ACUTE PAIN MANAGEMENT
INTRODUCTION
The treatment and alleviation of pain constitute a basic human right that exists regardless of age. Pain is defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage. Previous experience and management of pain, even from very early stages in life, alter the responses and behavior toward further “painful” experiences and events. Hence, no two people experience pain the same way, which adds to the complexity of the management of pain.
Unfortunately, even when pain is obvious, children frequently receive no or inadequate treatment for pain and painful procedures. The newborn and critically ill child are especially vulnerable to receiving no treatment or under-treatment. The conventional notion that children neither respond to nor remember painful experiences to the same degree that adults do is inaccurate. Many of the nerve pathways essential for the transmission and perception of pain are present and functioning by 24–29 weeks of gestation. Research in newborn animals has revealed that failure to provide analgesia for pain results in “rewiring” of the nerve path-ways responsible for pain transmission in the dorsal horn of the spinal cord, resulting in increased pain perception of future painful insults. This confirms human newborn research that found that the failure to provide anesthesia or analgesia for newborn circumcision resulted not only in short-term physiologic perturbations but also in longer-term behavioral changes.
Nurses are traditionally taught or cautioned to be wary of physicians’ orders and patients’ requests for pain management, as well. The most common prescription order for potent analgesics, “to give as needed” (pro re nata, PRN), in reality means “to give as infrequently as possible.” The PRN order also means that either the patient must know or remember to ask for pain medication or the nurse must be able to identify when a patient is in pain. Neither requirement may be met by children in pain. Children less than 3 years of age and critically ill children may be unable to adequately verbalize when they are in pain or where they hurt. Moreover, they may be afraid to report their pain. Several studies have documented the inability of nurses, physicians, and parents/guardians to correctly identify and treat pain, even in postoperative pediatric patients.
Societal fears of opioid addiction and lack of advocacy are also causal factors in the undertreatment of pediatric pain. Unlike adult patients, pain management in children is often dependent on the ability of parents/guardians to recognize and assess pain and on their decision whether to treat or not. Parental misconceptions concerning pain assessment and pain management may therefore also result in inadequate pain treatment. Even in hospitalized patients, most of the pain that children experience is managed by their parents/guardians. Parents/guardians may fail to report pain either because they are unable to assess it or are afraid of the consequences of pain therapy. In one study, false beliefs about addiction and the proper use of acetaminophen and other analgesics resulted in the failure to provide analgesia to children. In another, the belief that pain was useful or that repeated doses of analgesics lead to medication underperformance resulted in the failure of the parents/guardians to provide or ask for prescribed analgesics to treat their children’s pain. Parental/guardian education is therefore essential if children are to be adequately treated for pain.
All of these factors make children an extremely vulnerable group. Fortunately, the past 25 years have seen substantial advances in research and interest in pediatric pain management and in the development of pediatric pain services, primarily under the direction of pediatric anesthesiologists. Pain service teams provide pain management for acute, postoperative, terminal, neuropathic, and chronic pain. Nevertheless, the assessment and treatment of pain in children are important aspects of pediatric care, regardless of who provides it. Failure to provide adequate control of pain amounts to substandard and unethical medical practice.
PAIN ASSESSMENT
The perception of pain is a subjective, conscious experience; operationally, it can be defined as “what the patient says hurts” and existing “when the patient says it does.” Infants, preverbal children, and children between the ages of 2 and 7 years may be unable to describe their pain or their subjective experiences. This has led many to conclude incorrectly that children do not experience pain in the same way that adults do. Clearly, children do not have to know (or be able to express) the meaning of an experience to have an experience. Therefore, because pain is essentially a subjective experience, it is becoming increasingly clear that the child’s perspective of pain is an indispensable facet of pediatric pain management and an essential element in the specialized study of childhood pain. Sometimes there is an overreliance on objective assessments of pain, whether from a healthcare professional or parental/guardian assessment. This objective assessment, though sometimes important, should remain only a minor partner in the assessment and management of pain, as objective assessments are also subject to bias and preconceived notions. Indeed, pain assessment and management are inter-dependent, and one is essentially useless without the other. The goal of pain assessment is to provide accurate data about the location and intensity of pain, as well as the effectiveness of measures used to alleviate or eradicate it.
Instruments currently exist to assess pain in children of all ages. Indeed, the sensitivity and specificity of these instruments have been widely debated and have resulted in a plethora of studies to validate their reliability and validity. The most commonly used instruments measure the quality and intensity of pain and are “self-report measures” that make use of pictures or word descriptors to describe pain. Pain intensity or severity can be measured in children as young as 3 years of age by using either the Oucher scale (developed by Judith E. Beyer, RN, PhD; Antonia M. Villarreal, RN, PhD; and Mary J. Denyes, RN, PhD)—a two-part scale including both a numeric scale (from 0 to 100) and a photographic scale of six photographs of a young child’s face expressing increasing degrees of discomfort—or a visual analog scale—a 10-cm line with a distraught, crying face at one end and a smiling face at the other. The visual analog scale has been validated by both sex and race. In our practice, we use the six-face Wong-Baker FACES Pain Rating Scale (developed by Dr. Donna Wong and Connie M. Baker), primarily because of its simplicity (Figure 1). This scale is attached to the vital sign record, and nurses are instructed to use it or a more age-appropriate self-report measure whenever vital signs are taken.
NYSORA Tips
- Regular assessment using appropriate pain assessment tools, involving the patient and carers in decision making, and being as flexible as possible to the patient’s needs all play a vital role in achieving a successful outcome.
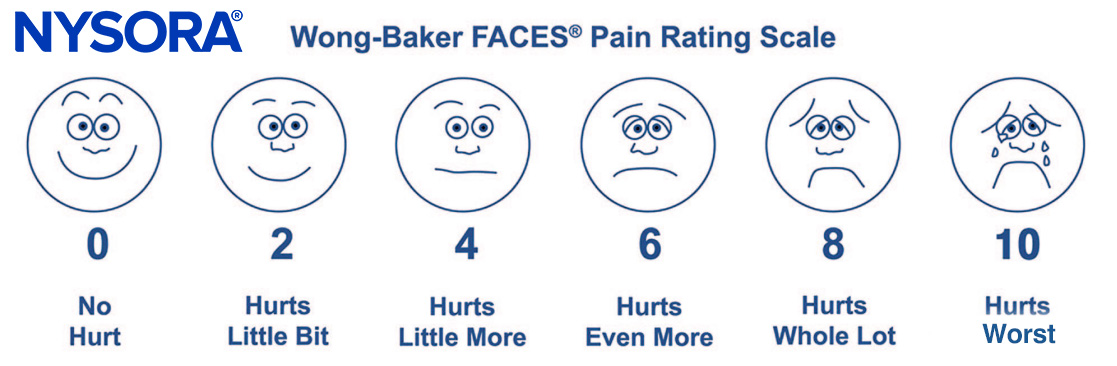
FIGURE 1. The six-face Wong-Baker FACES Pain Rating Scale. (Wong-Baker FACES Foundation (2015). Wong-Baker FACES® Pain Rating Scale. Retrieved January 28, 2017 with permission from http://www.WongBakerFACES.or.)
