Christiana C. Burt, Sanford M. Littwin, Jolaade Adebayo, Navin A. Mallavaram, and Daniel M. Thys
INTRODUCTION
The decision to utilize regional anesthesia is dependent on many factors. Patient characteristics, the type of surgery proposed, and the potential anesthetic risks will all have an impact on anesthetic choice and perioperative management. In patients with cardiovascular disease, regional anesthesia techniques (either alone or in conjunction with general anesthesia) can offer the potential perioperative benefits of stress response attenuation, cardiac sympathectomy, earlier extubation, shorter hospital stay, and intense postoperative analgesia. However, the decision to utilize regional anesthesia should be made with caution in some circumstances. The aim of this chapter is to provide an overview of the physiological effects of different regional anesthesia techniques on the cardiovascular system, to examine the role of regional anesthesia in cardiac surgery and noncardiac surgery and to provide an overview of the physiological requirements of patients with different types of cardiac and vascular disease.
THE CARDIOVASCULAR EFFECTS OF REGIONAL ANESTHESIA
Thoracic Epidural Anesthesia
High thoracic epidural anesthesia (TEA) from T1–T5 blocks the cardiac afferent and efferent sympathetic fibers with a loss of chronotropic and inotropic drive to the myocardium and reduced perception of cardiac pain.In healthy volunteers, there is some evidence that thoracic epidural block reduces left ventricular contractility as measured by transesophageal echocardiography and that this effect is present in high thoracic epidural block but not in low thoracic epidural block, which is consistent with a loss of inotropic drive to the myocardium with high epidural block. During exercise, it has been reported that TEA does not affect oxygen consumption (VO2) but does reduce systemic arterial blood pressure compared to control subjects. Another study compared the cardiovascular effects of 0.5% bupivacaine administered via the thoracic epidural route against the effects when administered via the intramuscular route and found no significant difference and postulated whether the effects of epidural anesthesia may in part be due to systemic effects. However, their conclusions are limited by the low number (9) of subjects enrolled.
Several studies have documented the effects of TEA on cardiovascular function in patients with heart disease. In a small study of 10 patients scheduled for thoracotomy, a TEA with a mean analgesic level of C7 to T5 had only minor effects on the cardiovascular system. In patients with severe coronary artery disease and unstable angina pectoris, Blomberg et al observed that TEA relieved chest pain. It also significantly decreased heart rate and systolic arterial, pulmonary arterial, and pulmonary capillary wedge pressures without any significant changes in coronary perfusion pressure, cardiac output, stroke volume, or systemic or pulmonary vascular resistances. The investigators also found that TEA may increase the diameter of stenotic epicardial coronary arteries in patients with coronary artery disease without causing a dilation of coronary arterioles.
Intraoperatively, during abdominal aortic aneurysm surgery, Reinhart et al observed a lower cardiac index and O2 delivery (QO2) in patients receiving TEA and general anesthesia (GA) than in those receiving GA alone; VO2 was similar. They also reported that the oxygen supply–demand ratio (QO2/VO2) was less in the TEA group throughout the perioperative period and about 30% below baseline values during early recovery. The authors attributed the reduced adaptation of cardiac output to tissue O2 needs during TEA to negative inotropic and chronotropic effects of sympathetic block. In patients on chronic β-adrenergic blocking medication, TEA has been reported to induce a moderate decrease in mean arterial pressure and coronary perfusion pressure, but without producing clinically significant cardiovascular effects.
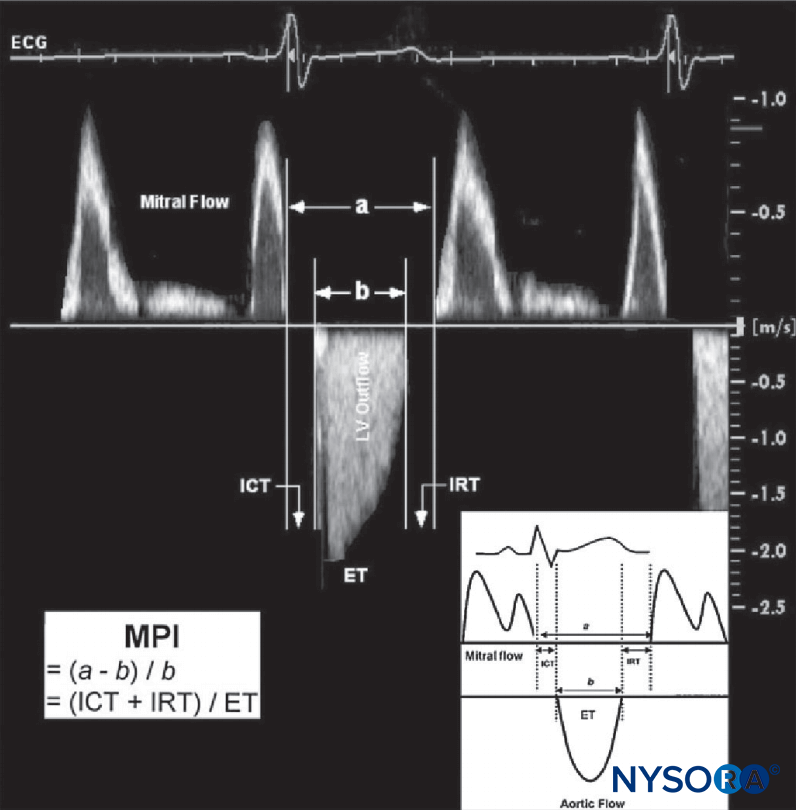
Conversely, improvements in parameters of cardiac function have been reported, specifically in improved regional left ventricular function during coronary artery bypass surgery. This was attributed to the cardiac sympathectomy effect of the thoracic epidural. A separate study evaluating left ventricular systolic and diastolic function in patients with coronary artery disease found that TEA induced a significant improvement in left ventricular diastolic function, whereas indices of systolic function did not change (Figure 1).
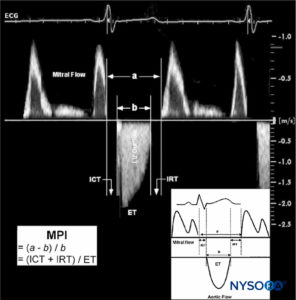
FIGURE 1. Estimation of the myocardial performance index (MPI; Tei index). MPI is calculated from two time intervals as a–b/b. Interval a: from cessation to next onset of mitral flow. Interval b: from onset to cessation of aortic flow. Time intervals a and b are indicated in milliseconds. A typical example of measuring the MPI using Doppler ECG registration of mitral and aortic flow velocity profiles is demonstrated. For illustrative purposes, the original Doppler tracings of mitral inflow and left ventricular (LV) outflow are plotted together. ET, ejection time of LV outflow; ICT, isovolumic contraction time; IRT, isovolumic relaxation time. (Reproduced with permission from Schmidt C, Hinder F, Van Aken H, et al: The effect of high thoracic epidural anesthesia on systolic and diastolic left ventricular function in patients with coronary artery disease. Anesth Analg. 2005 Jun;100(6):1561–1569.)
Hemodynamic changes during laryngoscopy and intubation can increase the risk of ischemia in some patients with cardiac disease. Licker et al. reported that patients who received TEA in addition to GA had smaller increases in mean arterial pressure and heart rate during laryngoscopy and tracheal intubation than those who received GA only; this would suggest that TEA affords hemodynamic protection during these maneuvers.
The effect of TEA on hemodynamic stability during open abdominal aortic surgery has been investigated, with findings of minimal effects on cardiac index (CI) and pulmonary capil-lary wedge pressure (PCWP) reported during aortic cross-clamping in a group who received GA with TEA as opposed to detrimental effects (decrease in CI and increase in PCWP) seen in the group who received GA only. Whether this outcome results in a difference in morbidity or mortality is unclear, though, with some groups reporting no difference in outcome, and one reporting detrimental effects in the epi-dural group with rebound myocardial ischemia seen on termination of the epidural.
TEA has been reported to be beneficial in morbidly obese patients undergoing gastric bypass surgery with better postoperative pain relief but no firm conclusions regarding cardiovascular function other than a significant reduction in SVR and intrapulmonary shunt compared to GA.
