culture of neuraxial anesthesia use, which may not be generalizable to all institutions.
Further prospective research could solidify optimal dosing and evaluate mepivacaine’s utility across broader surgical settings and institutions.
Implications for clinical practice
The findings from this study may influence pediatric anesthetic practice in several key ways:
- Support for routine use in children > 5 years:
Mepivacaine emerges as a practical, safe, and effective alternative for spinal anesthesia in older children and adolescents, particularly in outpatient settings where rapid recovery is paramount.
- Age- and weight-based guidelines:
Clinicians now have a dosing framework:
- 5–8 years: ~1.5–1.7 mg/kg
- 10–13 years: ~1.0–1.2 mg/kg
- 15–18 years: ~0.8–0.9 mg/kg
- Encouragement of tailored anesthesia:
This study underscores the importance of individualized dosing, integrating patient age, weight, and procedural factors rather than relying solely on fixed dose-per-weight formulas.
Conclusion
This expansive chart review provides a much-needed blueprint for integrating mepivacaine into pediatric spinal anesthesia protocols. As anesthesiologists seek shorter-acting, recovery-friendly agents in a fast-paced surgical environment, mepivacaine offers the right balance of efficacy and safety for older children and teens.
With detailed, age- and weight-specific data now available, clinicians can confidently incorporate this intermediate-acting agent into their spinal anesthesia toolkit, improving outcomes, minimizing complications, and aligning with the growing push for rapid recovery in pediatric outpatient surgery.
Key takeaways:
- Age-adjusted dosing ensures safety and efficacy.
- Low complication and conversion rates validate feasibility.
- Shorter-acting agents like mepivacaine may improve pediatric perioperative outcomes.
Clinical implications:
Mepivacaine may soon become a preferred agent for spinal anesthesia in pediatric ambulatory orthopedic surgeries, providing an optimal balance between anesthesia duration and rapid recovery.
For more information, refer to the full article in Regional Anesthesia & Pain Medicine.
Carley M et al. Mepivacaine dosing for spinal anesthesia in pediatric orthopedic surgery: a retrospective chart review. Reg Anesth Pain Med. 2025 Mar 5;50:264-270.
Read more about spinal anesthesia with the Regional Anesthesia Module on the NYSORA360 website!