Pain assessment in preverbal children poses challenges as they are unable to self-report. There are many pain assessment tools available in this age group, but none are ideal. The CRIES pain score is frequently used to score pain in neonates (Table 1). Children with developmental delay, complex needs, and or in intensive care need special pain assessment tools to monitor pain. Most such tools incorporate physiological parameters for stress (cardiac, respiratory, and endocrine) with behavioral changes associated with pain (facial expressions, crying, body and limb movements). A separate pain assessment scale called the Paediatric Pain Profile (PPP) is available at our institution for use in children with complex needs. It is used primarily by parents/guardians to measure their child’s pain and incorporates scoring of the aforementioned behavioral changes. Irrespective of the pain assessment tool used in these patient groups, it is important that healthcare professionals understand what causes pain, appreciate that pediatric patients perceive pain, and have a variety of assessment methods and treatments in their armamentarium to achieve effective pain control.
TABLE 1. CRIES pain scale for babies from 32 weeks’ gestational age.
| 0 | 1 | 2 | |
|---|---|---|---|
| Crying Characteristic cry of pain is high pitched | No cry or cry that is not high pitched | High-pitched cry but infant is consolable | High-pitched cry and infant is inconsolable |
| Requires O2 to maintain SaO2 > 95 Consider other changes in oxygenation | No | Requires O2 < 30% | Requires O2 > 30% |
| Increased vital signs Take BP last as this may cause difficulty with other assessments | HR and BP +/– 10% of baseline | 10%–20% increase in BP or HR | > 20% increase in HR or BP |
| Expression Grimace characterized by brow bulge, eyes shut, open mouth, deepening nasolabial furrow | Neutral | Grimace | Grimace/grunt |
| Sleeplessness Based on state during the hour preceding the assessment | No | Wakes frequently | Constantly awake |
| Instructions: Each of the five categories is scored 0, 1, or 2, resulting in total score between 0 and 10. | |||
PAIN MANAGEMENT
Acute pediatric pain management is increasingly characterized by a multimodal or “balanced” approach in which smaller doses of opioid and nonopioid analgesics, such as nonsteroidal anti-inflammatory drugs (NSAIDs), local anesthetics, N-methyl-D-aspartate (NMDA) antagonists, and α2-adrenergic agonists, are combined to maximize pain control and minimize drug-induced adverse side effects. Pain management also includes management of both patient and parental/guardian expectations and being knowledgeable, open, and frank about what to expect during the course of the postoperative and rehabilitation periods. It should be recognized that certain procedures “hurt” more than others and that, in spite of our best efforts, it is not always possible to achieve “no pain,” although that should always be the aim. At the same time, preoperative discussion of the various analgesic strategies available and reassurance that the patient “will be looked after” go a long way to achieving a satisfactory outcome for all concerned. In addition, a multimodal approach utilizes nonpharmacological, complementary, and alternative medicine therapies, as well. These techniques include distraction, guided imagery, transcutaneous nerve stimulation, acupuncture, therapeutic massage, among others.
NYSORA Tips
- The aim of acute pain management is providing a comfortable/pain-free perioperative period in order to facilitate early ambulation and rehabilitation.
- Preoperative discussions with the patient and his or her parents/guardians detailing the achievable outcome, expected course, and various available pain management modalities play an important role in achieving a satisfactory outcome for all concerned.
- A multimodal approach to pain management achieves the best results.
- If possible, regional anesthesia/analgesia should be part and parcel of any multimodal analgesia regime.
NYSORA Tips
Alternative medicine pain therapy:
- Distraction
- Guided imagery
- Transcutaneous nerve stimulation
- Acupuncture
- Therapeutic massage
Procedural pain is often a forgotten and ignored aspect of pain management in children admitted to hospital. Various interventions and procedures, some of which are done repeatedly, may inflict pain or are perceived as painful by an anxious child (eg, cannulation, phlebotomy, lumbar puncture, and wound dressing and cleaning). It is essential to practice how to explain procedures and how to prepare and reassure the child and parents/guardians. Simple techniques such as local anesthetic creams and play/distraction therapy can help in many situations. Some patients may also need formal psychological intervention and support or pharmacological aids such as sedation or nitrous oxide (N2O), all of which require time and planning. Lastly, if conscious sedation or N2O is required, adequate monitoring and emergency equipment, including oxygen, suction, and appropriate personnel, should be immedi-ately available.Pain management strategies for day case surgery should include local anesthetic infiltration, regional blocks, simple analgesics (eg, acetominophen/paracetamol, NSAIDS, and “milder” opioids such as codeine or tramadol, if necessary). “Strong” opioids should be avoided, although they are not con-traindicated. Ultrasound-guided regional anesthesia is increasing in popularity, providing safer and more effective regional anesthesia.
NYSORA Tips
Pain management strategies for day case surgery:
- Local anesthetic infiltration
- Regional anesthesia
- Nonopioid analgesics (acetaminophen, NSAIDs)
- Mild opioids (codeine, tramadol) when necessary
- More potent opioids should be avoided when possible but are not contraindicated
Anesthesiologists must work closely with surgical colleagues to identify appropriate day case surgeries and develop patient care pathways with standard analgesia management plans for specific procedures.
For major surgery, in addition to all the above mentioned analgesics, opioid and/or local anesthetic infusions may be necessary. These can be complemented by other treatment modalities including ketamine, clonidine, or diazepam for muscle spasm after orthopedic surgeries; gabapentin for acute pain; intraoperative magnesium; and the addition of dexamethasone systemically or to a local anesthetic for nerve blocks.
Analgesics with Antipyretic Activity or Nonopioid (“Weaker”) Analgesics
The “weaker” or milder analgesics with antipyretic activity, of which acetaminophen, ibuprofen, naproxen, and diclofenac are the classic examples, make up a heterogeneous group of NSAIDs that are nonopioid analgesics (Table 2).
They provide pain relief primarily by blocking peripheral and central prostaglandin production by inhibiting cyclooxygenase types I and II. These analgesic agents are primarily administered enterally via the oral or rectal route and are particularly useful for inflammatory, bone, and rheumatic pain. Parenterally administered acetaminophen and NSAIDs, such as ketorolac, are available for use in children in whom the oral or rectal routes of administration are not possible. Unfortunately, regardless of dose, the nonopioid analgesics reach a “ceiling effect,” above which pain cannot be relieved by these drugs alone. Because of this, these weaker analgesics are considered the basic building blocks in a multimodal therapeutic approach and are often administered in combination forms with opioids such as codeine, oxycodone, hydrocodone, or tramadol. Aspirin has been largely abandoned in pediatric practice because of its possible role in Reye syndrome, its effects on platelet function, and its gastric irritant properties.