The clinical effect of cardiac sympathectomy and peripheral vasodilation caused by TEA appears to vary between populations of patients. The level of sympathetic block that follows a TEA depends in part on the degree of sympathetic tone before the block, which may explain some of the different effects on the cardiovascular system reported by different studies. In addition, the effect on cardiac function will depend on the exact nature of the patient’s cardiovascular disease. This is explored in more detail later in this chapter.
Lumbar Epidural Anesthesia
Lumbar epidural anesthesia (LEA) predominantly results in a drop in systemic vascular resistance via peripheral vasodilation, without the effects of cardiac sympathectomy that occur with high TEA. The influence of LEA without cardiac sympathectomy on global and regional left ventricular function was investigated prior to surgery in healthy subjects and in patients suffering from stable mild, effort-related angina.19 In both groups, epidural block was performed with 10 mL of 0.5% bupivacaine. Radionuclide angiography was used to determine cardiac output, left ventricular ejection fraction, and end-systolic and end-diastolic volumes and to analyze left ventricular wall motion. Throughout the procedure, patients with a history of angina exhibited neither chest pain nor electrocardiographic evidence of myocardial ischemia. At control, left ventricular ejection fraction (LVEF) and systolic pressure–volume ratio (SPVR) were lower in the patients with angina. These patients also had evidence of regional left ventricular dysfunction. Epidural block without volume loading resulted in slight improvements in LVEF and regional function. Such changes were not observed in normal patients. After volume loading, the improvements in ventricular function subsided. These observations led the authors to conclude that lumbar epidural anesthesia may improve global and regional ventricular function in patients with angina provided volume loading is limited.
In hypertensive patients, LEA has been shown to cause decreases in mean arterial pressure with associated decreases in systemic vascular resistance and cardiac output.
The importance of good pain relief in reducing ischemic episodes has been studied in elderly patients undergoing surgery for hip fracture with a reduction in ischemic episodes shown in the groups that received continuous epidural analgesia preoperatively. In addition, lumbar epidural anesthesia may reduce the risk of arterial thrombotic complications in patients undergoing lower extremity revascularization, and this may be as a result of prevention of the postoperative inhibition of fibrinolysis. However, other studies report no difference in major morbidity and mortality in patients with high cardiac risk undergoing peripheral vascular surgery with or without lumbar epidural anesthesia.
The successful use of lumbar epidural anesthesia has been reported in obstetric patients with a variety of types of cardiac disease.
Intrathecal Anesthesia
Intrathecal anesthesia using local anesthetic agents and/or opioids has been investigated in the context of cardiac and noncardiac surgery. Intrathecal anesthesia can be expected to produce profound vasodilation as well as motor and sensory block below the level of action.The hemodynamic response to lumbar spinal anesthesia using single-shot hyperbaric bupivacaine or lidocaine with morphine has been evaluated in cardiac surgical patients. It was observed that the induction of GA produced a decrease in mean arterial pressure and that the addition of spinal anesthesia produced a decrease in heart rate. Heart rate and mean arterial pressure did not change with sternotomy (suggesting good-quality analgesia).
In mixed populations (some with documented ischemic heart disease, some without), there has been no difference found in episodes of myocardial ischemia between patients receiving general anesthesia and patients receiving spinal anesthesia for transurethral surgery, although there was a relatively high rate of silent ischemia in both groups in both studies. An interesting study looking at hemodynamics and markers of myocardial ischemia in patients with coronary artery disease undergoing elective hip surgery found that while the number of patients who experienced episodes of ST segment depression did not differ between those who received incremental spinal anesthesia, single-shot spinal anesthesia, or general anesthesia, 56% of hypotensive patients developed ST-segment depression compared to only 10% of normoten-sive patients (P < 0.003). The incidence of hypotension and myocardial ischemia was lowest in the group receiving incremental spinal anesthesia.
Various investigators have reported on the effects of different doses of intrathecal local anesthetic as a single-shot spinal. A dose of 7.5 mg hyperbaric bupivacaine in combination with 5 mcg sufentanil has been reported to produce reliable anesthesia for repair of hip fractures in elderly patients with few episodes of hypotension and little need for vasopressor support of blood pressure. Other investigators have reported 4 mg bupivacaine with 20 mcg fentanyl to be effective in the same population.
Intrathecal anesthesia has not been reliably shown to have an effect on the stress response to surgery in terms of levels of serum catecholamines and serum cortisol. Some studies have reported a lower stress response during coronary artery bypass grafting (CABG) surgery in patients who received intrathecal bupivacaine in addition to general anesthesia compared to those who received general anesthesia and intravenous opioid (Figure 2), whereas other authors have reported no attenuation of the stress response.
Intrathecal opioid in addition to GA has been studied for elective abdominal aortic surgery. The addition of intrathecal opioid provided more intense analgesia compared to PCA during the first 24 hours postoperatively, but there was no dif-ference between the groups in the incidence of combined major cardiovascular, respiratory, and renal complications or mortality.
A group of patients judged to be at high risk for postoperative myocardial ischemia undergoing either elective hip arthroplasty or peripheral vascular surgery were randomized to receive either spinal anesthesia or general anesthesia. There was no significant difference between the groups in the incidence of myocardial ischemia during or after surgery.
A number of case reports have reported on the usefulness of spinal anesthesia in obstetric patients with a variety of cardiac diseases. Velickovic et al. successfully used continuous spinal anesthesia for two patients with recurrent peripartum cardiomyopathy presenting in congestive heart failure for emergent cesarean section. In one patient, a continuous spinal not only provided adequate anesthesia but also markedly reduced the patient’s symptoms. Others have reported similar success with spinal anesthesia in obstetric patients with hypertrophic obstructive cardiomyopathy, severe pulmonary stenosis, and coronary artery disease.
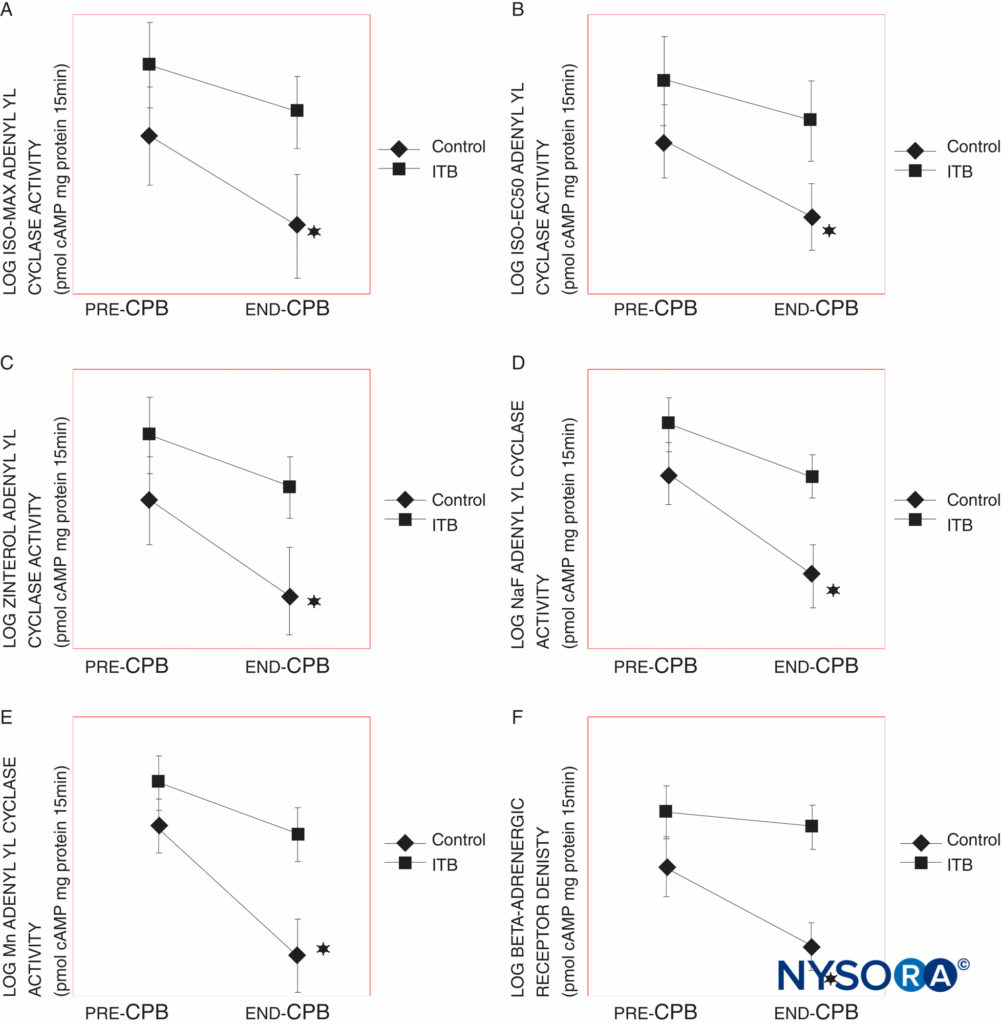
FIGURE 2. A: Maximal isproteronol (ISO MAX), B: 50% maximal isoproterenol (ISO EC50), C: zinterol, D: sodium fluoride (NaF)-stimulated, and E: manganese (Mn)-stimulated -adrenergic receptor (AR) responsiveness, as measured by adenylyl cyclase activity in control and intrathecal bupivacaine (ITB) groups with cardiopulmonary bypass (CPB) times from 61–120 min. The control group shows a significant decline in adenylyl cyclase activity in each of these measures, whereas the ITB group does not. F: AR density in control and ITB groups with CPB times from 61–120 min. The control group shows a significant decline in AR density at P 0.02. Adenylyl cyclase activity and AR density (AR Bmax) are reported as picomoles of cyclic adenosine monophosphate per milligram of protein per 15 min and femtomoles per milligram of protein, respectively. The data were log-transformed. Results are expressed as mean SEM (*P < 0.05, ‡P < 0.005).