NYSORA Tips
- The nonopioid analgesics have a “ceiling effect,” above which pain cannot be relieved by these drugs alone, regardless of dose.
- Nonopioid analgesics are considered the basic building blocks in a multimodal therapeutic approach and are often administered in combination forms with opioids such as codeine, oxycodone, hydrocodone, or tramadol.
The most commonly used nonopioid analgesic in pediatric practice remains acetaminophen. Unlike NSAIDs, acetaminophen works primarily centrally and has minimal, if any, anti-inflammatory activity. When administered in normal doses (10–15 mg · kg–1, PO), acetaminophen is extremely safe and has very few serious side effects. It is an antipyretic and, like all enterally administered NSAIDs, takes about 30 minutes to provide effective analgesia. Several investigators have reported that when administered rectally, acetaminophen should be given in significantly higher doses than previous recommendations had suggested. However this author does not use acetaminophen loading doses when the drug is administered rectally. Regardless of route of delivery, to prevent hepatotoxicity, the daily maximum acetamino-phen dose in the preterm neonate, term neonate, and older child is 30, 60, and 80 mg/kg, respectively (Table 3). The maximum adult dose is 4 g/day.
The discovery of at least two cyclooxygenase (COX) isoenzymes, referred to as COX-1 and COX-2, has increased our knowledge of NSAIDs. These two COX isoenzymes share structural and enzymatic similarities but are uniquely regulated at the molecular level and may be distinguished by their functions. Protective prostaglandins, which preserve the integrity of the stomach lining and maintain normal renal function in a compromised kidney, are synthesized by COX-1. COX-2 is an inducible isoform. The inducing stimuli include pro-inflammatory cytokines and growth factors, implying a role for COX-2 in both inflammation and control of cell growth. In addition to the induction of COX-2 in inflammatory lesions, COX-2 is present constitutively in the brain and spinal cord, where it may be involved in nerve transmission, particularly for pain and fever.
TABLE 2. Dosing guidelines for commonly used nonopioid analgesics (Institutional or national guidelines may vary.)
| Premature Neonates (32–36 weeks Postmenstrual Agea) | Term Neonates (> 36–44 weeks Postmenstrual Agea) | Infants and Children (> 44 weeks Postmenstrual Agea and up to 50 kg) | > 12 Years (and Weight > 50 kgb) | |
|---|---|---|---|---|
| Acetaminophen (Paracetamol) | ||||
| Acetaminophen (Paracetamol) | 15 mg/kg PO/PR every 8 hours (max 60 mg/kg/day) | 15 mg/kg PO/PR every 6 hours (max 60 mg/kg/day) | 15–20 mg/kg PO/PRbc every 4–6 hours (max 90 mg/kg/day) | 1g PO/PR every 4–6 hours (max 4 g/day) |
| IV acetaminophen | 7.5 mg/kg IV every 8 hours (max 25 mg/kg/day) | 7.5 mg/kg IV every 6 hours (max 30 mg/kg/day) | 15 mg/kg IVb every 6 hours (max 60mg/kg/day) | 15 mg/kg IV (maximum 1 g) every 6 hours |
| Nonsteroidal anti-inflammatory drugs (NSAIDs) Prescribe one drug only. | ||||
| Ibuprofen | Not recommended | Not recommended | Less than 3 months of age: 5 mg/kg PO every 8 hours From 3 months of age: 10 mg/kg PO (maximum 400 mg) every 8 hours (max 30 mg/kg/day) | 400 mg PO every 8 hours |
| Diclofenac | Not recommended | Not recommended | From 6 months of age: 1 mg/kg PO/PR every 8 hours | 50 mg PO/PR every 8 hours |
| Naproxen | Not recommended | Not recommended | 5 mg/kg every 12 hours | 5mg/kg every 12 hours (max 1g/day) |
bDoses based on weight in obese patients or based on age in underweight patients may need to be reduced to avoid overdosage.
cA higher dose of acetaminophen 20 mg/kg PO/PR every 6 hours may be used when pain is not controlled with the standard dose (15 mg/kg) when no contraindications exist. This dose should be reviewed every 24 hours. Loading doses are not recommended in order to minimize the potential for error
Prostaglandins made by COX-2 are also important in ovulation and in the birth process. The discovery of COX-2 has made possible the design of drugs that reduce inflammation without removing the protective prostaglandins in the stomach and kidney made by COX-1. In fact, developing a more specific COX-2 inhibitor has been an important goal of much drug research because this class of drug has all of the anti-inflammatory and analgesic properties that one desires in a drug with none of the gastrointestinal and antiplatelet side effects. Unfortunately, the growing controversy regarding the potential adverse cardiovascular risks of the prolonged use of COX-2 inhibitors has dampened much of the enthusiasm for these drugs and has led to the removal of rofecoxib from the market by its manufacturer. Other NSAIDs, especially diclofenac, are now facing similar scrutiny. Many orthopedic surgeons are also concerned about the negative effect of all NSAIDs on bone growth and healing. While some pediatric orthopedic surgeons have recommended that these drugs not be used in their patients in the postoperative period, it is this author’s view that in spite of the controversies, NSAIDs remain effective and useful drugs in pediatric acute pain management when used wisely and for short duration.
TABLE 3. Opioid analgesic initial dosage guidelines (institutional or national guidelines may vary.)
| Equianalgesic Dose (mg) | Usual Starting IV Dose and Interval | Usual Starting Oral Dose and Interval | |||||
|---|---|---|---|---|---|---|---|
| Drug | IV, IM, SC | Oral | < 50 kg | > 50 kg | IV/Oral Ratio | < 50 kg | > 50 kg |
| Codeine | 120 | 200 | NR | NR | 1:2 | 0.5–1a mg/kg every 4-6 hours | 0.5–1a mg/kg every 4–6 hours |
| Fentanyl | 0.1 | NAb | Bolus: 0.5–1 mcg/kg, 0.5–2 h (max 50 mcg) NCA/PCA (drug concentration: 1 mcg/kg/mL, max 50 mcg/mL) NCA: Bolus: 0.5–1 mcg/kg, 30 min–1 h; infusion: 0.5–1 mcg/kg/h PCA: Bolus: 0.5 mcg/kg, 10 min–1 h; infusion: 0.5–1 mcg/kg/h | NA | NA | NA | |
| Hydrocodone | NA | 10–20 | NA | NA | NA | 0.1 mg/kg every 3–4 hours | 5–10 mg every 3–4 hours |
| Hydromorphone | 1.5–2 | 3–5c | Bolus: 0.02 mg/kg, 0.5–2 h; infusion: 0.004 mg/kg/h | Bolus: 1 mg, 0.5–2 h; infusion: 0.3 mg/h | 1:2 | 0.03–0.08 mg/kg every 4 hours | 2–4 mg every 4 hours |
| Methadone | 10 | 10–20 | 0.1 mg/kg every 4–8 hours | 5–10 mg every 4–8 hours | 1:2 | 0.2 mg/kg every 4–8 hours | 10 mg every 4–8 hours |
| Morphine | 10 | 30–50 | Bolus: 0.03–0.1 mg/kg, 0.5–2 h (max 10 mg) NCA/PCA(drug concentration 20mcg/kg/mL, max 1mg/mL)d NCA: Bolus: 20 mcg/kg, 15 min–1 h; infusion: 20 mcg/kg/h PCA: Bolus: 20 mcg/kg (max 1 mg), 5min; infusion: 4 mcg/kg/h | 1:2–3 | 0.2–0.3 mg/kg every 4–6 hours Sustained release: 0.4–0.5 mg/kg every 8–12 hours | 15 mg/kg every 4–6 hours Sustained release: 30 mg every 8–12 hours |
|
| Oxycodone | NA | 10–20 | NA | NA | NA | 0.1 mg/kg every 3–4 hours | 5–10 mg every 3–4 hourse |
bOral transmucosal route available: dose 10–15 mcg/kg.
cThe equianalgesic oral dose and parenteral/oral dose ratio are not well established.
dFor neonates and infants younger than 13 weeks, the drug concentration is to be halved: bolus 5 mcg/kg,1 h; infusion 5–10 mcg/kg/h.
eA sustained-release preparation is available.
Opioid Drug Selection
Many factors are considered when deciding which is the appropriate opioid analgesic to administer to a pediatric patient in pain. These include pain intensity, patient age, coexisting disease, potential drug interactions, treatment history, physician preference, patient preference, and route of administration. Some opioids are preferred over others, and some may be unavailable depending on institution, country, or continent, for reasons not entirely understood. The idea that some opioids are “weak” (eg, codeine) and others “strong” (eg, morphine) is outdated. All are capable of treating pain regardless of intensity if the dose is adjusted appropriately (Table 4). At equipotent doses, most opioids have similar effects and side effects. Meperidine (pethidine) at an equianalgesic dose has the same side effect profile as morphine; however, it is no longer commonly prescribed.
TABLE 4. Maximum local anesthetic dosing guidelines.
| Drug | Dose mg/kg Without Epinephrine | Dose mg/kg with Epinephrine | Duration in Hours | Contra-Indications | Comments |
|---|---|---|---|---|---|
| Bupivacainea | 2.5 | 3 | 3–6 | Reduce dose by 50% in neonates | |
| Chloroprocaineb | 8 | 10 | 1 | Plasma cholinesterase deficiency | Short-acting, rapid metabolism, useful in neonates and possibly patients with seizures or liver disease |
| Lidocaine | 5 | 7 | 1 | ||
| Ropivacainec | 3 | never mixed | 3-6 | Less cardiotoxicity than bupivacaine |
bIn neonatal epidural continuous infusion: 10–15 mg/kg/h.cInfusion rates is 0.5 mg/kg for pediatric patients older than 4-6 months of age.
Commonly Used Oral Opioids: Codeine, Oxycodone, Hydrocodone, Morphine, and Tramadol
Codeine, oxycodone, and hydrocodone are opioids that are frequently used to treat pain in children and adults, particularly less severe pain and when patients are being converted from parenteral to enteral opioids (see Table 3). Morphine is commonly used in regimens for chronic pain (eg, cancer). Codeine, oxycodone, and hydrocodone are most commonly administered in oral form, usually in combination with acetaminophen or aspirin. Unfortunately, very few, if any, pharmacokinetic or dynamic studies have been performed in children, and most dosing guidelines are anecdotally based. In equipotent doses, codeine, oxycodone, hydrocodone, and morphine are equal both as analgesics and respiratory depressants (see Table 3). In addition, these drugs share common effects on the central nervous system with other opioids, including sedation, respiratory depression, and stimulation of the chemoreceptor trigger zone in the brain stem, the latter particularly the case for codeine. There are fewer nausea and vomiting side effects with oxycodone and hydrocodone. Codeine, hydrocodone, and oxycodone have a bioavailability of approximately 60% after oral ingestion. The analgesic effects occur as early as 20 minutes after ingestion and reach a maximum after 60–120 minutes. The plasma half-life of elimination is 2.5–4 hours. Codeine undergoes nearly complete metabolism in the liver before its final excretion in urine. Approximately 10% of codeine is metabolized into morphine (CYP2D6), and it is this 10% that is responsible for codeine’s analgesic effect. Interestingly, approximately 10% of the population and most newborn infants cannot metabolize codeine into morphine, and in these patients, codeine produces little, if any, analgesia.
Codeine needs special mention, as its use has come under increased scrutiny at the time of writing this chapter. A few instances of fatalities and life-threatening episodes of respiratory depression have been reported in children who are cytochrome P450 CYP2D6 “ultra-rapid metabolizers” and were given codeine after tonsillectomy or adenoidectomy in the treatment of obstructive sleep apnea. The CYP2D6 enzyme is subject to genetic polymorphism. Having multiple gene copies, some patients metabolize codeine more rapidly (and are thus termed “ultra-rapid metabolizers”) and therefore have an increased risk of experiencing morphine toxicity, ie, respiratory depression. The prevalence of this varies with ethnicity, from as low as 0%–2% in Asians to as high as 10%–16% in Ethiopians and Saudi Arabians.
The current position on codeine use is as follows. The U.S. Federal Drug Administration (FDA), the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medi-cines Agency (EMA), and the U.K. Medicines and Healthcare Products Regulatory Agency (MHRA) have recommended restrictions on the use of codeine in children. These include the following:
- Restrict the use of codeine to children over 12 years of age (EMA and MHRA)
- Avoid codeine use in patients under 18 years of age who are undergoing tonsillectomy or adenoidectomy, especially for obstructive sleep apnea (EMA and FDA).
- In all other cases, use codeine only if required. It should be prescribed on an “as-needed basis” only, with the dose restricted to 0.5 mg/kg (maximum 30 mg) every 6 hours, and limited in treatment duration.
- Patient receiving codeine should be closely monitored for respiratory depression; nurses and parents/guardians should be advised to watch for signs of morphine overdose.
For many years codeine has been more or less universally used in pediatric practice for the relief of moderate pain, as a step-down medication, and as a take-home medication on discharge.Possible dilemmas include the following:
- Due to its widespread use, various “child-friendly” preparations and formulations exist to provide versatile delivery systems for all ages. However, recent developments may have led to discouraging the development of versatile formulations of other similar-“strength” opioids. Thus, many countries are left with limited or no suitable alternatives to codeine.
- Licensing and use of alternative drugs is lagging in certain countries. For instance, tramadol is not licensed in the U.K. for patients below 12 years of age.
- There are few data to determine if any the available alternatives are as effective as codeine.
- Although morphine is the most logical alternative to codeine, issues of concern include controlled drug regulations in certain countries, institutional and local practices, and the reluctance of some healthcare professionals to prescribe oral morphine due to social concerns and the perceived potential for abuse.