Thoracic Blocks (Paravertebral and Intercostal)
The extent of a single percutaneous paravertebral injection was studied in 6 patients with chronic chest wall pain. It was shown that a large unilateral somatic and sympathetic block was obtainable. No significant postural changes in blood pressure were seen, but there was a small but significant decrease in supine heart rate. While it has been shown that paravertebral blocks can spread to the epidural space, a systematic review and meta-analysis concluded that paravertebral block causes less hypotension than epidural block after thoracotomy.
There is anecdotal evidence that paravertebral blocks can be beneficial in patients with ischemic heart disease. Ho et al. reported on the intraoperative resolution of ST-segment depression after a right thoracic PVB, although it is possible that this would have resolved spontaneously without PVB.
Intercostal nerve blocks have similarly been reported to be safe with no adverse hemodynamic consequences with an ultrasound-assisted approach to blocking the intercostal nerves in the midaxillary line for nonreconstructive breast and axilla surgery.
Upper Extremity Regional Anesthesia and Cardiac Disease
Cervical Plexus block
Several studies have investigated the difference in outcome after carotid endarterectomy (CEA) between general anesthesia (GA) and regional anesthesia (RA) in the form of deep and/or super-ficial cervical plexus block. As with other types of vascular surgery, CEA patients are more likely to be hypertensive, diabetic, and at increased risk for cardiac morbidity. In the context of this surgery, however, there are other causes of hemodynamic instability in addition to the effects of pain, specifically baroreceptor stimulation and sensitivity and impaired arterial pressure regulation following cerebrovascular accident (CVA).Although greater hemodynamic stability and reduced cardiovascular complications have been reported with the use of cervical plexus block compared to GA alone, and a meta-analysis including prospective and retrospective studies reported reduced incidences of stroke, myocardial infarction (MI), and death with the use of cervical plexus block without GA, these findings were potentially confounded by bias relating to the use of GA in higher-risk patients. A recent multicenter, randomized, prospective, controlled trial (General Anaesthetic versus Local Anaesthetic for Carotid Surgery [GALA]), which included over 3500 patients randomly assigned to surgery under GA or RA, showed no difference in stroke, MI, or death in the first 30 days after surgery (among other outcomes). It is possible, though, that the highest-risk patients may not have been included in the study and that the study did not address whether there is any difference between GA or RA in higher-risk groups of patients.
Brachial Plexus block
Most of the literature investigating the physiological consequences of interscalene brachial plexus block (BPB) has focused on its effect on the phrenic nerve and ventilatory function. A reasonable conclusion from the literature on hemodynamic effects would appear to be that significant hemodynamic effects occur as a result of intravascular absorption of local anesthetic rather than from the block itself. Continuous interscalene BPB performed with 1.25 mg/kg of 0.5% bupivacaine in 24 patients followed by an infusion of 0.25% bupivacaine at a dose of 0.25 mg/kg/h was reported to result in no hemodynamic problems after 30 minutes and in an undetectable concentration of free plasma bupivacaine after 24 hours of infusion. Interscalene BPB alone for shoulder surgery was found to be more hemodynamically stable than interscalene BPB combined with GA, with a significant decrease in mean arterial pressure (MAP) when the patient was moved into the sitting position with block and GA; heart rate remained stable in both groups. The addition of clonidine to interscalene BPB appears to have haemodynamic effects, with Culebras reporting that a dose of 150 mcg added to 40 mL of 0.5% bupivacaine resulted in a reduction in heart rate and blood pressure but did not prolong the duration of analgesia. Hypotension and bradycardia were also reported to be more common with a dose of 2 mcg/kg clonidine compared to a dose of 1 mcg/kg clonidine added to 30 mL 0.5% bupivacaine.
Lower Extremity Regional Anesthesia and Cardiac Disease
Lower extremity peripheral nerve blocks are associated with minimal hemodynamic disturbances. Fanelli et al. compared the hemodynamic changes induced by unilateral spinal anes-thesia to the changes induced by combined sciatic–femoral nerve block in 20 ASA I–II patients undergoing elective orthopedic surgery with tourniquet. Both groups had adequate anesthesia for the surgery. The combined sciatic–femoral nerve block group (obtained with 7 mg/kg of 2% mepivacaine) resulted in no significant hemodynamic changes, whereas the spinal anesthesia group (8 mg of hyperbaric 0.5% bupivacaine administered intrathecally) experienced small but significant differences in MAP, cardiac index, and stroke volume.
There are several case reports detailing the successful use of lower extremity peripheral nerve blocks in high-risk patients. Chia et al. presented the practical benefits of a combined sciatic–femoral nerve block on a 56-year-old man with severe sepsis and recent myocardial infarction requiring an urgent above-knee amputation. Ho et al. have reported on the use of a combined paravertebral lumbar plexus and parasacral sciatic nerve block for the reduction of a hip fracture in an elderly patient with severe aortic stenosis. Tanaka et al. have described the use of a psoas compartment block (PCB) in a 72-year-old female with severe heart failure due to rheumatoid myocarditis who required an open reduction of a left femoral neck (tro-chanteric) fracture. With the patient in the lateral position with the fractured side up, the block was performed at L3/4’ using a 22-gauge Tuohy needle to inject 10 mL of normal saline and 20 mL of 2% mepivacaine. No complications were reported. Rizzo et al. used regional anesthesia to anesthetize a 32-year-old male patient suffering from Eisenmenger’s syndrome with left-type-only ventricle, who needed an extirpation of meniscus by arthroscopic surgery. The investigators used sciatic, femoral, and lateral cutaneous thigh nerves blocks with ropivacaine without complications.
REGIONAL ANESTHESIA AND CARDIAC SURGERY
Several authors have examined the relationship between regional anesthesia and outcome in cardiac surgery. A selection of the literature looking at thoracic epidural anesthesia is summarized in Table 1, and a summary of the literature looking at intrathecal anesthesia is summarized in Table 2. One consistent conclusion from the literature is that the quality of pain relief overall appears to be better with regional anesthesia compared to intravenous morphine. Some studies also report a reduction in the incidence of atrial fibrillation and other supra-ventricular dysrhythmias with regional anesthesia versus intravenous morphine. Further conclusions regarding outcome in terms of patient morbidity and mortality with or without regional anesthesia are unclear, which is likely related to the nature of the surgery itself and the greater influence of this on parameters such as cardiac function and dysrhythmias.
TABLE 1. Studies investigating thoracic epidural anesthesia in cardiac surgery.
| Author | Year | Population Studied | Type of Technique | Conclusion |
|---|---|---|---|---|
| Richter et al75 | 2002 | 37 patients with refractory angina | TEA | Decreased frequency of angina attacks and nitroglycerin intake. Increased self-rated quality of life. |
| Olausson et al76 | 1997 | 40 patients with severe refractory unstable angina | TEA vs standard anti-anginal therapy | Lower incidence of myocardial ischemia. Shorter duration of ischemic episodes in TEA group. |
| Salvi et al77 | 2004 | 106 patients undergoing OPCAB | TEA + GA | TEA with GA is a feasible technique for OPCAB with intense postoperative analgesia. |
| Kessler et al78 | 2005 | 90 patients undergoing OPCAB | TEA (30) vs TEA + GA (30) vs GA (30) | GA + TEA was the most comprehensive technique, providing good hemodynamic stability and reliable postoperative analgesia. |
| Stritesky et al79 | 2004 | 129 patients undergoing awake on-and-off pump cardiac surgery | TEA | 10 conversions to GA intraoperatively. TEA provided rapid recovery from cardiac surgery. |
| Hansdottir et al68 | 2006 | 97 patients undergoing elective cardiac surgery | GA + TEA (48) vs GA + IV morphine (49) | Shorter time to extubation (2.3 h vs 7.3 h) in the TEA group. No differences in postoperative analgesia, lung volume, degree of ambulation, cardiac morbidity, neurologic outcome, length of ICU stay, or length of hospital stay (LOS). |
| Kessler et al80 | 2002 | 20 patients undergoing OPCAB | TEA | 3 required conversion to GA.High degree of reported patient satisfaction. |
| Anderson et al81 | 2002 | 10 patients undergoing OPCAB via left anterior thoracotomy | TEA | 1 required conversion to GA; 2 required brief periods of assisted ventilation. High degree of patient satisfaction. |
| Noiseaux et al82 | 2008 | 15 patients undergoing OPCAB | TEA + femoral NB | 3 required conversion to GA; 5 experienced postoperative AF. |
| Barrington et al83 | 2005 | 120 patients undergoing CABG | GA vs GA + TEA | No difference in troponin T postoperatively. The TEA group had better analgesia and reduced time to extubation. |
| Kendall et al84 | 2004 | 30 patients undergoing OPCAB | Propofol vs isoflurane vs isoflurane + TEA | No difference in mean troponin T at 24 hours postoperatively. |
| Loick et al85 | 1999 | 70 patients undergoing CABG | GA + TEA vs GA + IV clonidine vs control group | TEA + GA had a beneficial effect on perioperative stress response and decreased postoperative myocardial ischemia as measured by troponin T. |
| Fillinger et al86 | 2002 | 60 patients undergoing cardiac surgery with bypass (prospective RCT) | GA + IV opioid vs GA + TEA | No differences in time to extubation, duration of ICU stay, length of hospital stay, pain control, urinary free cortisol, cardiopulmonary complication rate, or total hospital charges. |
| Scott et al87 | 2001 | 420 patients undergoing CABG (prospective RCT) | GA vs GA + TEA | The TEA group experienced less supraventricular dysrhythmia, better maximal inspiratory lung volume, earlier extubation, less respiratory tract infection, less acute confusion, less acute renal failure, and no neurological complications associated with TEA. |
| Turfrey et al88 | 1997 | 218 patients undergoing CABG (retrospective) | GA vs GA + TEA | The TEA group experienced less dysrhythmia, a trend toward reduced respiratory complications, reduced time to extubation, and no serious neurological complications from the use of TEA. |
| Karagoz et al89 | 2003 | 137 patients undergoing CABG | TEA alone | 5 converted to GA; no mortality; mean LOS in hospital 1 day. |
| Liu SS et al90 | 2004 | Meta-analysis: TEA: 15 trials, 1178 patients undergoing CABG; IT: 17 trials, 668 patients undergoing CABG and CABG-valve. | TEA vs GAIT morphine vs GA | No difference in mortality or MI with central neuraxial analgesia vs GA, but faster tracheal extubation and decreased respiratory complications, cardiac dysrhythmias, and pain scores with neuraxial analgesia. |
| Hemmerling et al91 | 2004 | 30 patients undergoing AVR, AVR + CABG, ascending aorta repair, and PFO repair | TEA + GA | All patients extubated within 15 minutes of end of surgery; no complications related to TEA. |
| Klokocovnik et al92 | 2004 | Case report: 1 patient undergoing awake minimally invasive AVR | TEA alone | Operation proceeded uneventfully; discharge on day 2; no complications within 30 days. |
| Slin’ko93 | 2000 | 55 children aged 1–14 years undergoing cardiac surgery using cardiopulmonary bypass | TEA using lidocaine and fentanyl + GA vs TEA using lidocaine and clonidine + GA | Endocrine stress response decreased in lidocaine–clonidine group compared to lidocaine–fentanyl group. |
| Peterson et al94 | 2000 | 220 children undergoing cardiac surgery (retrospective review) | GA + TEA vs GA + LEA vs GA + caudal vs GA + IT | Extubation in operating room achieved for 89% patients. Lower rate of adverse events following use of TEA compared to others. |
| Hammer et al95 | 2000 | 50 children undergoing open heart surgery (retrospective) | GA + TEA vs GA + IT | No significant differences in incidence of clinically significant changes in vital signs, O2 desaturation, hypercarbia, or vomiting. |
| Royse et al96 | 2003 | 76 patients undergoing CABG with cardiopulmonary bypass (prospective) | GA + TEA (37) vs GA + IV morphine (39) | The TEA group experienced significantly less pain on days 1–2 postoperatively, earlier extubation, improved cooperation with physiotherapy, and a reduced risk of depression and posttraumatic stress. |
| Liem et al97 | 1992 | 54 patients undergoing uncomplicated CABG (retrospective) | GA + TEA (27) vs GA + IV opioid | The TEA group experienced better intraoperative and postoperative pain management and earlier waking and extubation. |
| Hemmerling et al98 | 2004 | 100 patients undergoing OPCAB (prospective audit) | GA + TEA vs GA + IV opioid | Immediate extubation is possible using either TEA or IV opioid for pain relief. TEA results in lower pain scores compared to morphine PCA. |
| Bois et al99 | 1997 | 124 patients undergoing aortic surgery (prospective) | GA + TEA vs GA + IV opioid | The TEA group experienced better postoperative pain control. No difference in incidence of early myocardial ischemia. |
| Ho et al100 | 2002 | 244 patients undergoing CABG (retrospective survey on persistent pain) | GA + TEA vs GA + IV opioid | No difference in frequency or intensity of persistent pain postoperatively. |
| Jensen et al101 | 2004 | 49 patients undergoing cardiac valve surgery | GA + TEA (35) vs GA + IV opioid (14) | TEA provided excellent analgesia in the peri- and postoperative periods. No protective effect on chronic poststernotomy pain. |
| Pastor et al64 | 2003 | 714 patients undergoing CABG with cardiopulmonary bypass (prospective observational) | GA + TEA | TEA inserted in operating room just before surgery. Protocol for management followed. No epidural hematomas detected. |
| Sanchez et al102 | 1998 | 558 patients undergoing CABG | GA + TEA | TEA inserted the day before surgery and left in for 5 days. No documented neuraxial hematomas. |
Many studies examining the time to extubation after cardiac surgery did so in the days before fast-tracking protocols became widespread and as such are less applicable to the majority of practice today. Having said that, regional anesthesia undoubtedly had a role to play in the early days of cardiac fast-tracking and still does in many centers, with a seemingly low risk of complications. It is, however, the potential risk of neuraxial complications in the face of full systemic heparinization and the lack of conclusive data on hard patient outcome measures that has resulted in continued debate and differing practices across centers with regard to the use of neuraxial anesthesia in cardiac surgery. This is illustrated by a survey of 892 cardiac anesthesiologists, in which only 68 (7.6%) reported the use of spinal techniques.
The timing of insertion and removal of the epidural catheter in cardiac surgery is still controversial. Some clinicians will insert the epidural 60–90 minutes before heparinization in off-pump coronary artery bypass (OPCAB) but will insert it a day before surgery in planned cardiopulmonary bypass cases. This method presumably relies on a high success of OPCAB and a low conversion rate to cardiopulmonary bypass intraoperatively. However, it also raises a question regarding the efficient use of resources, as the insertion of an epidural the day before surgery will, in most cases, necessitate admission to hos-pital the day before surgery. Insertion in the operating room without reported epidural hematomas has also been described. Removal of the epidural catheter after surgery requires normalization of coagulation for a period of time. In patients requiring anticoagulation post-cardiac surgery, this practice increases the risk of thromboembolic consequences.