- For institutions and countries where other safe, effective, and versatile formulations of codeine alternatives, such as tramadol, oxycodone, and buprenorphine, are available, local interim guidelines may need to be agreed upon (with or without the continued use of codeine) in order to continue to provide safe and effective analgesia to the pediatric population.
- These issues may encourage pharmaceutical companies to develop more “child-friendly” analgesic products and encourage similar research of other opioids to validate their efficacy in children.
- These concerns may also lead to the development of commercially viable patient genotyping.
Like codeine and oxycodone, morphine is very effective when given orally, but only about 40% of an oral dose of morphine reaches the systemic circulation. In the past, this led many to inappropriately conclude that morphine is ineffective when administered orally; instead, the lack of efficacy was simply the result of inadequate PO dosing. Therefore, when converting a patient’s required intravenous morphine dose to an oral maintenance dose, one must multiply the intravenous dose by a factor of 2 to 3.
NYSORA Tips
- When converting a patient’s required intravenous morphine dose to an oral maintenance dose, multiply the intravenous dose by a factor of 2 to 3.
Whereas oral morphine is prescribed alone, oral codeine, hydrocodone, oxycodone, and tramadol are usually prescribed in combination with either acetaminophen or aspirin. Acetaminophen potentiates the analgesia produced by codeine (and other opioids) and allows the use of a smaller dose of the opioid with satisfactory analgesia. In all “combination preparations,” beware of inadvertently administering a hepatotoxic dose of acetaminophen when increasing opioid doses for uncontrolled pain. Because of this concern, it is preferred to prescribe the opioid and acetaminophen (or ibuprofen) separately.Although it is an effective analgesic when administered parenterally, intramuscular codeine has no advantage over morphine or any other opioid; hence, its use is discouraged. Similar to codeine, tramadol is used to treat moderate to severe pain. Although tramadol is often categorized as a μ-receptor agonist, it has multiple proposed mechanisms of action. It is also a serotonin releaser, a norepinephrine reuptake inhibitor, and an NMDA receptor antagonist. The licensing age of tramadol varies by country, but it has been studied in children as young as 1 year. Tramadol is prescribed in a dose of 1–2 mg/kg every 6 hours to a maximum of 400 mg/day (in 4 divided doses for patients over 50 kg).
Hydrocodone is prescribed in a dose of 0.05–0.1 mg/kg. An elixir is available as 2.5 mg/5 mL combined with acetaminophen 167 mg/5 mL. As a tablet, it is available in hydrocodone doses between 2.5 mg and 10 mg, combined with 500–650 mg acet-aminophen. Oxycodone is prescribed in a dose of 0.05–0.1 mg/kg. Unfortunately, an elixir is not available in most pharmacies. When it is, it is prepared as either 1 mg/mL or 20 mg/mL. This can obviously result in catastrophic dispensing errors. In tablet form, oxycodone is commonly available as a 5-mg tablet or as Tylox (500 mg acetaminophen and 5 mg oxycodone) or Percocet (325 mg acetaminophen and 5 mg oxycodone). Oxycodone is also available without acetaminophen in a sustained-release tablet for use in chronic pain. Like many other timed-release tablets, it must not be crushed and therefore cannot be administered through a gastric tube. Breaking the tablet results in the immediate release of a huge amount of oxycodone. Like sustained-release morphine (see below), sustained-release oxycodone is only for use in opioid-tolerant patients with chronic pain, not for routine postoperative pain. Also, note that in patients with rapid gastrointestinal transit, sustained-release preparations may not be absorbed at all (liquid methadone may be an alternative).
Oral morphine is available as a liquid in various concentrations (as much as 20 mg/mL), a tablet (eg, MSIR [morphine sulfate immediate release], available in 15- and 30-mg tablets), and in a sustained-release preparation. Because it is so concentrated, the liquid is particularly easy to administer to children and severely debilitated patients. Indeed, in terminal patients who cannot swallow, liquid morphine provides analgesia when simply dropped into the patient’s mouth.
Patient- and Parent / Nurse-Controlled Analgesia
Among the many reasons for the undertreatment of pediatric pain is the lack of familiarity of physicians (and nurses) with appropriate drugs, drug dosing, and routes of administration. When drugs are given on demand (PRN), there is a lag between the time of the patient’s request and the nurse’s response and the preparation and administration of analgesia. In moderate to severe pain, around-the-clock administration interval administration (eg, q4h) is not always the answer either, however, because of the great individual variation in pain perception and opioid metabolism. Indeed, fixed doses and time intervals make little sense. Based on the pharmacokinetics of opioids, it should be clear that intravenous boluses of morphine may need to be given at intervals of 1–2 hours to avoid marked fluctuations in plasma drug levels.Continuous intravenous infusions may provide steady analgesic levels, are preferable to intramuscular injections, and have been used with great safety and effectiveness in children. However, they are not a panacea because the perception and intensity of pain are not constant. For example, a postoperative patient may be very comfortable resting in bed and require little adjustment in pain management. But this same patient may experience excruciating pain when coughing, voiding, or getting out of bed. Thus, rational pain management requires some form of titration to effect whenever any opioid is administered. To give patients (in some cases nurses and, rarely, parents/guardians) some measure of control over pain therapy, demand analgesia, or patient-controlled analgesia (PCA), devices have been developed. These devices are microprocessor-driven pumps with a button that the patient presses to self-administer a small dose of opioid.
PCA devices allow patients to administer small amounts of an analgesic whenever they feel a need for more pain relief. The opioid, usually morphine, hydromorphone, or fentanyl, is administered either intravenously or subcutaneously. The dosage of opioid (with or without background infusion), number of boluses per hour, and the time interval between boluses (the “lockout period”) are programmed into the equipment by the pain service physician or nurse to allow maximum patient flexibility and a sense of control with minimal risk of overdosage. Generally, when older patients know that if they have severe pain, they can obtain relief immediately, many prefer dosing regimens that result in mild to moderate pain in exchange for fewer side effects such as nausea or pruritus. Typically, morphine is prescribed, 20 mcg/kg per bolus (or hydromorphone 3–4 mcg/kg/h or fentanyl 0.5 mcg/kg/h), with a 5- to 15-minute lockout interval between each bolus. Variations include larger or smaller boluses, shorter or longer time intervals, and varying background infusion; these tend to be based on institutional practice and preferences.The PCA pump computer stores within its memory the number of boluses the patient has received as well as the number of attempts the patient has made to receive boluses. This allows the physician to evaluate how well the patient understands the use of the pump and provides information to program the pump more efficiently. Most PCA units allow low “background” continuous infusions (eg, morphine 2–30 mcg/kg/h, hydromorphone 3–4 mcg/kg/h, fentanyl 0.5-1 mcg/kg/h) in addition to self-administered boluses. A continuous background infusion is particularly useful at night and often provides more restful sleep by preventing the patient from awakening in pain. However, it also increases the potential for overdosage. Although the adult literature on pain does not support the use of continuous background infusions, our experience has been that continuous infusions are essential for good pain management in the pediatric patient. Indeed, in our practice, we almost always use continuous background infusions when we prescribe PCAs or nurse-controlled analgesia (NCA).