TABLE 2. Studies investigating intrathecal anesthesia in cardiac surgery.
| Author | Year | Population Studied | Type of Technique | Conclusion |
|---|---|---|---|---|
| Vanstrum et al103 | 1988 | 30 patients undergoing CABG (prospective, randomized) | GA + 0.5 mg IT morphine (16) vs GA + placebo (14) | The IT morphine group required less IV morphine and less sodium nitroprusside. No difference in pain scores. |
| Vanstrum et al103 | 1994 | 18 patients undergoing CABG (case series) | GA + IT bupivacaine and morphine | No change in heart rate or MAP with sternotomy. Postoperative analgesic requirements were minimal. |
| Bettex et al104 | 2002 | 24 patients undergoing elective cardiac surgery (prospective, randomized) | GA + IT sufentanil and morphine vs GA + IV sufentanil | The IT sufentanil and morphine group experienced earlier extubation, a reduced need for IV opioids postoperatively, and improved postoperative maximal inspiratory capacity. |
| Mehta et al105 | 2004 | 100 patients undergoing elective OPCAB (prospective, randomized) | GA + IT morphine 8 mcg/kg vs GA + placebo | The IT morphine group experienced better postoperative analgesia, better lung function as measured by spirometry, and earlier extubation. |
| Fitzpatrick et al106 | 1988 | 44 patients undergoing CABG | GA + IT morphine 1 mg (15), GA + IT morphine 2 mg (15) vs GA + IV morphine | he IT morphine groups reported lower pain scores, required less supplementary IV morphine, and had better PEFRs. Mean PaCO2 was significantly higher in patients given 2 mg IT morphine. |
| Latham et al107 | 2000 | 40 patients undergoing elective CABG or valve surgery | GA + IV remifentanil + IT morphine vs GA + IV sufentanil | No difference between regimens in hemodynamic stability or recovery profile. |
| Alhashemi et al108 | 2000 | 50 patients undergoing elective CABG (prospective) | GA + 250 mcg IT morphine vs GA + 500 mcg IT morphine vs GA + placebo | The IT morphine group experienced decreased postoperative IV morphine requirements and no clinically relevant effect on extubation time. The study suggests 250 mcg IT morphine is the optimal dose to provide significant analgesia without delaying extubation. |
| Chaney et al109 | 1997 | 40 patients undergoing cardiac surgery (prospective, randomized) | GA + IT morphine 10 mcg/kg (19) vs GA + IT placebo (21) | No significant difference in postoperative IV morphine use. 3 IT morphine patients experienced prolonged ventilatory depression and delayed extubation. |
| Finkel et al110 | 2003 | 30 children aged 7 months to 13 years undergoing open heart surgery | GA + IT hyperbaric tetracaine with morphine | All age groups tolerated the technique well hemodynamically. |
| Pirat et al111 | 2002 | 30 children aged 6 months to 6 years undergoing cardiac surgery (prospective, randomized) | GA + IT fentanyl (10) vs GA + IV fentanyl (10) vs GA + combined IT and IV fentanyl | The combined group was the only group to experience nonsignificant rises in HR and MAP from presurgery to poststernotomy. A single IT injection of fentanyl 2 mcg/kg offered no benefit over IV fentanyl with regard to hemodynamic stability and reduced stress response. |
| Williams et al112 | 1997 | 15 children undergoing PDA repair (series) | IT tetracaine | 2 patients required supplemental isoflurane. Minimal changes in blood pressure noted. |
| Chaney et al36 | 1996 | 60 patients undergoing CABG (prospective, randomized) | GA + IT morphine vs GA + IT placebo | No significant differences in perioperative epinephrine and norepinephrine levels. The IT morphine group required significantly less postoperative IV morphine. |
| Hall et al37 | 2000 | 25 patients undergoing CABG (prospective) | GA + IT morphine vs GA + IV morphine | IT morphine partially attenuated the postsurgical stress response (measured via cortisol and plasma epinephrine levels). |
| Zarate et al113 | 2000 | 20 patients undergoing elective CABG or valve replacement | GA + IT morphine + remifentanil vs GA + sufentanil | Remifentanil combined with IT morphine provided superior pain control after cardiac surgery compared to a sufentanil-based technique. |
| Boulanger et al114 | 2002 | 62 patients undergoing elective cardiac surgery | GA + IT morphine + PCA vs GA + IT placebo + PCA vs GA + SC morphine | The IT group experienced a tendency toward longer extubation times. Comparable pain scores in all 3 groups. |
A systematic review and meta-analysis from 12 published cohorts, including over 14,000 patients, suggested the maxi-mum risk for transient neurological injury following the use of thoracic epidural to be 1 in 1700 for cardiac and vascular surgery. There were no reported cases of epidural hematoma or permanent neurological injury in this analysis, although there are case reports in the literature.
As with all regional techniques, there is the possibility of failure. Hansdottir et al. reported a 5.2% (3 out of 52) failure rate of insertion and a 12.7% (7 out of 55) failure rate of placed catheters.
The use of neuraxial anesthesia in the form of epidural and spinal anesthesia in cardiac surgery is controversial. An interesting analysis suggested that for each episode of neurologic complication, the use of neuraxial anesthesia would prevent 20 myocardial infarctions and 76 episodes of atrial fibrillation. Whether this is an acceptable trade-off is the key question. Paravertebral and intercostal block, however, do not carry the same risks as neuraxial anesthesia and may be useful additions to postoperative analgesia (Table 3).
TABLE 3.
| Author | Year | Population Studied | Type of Technique | Conclusion |
|---|---|---|---|---|
| Canto et al115 | 2003 | 111 patients undergoing elective cardiac surgery using CP bypass (case series) | GA + 2 paravertebral catheters | Good hemodynamic stability, good postoperative analgesia, short times to tracheal extubation. |
| Exadaktylos et al116 | 2004 | 9 patients undergoing MIDCAB (case series) | GA + preoperative ipsilateral intercostal nerve blocks | All were extubated within 15 minutes and experienced good analgesia. |
| McDonald et al117 | 2005 | 17 patients undergoing cardiac surgery via midline sternotomy (prospective, randomized) | GA + parasternal block vs GA + placebo | The parasternal block with levobupicaine group used significantly less morphine in the first 4 hours postoperative; no patients needed rescue pain medication. |
| Behnke et al118 | 2002 | 43 patients undergoing MIDCAB (prospective, randomized) | GA + ICB vs GA + PCA | The ICB group experienced better pain relief. |
| Dowling et al119 | 2003 | 35 patients undergoing CABG (prospective, randomized) | GA + bilateral ICB with ropivacaine (16) vs GA + bilateral ICB with saline (19) | The ICB with ropivacaine group reported significantly lower pain scores and experienced a decrease in hospital LOS. |
| Dhole et al120 | 2001 | 41 patients undergoing MIDCAB (prospective, randomized) | GA + TEA vs GA + left-sided paravertebral catheter | No significant differences in pain scores or supplemental analgesia requirement.CI was higher in the TEA group.The PVB group had lower respiratory rates. |
THE PATIENT WITH CARDIAC DISEASE PRESENTING FOR NONCARDIAC SURGERY
Cardiac disease is a blanket term that covers a wide range of pathology. In deciding whether to utilize regional anesthesia, a consideration of the type and severity of cardiac disease is essen-tial in predicting the likely or possible response to a regional anesthetic. Table 4 lists the recommendations for active cardiac conditions for which it is recommended that the patient undergo evaluation and treatment before noncardiac surgery.
TABLE 4. Active cardiac conditions for which the patient should undergo evaluation and treatment before noncardiac surgery (class 1, level of evidence: B).
| Condition | Examples |
|---|---|
| Unstable coronary syndromes | Unstable or severe angina (CCS class III or IV) Recent MI (7–30 days) |
| Decompensated heart failure | NYHA class IV Worsening or new-onset heart failure |
| Significant dysrhythmias | High-grade atrioventricular block Mobitz II atrioventricular block Third-degree atrioventricular block Symptomatic ventricular dysrhythmias Supraventricular dysrhythmias with uncontrolled ventricular rate (HR > 100 at rest) Symptomatic bradycardia Newly recognized ventricular tachycardia |
| Severe valvular disease | Severe aortic stenosis (mean pressure gradient > 40 mm Hg, aortic valve area < 1cm2 or symptomatic) Symptomatic mitral stenosis (progressive dyspnea on exertion, exertional presyncope or heart failure) |
The Patient with Hypertension/Left Ventricular Hypertrophy
Severe long-standing hypertension is not only associated with a shift in the autoregulatory curve for many vascular beds but also commonly results in concentric left ventricular (LV) hypertrophy as a result of long-standing increased systemic vascular resistance (SVR) and pressure overload of the LV. Sudden drops in SVR must be avoided in these patients as such drops may not only compromise coronary perfusion and LV subendocardial perfusion but may also precipitate LV outflow tract obstruction as a result of systolic anterior motion (SAM) of the mitral valve or midcavity ventricular obstruction.
Diastole is the period of the cardiac cycle when the LV is perfused via the coronary arteries and when the LV chamber relaxes and fills. In general, tachycardia is poorly tolerated by hypertrophied hearts due to increased myocardial work, oxygen requirement, and a reduced diastolic time, which reduces both cardiac output via LV filling and coronary perfusion, further increasing the risk of myocardial ischemia. In addition, LV hypertrophy is usually associated with a degree of diastolic dys-function, making the maintenance of sinus rhythm (if possible) and avoidance of tachycardia even more important.
A regional technique may be very helpful in the avoidance of tachycardia due to pain. A localized technique that mini-mizes vasodilation may be preferable to central neuraxial tech-niques, although careful titration of these can avoid hemodynamic instability.
The Patient with Ischemic Heart Disease
Ischemic heart disease is synonymous with coronary artery disease. The American College of Cardiology Foundation (ACCF) has published guidelines on the diagnosis and management of patients with known stable ischemic heart disease, including indications for investigation and revascularization.70 There is also, however, a significant proportion of the population with undiagnosed, asymptomatic coronary artery disease. In 2010, the ACCF and the American Heart Association (AHA) published guidelines on how best to estimate cardiovascular risk in asymptomatic adults using a combination of history, examination, and investigation. Guidance is also available on the perioperative management of patients with ischemic heart disease presenting for noncardiac surgery.
Patients with ischemic heart disease may experience a range of complications, including myocardial infarction, dysrhythmias, heart failure, deteriorating ventricular function, and sudden death. Ischemic heart disease may also coexist with other cardiac pathologies, including valvular lesions and cardiomyopathies. The management of an individual patient will depend on the combination of these problems and the predominant lesion.The relationship between hypotension and an increase in myocardial ischemia in patients with known coronary artery disease has been demonstrated in a population of elderly patients undergoing hip surgery. In general terms, a patient with known coronary artery stenoses who has an otherwise normal, well-functioning heart will benefit from efforts to maintain preload (filling), prevent excessive vasodilation (causing a reduction in SVR), and prevent tachycardia, which would increase oxygen requirement by the myocardium while reducing the time available for coronary perfusion. A patient with coronary artery disease and poor LV systolic function (defined as an ejection fraction < 30%) can be challenging, as reducing systemic vascular resistance will reduce resistance to outflow and increase ejection fraction, but this must not done at the expense of coronary artery perfusion pressure. In this circumstance, careful titration of central neuraxial anesthesia and a reduced volume of total local anesthetic is advisable.
The Patient with Valvular Heart DiseaseRegurgitant Valvular Disease
In general, regurgitant valvular disease is improved symptomatically by peripheral vasodilation and worsened by peripheral vasoconstriction. Central neuraxial block and peripheral neur-axial block therefore tend to be well tolerated cardiovascularly and are ideal for preventing a worsening in regurgitant fraction as a result of peripheral vasoconstriction caused by pain and anxiety. Care needs to be taken, however, in patients with concomitant coronary artery disease or stenotic valvular disease.
Stenotic Valvular Disease
Aortic and mitral valve stenoses are much more prevalent in the adult population than tricuspid or pulmonary valve stenosis. Although it is recommended that a patient with severe aortic stenosis or symptomatic mitral stenosis be referred for investigation and management before undergoing noncardiac surgery, there may be emergency situations when this is not possible.
Aortic valve stenosis results in a fixed obstruction to LV systolic outflow and usually results in concentric LV hypertrophy. Sudden decreases in SVR need to be avoided as such decreases may compromise coronary perfusion. As with patients with LV hypertrophy from other causes, maintenance of filling status and avoidance of tachycardia and fast dysrhythmias is desirable.
Mitral stenosis results in a fixed obstruction to LV inflow. Particular care needs to be taken to maintain filling status and preload, but not excessively, as large boluses of fluid may result in pulmonary edema. Careful titration of regional anesthesia with a low threshold for invasive monitoring is desirable.
The Adult Patient with Congenital Heart Disease
The term congenital heart disease covers an extremely broad range of conditions, from relatively simple acyanotic lesions to complex cyanotic pathology requiring complex surgery. As pediatric congenital cardiac surgical techniques have advanced over the past few decades, more children with congenital heart disease are surviving into adulthood and presenting for noncardiac surgery where the use of regional anesthesia may be employed. Ideally, a patient with complex congenital heart disease should be managed in a specialist facility with support from clinicians and staff who are familiar with the patient, his or her condition, and his or her current medical status.While it is not the aim of this chapter to provide a comprehensive review of every type of congenital heart disease, there are a number of general issues that should be taken into consideration when planning the use of regional anesthesia in these patients:
- Abnormal anatomy, including alterations as a result of previous surgery (Table 5)
- The presence of anticoagulation
- Cardiac function, including the presence of dysrhythmias
- Dependence of the pulmonary circulation on passive venous return without right ventricular assistance (Fontan or hemi-Fontan physiology)
- The likely effect on cardiovascular stability of a reduction in systemic vascular resistance
- The need for additional monitoring intraoperatively and postoperatively (the site of insertion of invasive monitoring will require knowledge of abnormal anatomy, including thrombosed veins and arterial shunts)
The most common causes of low cardiac output in a patient with Fontan physiology are inadequate preload, elevated pulmonary vascular resistance, ventricular dysfunction, and dysrhythmias. The use of regional anesthesia may be very useful in these patients, as general anesthesia with positive pressure ventilation carries its own risks. Poorly controlled postoperative pain and poor respiratory effort may lead to life-threatening complications.
TABLE 5. Anesthetic relevance of previous surgical intervention in adult congenital heart disease.
| Anatomic Lesion/Surgical Correction | Brief Description of Lesion | Anesthetic Relevance |
|---|---|---|
| Blalock–Taussig shunt | Subclavian artery to pulmonary artery | Avoid blood pressure measurement (invasive and noninvasive) in affected arm. |
| Bidirectional Glenn (hemi-Fontan) | urgical connection of superior vena cava (SVC) to pulmonary artery | Blood flow through pulmonary circulation dependent on venous return and pulmonary vascular resistance. |
| Total cavopulmonary connection (Fontan type) | Surgical connection of SVC and inferior vena cava (IVC) to pulmonary artery | Blood flow through pulmonary circulation totally dependent on venous return and pulmonary vascular resistance. |
Modifications to regional anesthetic technique in patients with complex congenital heart disease include the following:
- Consider a reduction in the total dose of local anesthetic administered, particularly in patients with poor cardiac function or a history of dysrhythmias.
- Use a slow titration of or avoid narcotic or anxiolytic sedation in patients with Fontan or hemi-Fontan physiology. Avoidance of hypoxia and hypercarbia is imperative due to the risk of precipitating an acute rise in pulmonary vascular resistance and the corresponding reduction in cardiac output.
- Avoid a sudden reduction in systemic vascular resistance in the patient with Fontan or hemi-Fontan physiology. Carefully titrate the central neuraxial block with appropriate fluid administration and close monitoring.