PCA requires a patient with enough intelligence, manual dexterity, and strength to operate the pump. Thus, these devices were initially limited to adolescents, but the lower age limit in whom this treatment modality can be used continues to fall (currently around age 5–6 years). Contraindications to the use of PCA include inability to push the bolus button, inability to understand how to use the machine, and patient desire not to assume responsibility for his or her care.In patients considered below “competent” age, neonates, toddlers, and patients with complex needs, the practice of allowing surrogates such as nurses to initiate a PCA bolus is called nurse-controlled analgesia (NCA). This is standard practice in our institution. It has been demonstrated that nurses and, in rare cases, parents can be empowered to initiate PCA boluses and to use this technology safely in children, even in those younger than 1 year of age, the incidence of common opioid-induced side effects being similar to that observed in older patients. NCAs tend to have a slightly higher background infusion rate and longer lockout period than PCAs. For neonates and infants 1–3 months old, we use NCA morphine: a background infusion of 5 or 10 mcg/kg/h, respectively, with a bolus of 5 mcg/kg and a lockout of 60 minutes.
Interestingly, respiratory depression is very rare, but does occur, reinforcing the need for close monitoring and established nursing protocols. Difficulties with PCA include its increased cost, patient age limitations, and the bureaucratic obstacles (protocols, nurse education, storage arrangements) that must be overcome before its implementation.
Transmucosal, Intranasal, and Transdermal Fentanyl
Because fentanyl is extremely lipophilic, it can be readily absorbed across any biologic membrane, including the skin. Thus, it can be given painlessly by new, nonintravenous routes of drug administration, including the transmucosal (nose and mouth) and transdermal routes. The transmucosal route of fentanyl administration is extremely effective for acute pain relief. When given intranasally (2 mcg/kg), it produces rapid analgesia that is equivalent to intravenously administered fentanyl.
Alternatively, fentanyl has been manufactured in a candy matrix (Actiq) attached to a plastic applicator (it looks like a lollipop) for transoral/transmucosal absorption. As the child sucks on the candy, fentanyl is absorbed across the buccal mucosa and is rapidly (over 10–20 minutes) absorbed into the systemic circulation. If excessive sedation occurs, the fentanyl is removed from the child’s mouth by the applicator. This method is more efficient than ordinary oral-gastric intestinal administration because transmucosal absorption bypasses the efficient first-pass hepatic metabolism of fentanyl that occurs after enteral absorption into the portal circulation. Actiq has been approved by the FDA for use in children for premedication before surgery and for procedure-related pain (eg, lumbar puncture, bone marrow aspiration). It is also useful in the treatment of cancer pain and as a supplement to transdermal fentanyl. When administered transmucosally, fentanyl is given in doses of 10–15 mcg/kg, is effective within 20 minutes, and lasts approximately 2 hours. Approximately 25%–33% of the given dose is absorbed. Thus, when administered in doses of 10–15 mcg/kg, blood levels equivalent to 3–5 mcg/kg IV fentanyl are achieved. The major side effect, nausea and vomiting, occurs in approximately 20%–33% of patients who receive it.
The transdermal route is frequently used to administer chronically administered drugs, including scopolamine, clonidine, and nitroglycerin. Many factors, such as body site, skin temperature, skin damage, ethnic group, and age, affect the absorption of transdermally administered drugs. Placed in a selective semipermeable membrane patch, a reservoir of drug provides slow, steady-state absorption of drug across the skin. The patch is attached to the skin by a contact adhesive, which often causes skin irritation.The use of transdermal fentanyl has revolutionized adult cancer pain management. As fentanyl is painlessly absorbed across the skin, a substantial amount is stored in the upper skin layers, which then acts as a secondary reservoir. The presence of a skin depot has several implications: it dampens the fluctuations of fentanyl effect, it needs to be reasonably filled before significant vascular absorption occurs, and it contributes to a prolonged residual fentanyl plasma concentration after patch removal. Indeed, the amount of fentanyl remaining within the system and skin depot after patch removal is substantial. At the end of a 24-hour period, approximately 30% of the total delivered dose from the patch remains in the skin depot. Thus, removing the patch does not stop the continued absorption of fentanyl into the body.
Because of the long onset time, inability to rapidly adjust drug delivery, and long elimination half-life, the use of transdermal fentanyl for acute pain management is controversial. As stated above, the safety of this drug delivery system is compromised even further because fentanyl continues to be absorbed from the subcutaneous fat for almost 24 hours after the patch is removed. In fact, the use of this drug delivery system for acute pain has resulted in the death of an otherwise healthy patient. Transdermal fentanyl is generally reserved for patients with chronic pain (eg, cancer) and those who are opioid tolerant. Even when transdermal fentanyl is appropriate, the vehicle imposes its own constraints. The lowest-dose fentanyl “patch” delivers 25 mcg fentanyl per hour; the others deliver 50, 75, and 100 mcg of fentanyl per hour. Patches cannot be physically cut into smaller pieces to deliver less fentanyl. This often limits usefulness in patients with lower body weights, and, as with other opioids, this drug delivery system has neither been tested nor approved for use in children.
A new noninvasive method of transdermal PCA is on the horizon. Using iontophoresis (electrotransport), small doses of fentanyl (40 mcg) can be self-administered across the skin (E-Trans, Alza Corporation). Transdermal PCA may offer logistic advantages for patients and nursing staff by eliminating the need for venous access, IV tubing, and specialized pumps.
Complications
Regardless of method of administration, all opioids produce common unwanted side effects, such as pruritus, nausea and vomiting, constipation, urinary retention, cognitive impairment, tolerance, and dependence. Indeed, many patients suffer needlessly from agonizing pain because they would rather suffer pain than experience these opioid-induced side effects. In addition, physicians are often reluctant to prescribe opioids because of these side effects and because of their fear of other less common, but more serious, side effects such as respiratory depression. Several clinical and laboratory stud-ies have demonstrated that low-dose naloxone infusions (0.25–1 mcg/kg/h) can treat or prevent opioid-induced side effects without affecting the quality of analgesia or opioid requirements.
Some opioids, when given concomitantly with selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs), or serotonin–norepinephrine reuptake inhibitors (SNRIs), have been associated with serotonin syndrome; these include fentanyl, oxycodone, hydrocodone, and tramadol.