SUMMARY
Regional anesthesia, by virtue of its ability to provide intense analgesia and in some circumstances avoid general anesthesia, plays an essential role in the management of patients with cardiovascular disease undergoing surgery. The method proposed should take into account not only the type of surgery being undertaken but also the combination of issues present in the individual patient. In general terms, patients with a poor ejection fraction and regurgitant valvular lesions respond well to peripheral vasodilation, as long as adequate preload and coronary artery perfusion are maintained. Patients with stenotic valvular lesions, severe coronary artery stenosis not amenable (or practical in an emergency) to revascularization preoperatively, and/or left ventricular hypertrophy can still benefit greatly from regional anesthesia in terms of avoiding an increase in myocardial work and oxygen demand caused by pain and tachycardia. However, caution is required in these patients, as a sudden or excessive reduction in peripheral vascular resistance, particularly with central neuraxial block, may precipitate a drop in myocardial perfusion and/or a drop in preload and cardiac output with severe consequences. In these patients, the decision to utilize regional anesthesia should be made with cau-tion and undertaken with appropriate monitoring. As in all other circumstances in which regional anesthesia is proposed, attention should be paid to anticoagulation, weighing the potential thromboembolic risks of stopping anticoagulation against the potential benefits. Skillfully performed major peripheral blocks for distal extremity (e.g. amputation, extrem-ity debridement, etc), can be life-saving in patients with severe cardiovascular disease, such.
REFERENCES
- Veering BT, Cousins MJ: Cardiovascular and pulmonary effects of epidural anaesthesia. Anaesth Intensive Care 2000;28:620–635.
- Goertz AW et al: Influence of high thoracic epidural anesthesia on left ventricular contractility assessed using the end-systolic pressure-length relationship. Acta Anaesthesiol Scand 1993;37:38–44.
- Niimi Y et al: Echocardiographic evaluation of global left ventricular function during high thoracic epidural anesthesia. J Clin Anesth 1997; 9:118–124.
- Ottesen S: The influence of thoracic epidural analgesia on the circulation at rest and during physical exercise in man. Acta Anaesthesiol Scand 1978;22:537–547.
- Wattwil M et al: Circulatory changes during high thoracic epidural anaesthesia—influence of sympathetic block and of systemic effect of the local anaesthetic. Acta Anaesthesiol Scand 1985;29:849–855.
- Hasenbos M et al: The influence of high thoracic epidural analgesia on the cardiovascular system. Acta Anaesthesiol Belg 1988;39:49–54.
- Blomberg S, Emanuelsson H, Ricksten SE: Thoracic epidural anesthesia and central hemodynamics in patients with unstable angina pectoris. Anesth Analg 1989;69:558–562.
- Blomberg S et al: Effects of thoracic epidural anesthesia on coronary arteries and arterioles in patients with coronary artery disease. Anesthesiology 1990;73:840–847.
- Reinhart K et al: Effects of thoracic epidural anesthesia on systemic hemodynamic function and systemic oxygen supply-demand relationship. Anesth Analg 1989;69:360–369.
- Stenseth R et al: The influence of thoracic epidural analgesia alone and in combination with general anesthesia on cardiovascular function and myocardial metabolism in patients receiving beta-adrenergic blockers. Anesth Analg 1993;77:463–468.
- Berendes E et al: Reversible cardiac sympathectomy by high thoracic epidural anesthesia improves regional left ventricular function in patients undergoing coronary artery bypass grafting: a randomized trial. Arch Surg 2003;138:1283–1290; discussion 1291.
- Schmidt C et al: The effect of high thoracic epidural anesthesia on systolic and diastolic left ventricular function in patients with coronary artery disease. Anesth Analg 2005;100:1561–1569.
- Licker M, Farinelli C, Klopfenstein CE: Cardiovascular reflexes during anesthesia induction and tracheal intubation in elderly patients: the influence of thoracic epidural anesthesia. J Clin Anesth 1995;7: 281–287.
- Her C et al: Combined epidural and general anesthesia for abdominal aortic surgery. J Cardiothorac Anesth 1990;4:552–557.
- Norris EJ et al: Double-masked randomized trial comparing alternate combinations of intraoperative anesthesia and postoperative analgesia in abdominal aortic surgery. Anesthesiology 2001;95:1054–1067.
- Davies MJ et al: Combined epidural and general anaesthesia versus general anaesthesia for abdominal aortic surgery: a prospective randomised trial. Anaesth Intensive Care 1993;21:790–794.
- Garnett RL et al: Perioperative ischaemia in aortic surgery: combined epidural/general anaesthesia and epidural analgesia vs general anaesthesia and i.v. analgesia. Can J Anaesth 1996;43:769–777.
- Gelman S et al: Thoracic epidural vs balanced anesthesia in morbid obesity: an intraoperative and postoperative hemodynamic study. Anesth Analg 1980;59:902–908.
- Baron JF et al: Left ventricular global and regional function during lumbar epidural anesthesia in patients with and without angina pectoris. Influence of volume loading. Anesthesiology 1987;66:621–627.
- Dagnino J, Prys-Roberts C: Studies of anaesthesia in relation to hypertension. VI: Cardiovascular responses to extradural block of treated and untreated hypertensive patients. Br J Anaesth 1984;56:1065–1073.
- Scheinin H et al: Epidural infusion of bupivacaine and fentanyl reduces perioperative myocardial ischaemia in elderly patients with hip fracture—a randomized controlled trial. Acta Anaesthesiol Scand 2000; 44:1061–1070.
- Matot I et al: Preoperative cardiac events in elderly patients with hip fracture randomized to epidural or conventional analgesia. Anesthesiology 2003;98:156–163.
- Perler BA et al: The influence of anesthetic method on infrainguinal bypass graft patency: a closer look. Am Surg 1995;61:784–789.
- Christopherson R et al: Perioperative morbidity in patients randomized to epidural or general anesthesia for lower extremity vascular surgery. Perioperative Ischemia Randomized Anesthesia Trial Study Group. Anesthesiology 1993;79:422–434.
- Rosenfeld BA et al: The effects of different anesthetic regimens on fibrinolysis and the development of postoperative arterial thrombosis. Perioperative Ischemia Randomized Anesthesia Trial Study Group. Anesthesiology 1993;79:435–443.
- Bode RH Jr et al: Cardiac outcome after peripheral vascular surgery. Comparison of general and regional anesthesia. Anesthesiology 1996; 84:3–13.
- Cohen MC et al: Types of anesthesia and cardiovascular outcomes in patients with congestive heart failure undergoing vascular surgery. Congest Heart Fail 1999;5:248–253.
- Goldszmidt E et al: Anesthetic management of a consecutive cohort of women with heart disease for labor and delivery. Int J Obstet Anesth 2010;19:266–272.
- Kowalewski RJ et al: Anaesthesia for coronary artery bypass surgery supplemented with subarachnoid bupivacaine and morphine: a report of 18 cases. Can J Anaesth 1994;41:1189–1195.
- Windsor A et al: Silent myocardial ischaemia in patients undergoing transurethral prostatectomy. A study to evaluate risk scoring and anaesthetic technique with outcome. Anaesthesia 1996;51:728–732.
- Edwards ND et al: Perioperative myocardial ischaemia in patients undergoing transurethral surgery: a pilot study comparing general with spinal anaesthesia. Br J Anaesth 1995;74:368–372.
- Juelsgaard P et al: Perioperative myocardial ischaemia in patients undergoing surgery for fractured hip randomized to incremental spinal, single-dose spinal or general anaesthesia. Eur J Anaesthesiol 1998;15: 656–663.
- Olofsson C et al: Low-dose bupivacaine with sufentanil prevents hypotension after spinal anesthesia for hip repair in elderly patients. Acta Anaesthesiol Scand 2004;48:1240–1244.
- Ben-David B et al: Minidose bupivacaine-fentanyl spinal anesthesia for surgical repair of hip fracture in the aged. Anesthesiology 2000;92: 6–10.
- Lee TW et al: High spinal anesthesia for cardiac surgery: effects on beta-adrenergic receptor function, stress response, and hemodynamics. Anesthesiology 2003;98:499–510.
- Chaney MA et al: Large-dose intrathecal morphine for coronary artery bypass grafting. Anesth Analg 1996;83:215–222.
- Hall R et al: Does intrathecal morphine alter the stress response following coronary artery bypass grafting surgery? Can J Anaesth 2000; 47:463–466.