Transition to Oral Medication
Successful transition from intravenous (or epidural) analgesics to oral medication depends on the clinician’s ability to provide alternative therapy that is palatable, acceptable, and above all, equally effective in treating pain. There are many advantages in providing pain medication by the oral route. Enteral therapy consists of a less invasive route of drug administration and enables children to more rapidly return to their normal lives. Moreover, oral medications are easier and less expensive to deliver than IV and epidural drugs.Certain criteria are essential for the successful transition to oral medication. Normal gastrointestinal function must be present before attempting enteral therapy. Thus, the child must be able to drink and/or eat (or have a functioning gastric tube). A child who is nauseated or vomits after eating will simply not tolerate oral analgesics. Second, severe pain is difficult, if not impossible, to control with oral analgesics alone. Therefore, oral analgesics should be reserved for the treatment of mild to moderate pain during the latter part of the recovery process. Assessment of the degree of pain and existing treatment modal-ities are steps that aid the transition process. Third, an oral formulation that is palatable and appropriate must be available. Finally, one must convert the current parenteral opioid dosing to a roughly equianalgesic oral dose.
This conversion is fairly straightforward even when patients are receiving multiple forms and doses of parenteral opioids. As a first step, convert the entire daily dose of administered opioids into IV morphine equivalents (Example 1). Then, convert that morphine dose to an equianalgesic dose of oral morphine (1:2) or other oral opioid, if desired. This formula actually underes-timates the bioequivalence of the drugs but is used to minimize the risk of overdose during the transition.
Example 1
A 5-year-old, 20-kg boy was the victim of a motor vehicle acci-dent and sustained a pelvic fracture. He has been on IV PCA morphine for 2 weeks and will be discharged home for further outpatient therapy and recovery. He receives morphine 2 mg/h and averages one bolus of 0.5 mg morphine every hour. He cannot swallow pills.
Step 1: 2 mg/h for 24 hours = 48 mg morphine/24 hours
Step 2: 0.5 mg/bolus for 24 boluses/day = 12 mg morphine
Step 3: Total 24-hour morphine = 48 mg + 12 mg = 60 mg
Step 4: 60 mg IV morphine = 120 mg PO morphine (actually, this represents a 25%–40% decrease in bioequivalence)
Step 5: Prescribe oral morphine 20 mg every 4 hours and an analgesic with antipyretic activity (eg, acetaminophen or ibuprofen).
Step 6: Stop the basal opioid infusion (PCA) immediately or concomitantly with oral dose; increase oral dose by 20%–25% if pain relief is deemed inadequate. If the opioid requirement is high, PCA can be used to provide “rescue” boluses only for the period of transition or to wean the background infusion/PCA doses to more manageable oral doses.
Local Anesthetics
Over the past 25 years, the use of local anesthetics and regional anesthetic techniques in pediatric practice has undergone a dramatic change. Unlike most drugs used in medical practice, local anesthetics must be physically deposited at their site of action by direct application. This requires patient cooperation and the use of specialized needles and equipment; because of this, children were long considered poor candidates for regional anesthetic techniques because of their overwhelming fear of needles. However, once it was recognized that regional anesthesia could be used as an adjunct, and not as a replacement for general anesthesia, its use has increased exponentially. Regional anesthesia offers the anesthesiologist and pain specialist many benefits. It modifies the neuroendocrine stress response, provides profound postoperative pain relief, ensures a more rapid recovery, and may shorten the hospital stay. Furthermore, because catheters placed in the epidural, upper or lower extremity, or lumbar plexi can be used for days or months, local anesthetics are increasingly being used not only for postoperative pain relief but also for medical (eg, sickle cell vasoocclusive crisis), neuropathic, and terminal pain relief. These techniques range from simple infiltration of local anesthetics to neuraxial blocks (eg, spinal and epidural analgesia). With the use of ultrasound guidance, regional anesthesia in the pediatric population has gained further popularity. Peripheral nerve blocks can also provide significant pain relief following many common pediatric procedures and have the potential to replace or provide an alternative to “gold standard” epidural treatment. This is particularly true in the neonatal population where paravertebral block for a thoracotomy or a transverse abdominis plane (TAP) block for a laparotomy can replace an epidural for effective pain relief and avoid the risks associated with neuroaxial block. To be used safely, a working knowl-edge of anatomy, limitations of technique, and differences in how local anesthetics are metabolized in infants and children is necessary. All aspects of local anesthetics are discussed in detail in previous chapters.
Other Adjutants in a Multimodal Analgesia Regime
Gabapentin
Gabapentin is well established in chronic pain management. A few studies have shown that perioperative gabapentin reduces acute postoperative opioid consumption in patients undergoing a variety of surgeries, including coronary artery bypass and knee arthroplasty. In one study, gabapentin was shown to reduce perioperative opioid use but not opioid-related side effects in pediatric patients undergoing posterior spinal fusion. Dosing varies from a single perioperative dose to treatment for 1–2 weeks. At our institution, we use gabapentin for spinal fusion surgeries and selected surgeries where postoperative pain relief is deemed challenging. We normally administer gabapentin at a dose of 5–10 mg/kg every 8 hours for 5 days. For patients with complex needs, doses may need to be revised down, as in some cases, gabapentin can produce noticeable sedation or drowsiness.
Ketamine
Ketamine is a well-known anesthetic that produces dissociative anesthesia but also provides a very good-quality analgesia at very low doses via its NMDA receptor antagonist activity. Healthcare professionals remain wary of ketamine, however, due to its unpleasant side effect of hallucinations and its recent implication in neuroapoptosis in the developing brain. These concerns have now led to the avoidance of ketamine use in patients under 1 year of age and has also decreased the popularity of ketamine as an additive in caudal and epidural blocks.
However, in older children and adolescents, ketamine is still widely used with good effect. Ketamine can also be added to morphine PCA in a 1:1 ratio. At our institution, a single low dose of ketamine (0.1–0.25 mg/kg) forms part of a balanced intraoperative analgesic regime for adenotonsillectomy and major surgeries. Ketamine infusion is also used as a second-line IV analgesic for complex and painful conditions and in cases of acute or chronic pain where other treatments have failed to produce effective analgesia. The infusion dose of ketamine used is 0.05–0.2 mg/kg/h (using a drug concentration of 0.1 mg/kg/mL, up to a maximum of 250 mg in 50 mL).
Magnesium
Magnesium is used in variety of medical emergencies and treatments. The intravenous use of magnesium has been reported to improve postoperative analgesia. Though the mechanism of action is not yet fully understood, the analgesic properties of magnesium are believed be due to the regulation of calcium influx into the cell and NMDA receptor antagonist activity. Evidence has been equivocal, however, and although relatively safe, magnesium is not without side effects. A recent meta-analysis concluded that perioperative intravenous magnesium reduces opioid consumption, and to a lesser extent pain scores, in the first 24 hours postoperatively without any reported serious adverse effects. The use of magnesium, either as a bolus or infusion (at a dose of 30–50 mg/kg), for major surgeries, particularly spinal fusions, and for other major orthopedic and general surgeries, is standard practice at our institution.
CHRONIC PAIN MANAGEMENT
THE TRANSITION FROM ACUTE TO CHRONIC PAIN
Acute pain has evolved as a vital defense mechanism, alerting the animal to injury and physical harm in order to stop the exposure to injury, such as with the pain reflex or to signal the need for rest to allow healing to occur. Chronic pain, however, serves no protective function. Chronic pain is considered to be pain that extends beyond the expected period of healing. There are increasing studies in the adult literature demonstrat-ing the development of postoperative chronic pain, which is then associated with significant negative consequences for the individual, in terms of both physical and mental health, and for the wider society, in terms of both economic and healthcare resource burdens. The incidence of chronic postoperative pain varies depending on surgery type, with estimates between 5% and 50% in the most common surgical procedures, including hernia repair, hip replacement, and cholecystectomy, versus up to 85% in amputations. Although the literature for the development of chronic pain postoperatively is sparse in pediatric age ranges, studies are beginning to indicate that it does occur, although its incidence may be lower than in the adult population.
Fortier studied 113 children between the ages of 2 and 17 years who had undergone general urological or orthopedic surgery and found that 13.3% reported chronic pain resulting from surgery. The surgeries most related to the development of chronic pain were orthopedic. Over one-quarter reported interference in sleep patterns and extracurricular activities, and 1 in 6 reported interference in school activities. Chronic pain in adults after inguinal surgery has a reported incidence of 5%–35%; however, in children, studies suggest the incidence may be lower. One particular surgery in adolescents associated with a high incidence of persistent pain is scoliosis, where estimates of 50% are reported. A study by Wong demonstrated a trend for those who experienced more severe postoperative pain to have a greater tendency toward developing persistent pain; only 39% of those with mild postoperative pain developed persistent pain compared with 74% of those with severe postoperative pain. Studies have indicated that persistent pain can be a complication for as long as 12 months after surgery; however, thoracotomy in childhood has been associated with pain persisting into adulthood up to 30 years later. This risk appears less when surgery takes place at a younger age and increases with the age at which surgery is performed. Although the mechanisms for the transition from acute to chronic pain are complex, some risk factors identified in the adult literature (in the absence of many studies in children) may also be relevant to the pediatric population. These include the presence of preoperative pain, the severity of acute postoperative pain, and open versus laparoscopic surgery. Good postoperative multimodal pain management therefore plays an important role in efforts to prevent the subsequent development of persistent pain in children.
NYSORA Tips
- Chronic pain can be a postoperative complication in the pediatric population.
- Chronic pain has a negative impact on sleep, activities, school attendance, and school achievement.
- Risks for the development of chronic pain include pre-operative pain, the severity of acute postoperative pain, and open versus laparoscopic surgery.
THE MANAGEMENT OF ACUTE-ON-CHRONIC PAIN
The management of patients undergoing surgery with preoperative chronic pain can be problematic, and the presence of preoperative pain increases pain perception postoperatively. Good preoperative preparation and planning are important, incorporating education for both the child and parents/guardians and involving them in decision making. Psychological interventions, such as cognitive behavioral therapy and decatastrophization can be beneficial preparation. Patients experiencing preoperative pain may be taking adjuvant analgesia medications, some of which should not be stopped abruptly, such as anticonvulsants (eg, gabapentin). Other medications may have significant interactions with commonly used analgesic medications; for example, the combination of amitriptyline and tramadol has resulted in serotonin syndrome. There are a small number of pediatric patients who present for surgery on long-term opioids, and not all of these patients will be palliative. The prolonged administrations of opioids may occur in children in intensive care units, those requiring frequent and repeated surgery (eg, for burns), those with frequent disease exacerbations (eg, sickle cell), adolescents using opioids illicitly, and in a small number of children with medically unexplained pain. Recommendations for the management of such patients are primarily extrapolated from the adult literature due to a lack of evidence in children (Table 5).
TABLE 5. Considerations for the perioperative management of children on long-term opioids.
| Educate and involve the child and his or her parents/guardians in preoperative planning. |
| Continue maintenance opioids or consider conversion to parenteral opioids |
| Supplementation with systemic opioid (oral or intravenous) may be necessary if a neuroaxial technique is used. |
| Titrate opioids postoperatively. |
| The dose may need to be 30%–100% higher than for opioid-naïve patients. |
| Use multimodal therapy, including adjuvant medications (eg, ketamine) and nonpharmacologic techniques. |
| Use regional anesthesia techniques where possible. |
| When transitioning from parenteral opioids to oral delivery calculate total dose requirement over 24 hours. Deliver 50% of the estimated oral dose as long-acting and the remainder as breakthrough. |
| Taper oral opioids to preoperative doses slowly, over a period of 2-4 weeks. - In the first 24 postoperative hours, reduce the dose by 20–40%. - Following the first 24 postoperative hours, reduce the dose by 5–20% (use a slower rate in the presence of withdrawal signs). |
| Ensure chronic pain service is involved in the transition to long-term pain management. |
SUMMARY
The past 25 years have seen an explosion in research and interest in pediatric pain management. In this brief review, we have tried to consolidate in a comprehensive manner some of the most commonly used agents and techniques in current practice.
Clinical updates
Hadland et al. (Pediatrics, 2024) reported the first American Academy of Pediatrics clinical practice guideline addressing opioid prescribing for acute pain in children and adolescents in outpatient settings. Multimodal analgesia is recommended as standard care, with opioids avoided as monotherapy. When indicated, immediate-release opioids at the lowest age- and weight-appropriate dose and for no more than 5 days are advised, except after major trauma or surgery. Codeine and tramadol are contraindicated in young children and high-risk adolescents. Naloxone co-prescribing, caregiver education on safe storage and disposal, and mitigation of inequities in pediatric pain treatment are core components of the guideline.
- Read more about the study HERE.
Trottier et al. (Paediatrics & Child Health, 2022) outlined evidence-based best practices for pediatric pain assessment and management in this Canadian Paediatric Society position statement. Pain assessment should occur early and be repeated regularly using developmentally appropriate, validated tools, with self-report prioritized whenever feasible. Behavioral scales are recommended for preverbal or cognitively impaired children, supplemented by caregiver input. Effective pain management integrates pharmacologic, physical, and psychological strategies in a family-centred model. Early pain treatment is shown to improve patient comfort and clinical evaluation without delaying diagnosis or care.
- Read more about the study HERE.
Gai et al. (Journal of Anesthesia, 2020) reviewed contemporary approaches to acute pain management in hospitalized children using a structured, multimodal framework. Developmentally appropriate pain assessment, prioritizing self-report and validated behavioral tools, underpins effective treatment. Scheduled non-opioid analgesics (acetaminophen and NSAIDs) are recommended as first-line therapy, with opioids reserved for moderate-to-severe pain. Clear guidance is provided for safe opioid dosing, PCA use, and monitoring for adverse effects. Adjunctive strategies—including ketamine infusions, gabapentinoids, regional anesthesia, and non-pharmacologic interventions—reduce opioid exposure while improving analgesia.
- Read more about the study HERE.