- Fleron MH et al: A comparison of intrathecal opioid and intravenous analgesia for the incidence of cardiovascular, respiratory, and renal complications after abdominal aortic surgery. Anesth Analg 2003;97: 2–12, table of contents.
- Backlund M et al: Factors associated with post-operative myocardial ischaemia in elderly patients undergoing major non-cardiac surgery. Eur J Anaesthesiol 1999;16:826–833.
- Velickovic IA, Leicht CH: Peripartum cardiomyopathy and cesarean section: report of two cases and literature review. Arch Gynecol Obstet 2004;270:307–310.
- Velickovic IA, Leicht CH: Continuous spinal anesthesia for cesarean section in a parturient with severe recurrent peripartum cardiomyopathy. Int J Obstet Anesth 2004;13:40–43.
- Okutomi T et al: Continuous spinal analgesia for labor and delivery in a parturient with hypertrophic obstructive cardiomyopathy. Acta Anaesthesiol Scand 2002;46:329–331.
- Ransom DM, Leicht CH: Continuous spinal analgesia with sufentanil for labor and delivery in a parturient with severe pulmonary stenosis. Anesth Analg 1995;80:418–421.
- Honig O et al: [Cesarean section with continuous spinal anesthesia in a cardiopulmonary high-risk patient]. Anaesthesist 1998;47:685–689.
- Cheema SP et al: A thermographic study of paravertebral analgesia. Anaesthesia 1995;50:118–121.
- Purcell-Jones G, Pither CE, Justins DM: Paravertebral somatic nerve block: a clinical, radiographic, and computed tomographic study in chronic pain patients. Anesth Analg 1989;68:32–39.
- Davies RG, Myles PS, Graham JM: A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural block for thoracotomy—a systematic review and meta-analysis of randomized trials. Br J Anaesth 2006;96:418–426.
- Ho AM et al: The resolution of ST segment depressions after high right thoracic paravertebral block during general anesthesia. Anesth Analg 2002;95:227–228, table of contents.
- Dieguez Garcia P et al: [Ultrasound-assisted approach to blocking the intercostal nerves in the mid-axillary line for non-reconstructive breast and axilla surgery]. Rev Esp Anestesiol Reanim 2013;60:365–370.
- Sternbach Y et al: Hemodynamic benefits of regional anesthesia for carotid endarterectomy. J Vasc Surg 2002;35:333–339.
- Guay J: Regional or general anesthesia for carotid endarterectomy? Evidence from published prospective and retrospective studies. J Cardiothorac Vasc Anesth 2007;21:127–132.
- Group, GTC et al: General anaesthesia versus local anaesthesia for carotid surgery (GALA): a multicentre, randomised controlled trial. Lancet 2008;372:2132–2142.
- Tuominen M et al: Continuous interscalene brachial plexus block: clinical efficacy, technical problems and bupivacaine plasma concentrations. Acta Anaesthesiol Scand 1989;33:84–88.
- Ozzeybek D et al: Comparison of the haemodynamic effects of interscalene block combined with general anaesthesia and interscalene block alone for shoulder surgery. J Int Med Res 2003;31:428–433.
- Culebras X et al: Clonidine combined with a long acting local anesthetic does not prolong postoperative analgesia after brachial plexus block but does induce hemodynamic changes. Anesth Analg 2001;92:199–204.
- Kohli S et al: Brachial plexus block: Comparison of two different doses of clonidine added to bupivacaine. J Anaesthesiol Clin Pharmacol 2013;29:491–495.
- Fanelli G et al: Cardiovascular effects of two different regional anaesthetic techniques for unilateral leg surgery. Acta Anaesthesiol Scand 1998;42:80–84.
- Chia N, Low TC, Poon KH: Peripheral nerve blocks for lower limb surgery—a choice anaesthetic technique for patients with a recent myocardial infarction? Singapore Med J 2002;43:583–586.
- Ho AM, Karmakar MK: Combined paravertebral lumbar plexus and parasacral sciatic nerve block for reduction of hip fracture in a patient with severe aortic stenosis. Can J Anaesth 2002;49:946–950.
- Tanaka Y, Negoro T: [Psoas compartment block for surgery of the femoral neck (trochanteric) fracture in a patient with severe heart failure due to rheumatoid myocarditis]. Masui 2000;49:1133–1135.
- Rizzo D et al: [Sciatic, femoral and cutaneous nerve block for arthroscopic meniscectomy in a patient with Eisenmerger’s syndrome. Case report]. Minerva Anestesiol 1999;65:733–736.
- Goldstein S et al: A survey of spinal and epidural techniques in adult cardiac surgery. J Cardiothorac Vasc Anesth 2001;15:158–168.
- Mehta Y, Arora D: Benefits and Risks of Epidural Analgesia in Cardiac Surgery. J Cardiothorac Vasc Anesth 2014;28:1057–1063.
- Pastor MC et al: Thoracic epidural analgesia in coronary artery bypass graft surgery: seven years’ experience. J Cardiothorac Vasc Anesth 2003; 17:154–159.
- Ruppen W et al: Incidence of epidural haematoma and neurological injury in cardiovascular patients with epidural analgesia/anaesthesia: systematic review and meta-analysis. BMC Anesthesiol 2006;6:10.
- Rosen DA et al: An epidural hematoma in an adolescent patient after cardiac surgery. Anesth Analg 2004;98:966–969, table of contents.
- Berman M et al: Safety and efficacy of aprotinin and tranexamic acid in pulmonary endarterectomy surgery with hypothermia: review of 200 patients. Ann Thorac Surg 2010;90:1432–1436.
- Hansdottir V et al: Thoracic epidural versus intravenous patient-controlled analgesia after cardiac surgery: a randomized controlled trial on length of hospital stay and patient-perceived quality of recovery. Anesthesiology 2006;104:142–151.
- Djaiani G, Fedorko L, Beattie WS: Regional anesthesia in cardiac surgery: a friend or a foe? Semin Cardiothorac Vasc Anesth 2005;9: 87–104.
- Fihn SD et al: 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012;60: e44–e164.
- Greenland P et al: 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010;56:e50–103.
- Fleisher LA et al: ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta-blocker therapy—a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Anesth Analg 2007;104:15–26.
- Fleisher LA et al: 2009 ACCF/AHA focused update on perioperative beta block incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation 2009; 120:e169–e276.
- Eagle SS, Daves SM: The adult with Fontan physiology: systematic approach to perioperative management for noncardiac surgery. J Cardiothorac Vasc Anesth 2011;25:320–334.
- Richter A et al: Effect of thoracic epidural analgesia on refractory angina pectoris: long-term home self-treatment. J Cardiothorac Vasc Anesth 2002;16:679–684.
- Olausson K et al: Anti-ischemic and anti-anginal effects of thoracic epidural anesthesia versus those of conventional medical therapy in the treatment of severe refractory unstable angina pectoris. Circulation 1997;96:2178–2182.
- Salvi L et al: High thoracic epidural anesthesia for off-pump coronary artery bypass surgery. J Cardiothorac Vasc Anesth 2004;18:256–262.
- Kessler P et al: Comparison of three anesthetic techniques for off-pump coronary artery bypass grafting: general anesthesia, combined general and high thoracic epidural anesthesia, or high thoracic epidural anesthesia alone. J Cardiothorac Vasc Anesth 2005;19:32–39.
- Stritesky M et al: On-pump cardiac surgery in a conscious patient using a thoracic epidural anesthesia—an ultra fast track method. Bratisl Lek Listy 2004;105:51–55.
- Kessler P et al: High thoracic epidural anesthesia for coronary artery bypass grafting using two different surgical approaches in conscious patients. Anesth Analg 2002;95:791–797, table of contents.
- Anderson MB et al: Thoracic epidural anesthesia for cardiac surgery via left anterior thoracotomy in the conscious patient. Heart Surg Forum 2002;5:105–108.
- Noiseux N et al: Coronary artery bypass grafting in the awake patient combining high thoracic epidural and femoral nerve block: first series of 15 patients. Br J Anaesth 2008;100:184–189.
- Barrington MJ et al: Epidural anesthesia for coronary artery bypass surgery compared with general anesthesia alone does not reduce biochemical markers of myocardial damage. Anesth Analg 2005;100: 921–928.
- Kendall JB et al: A prospective, randomised, single-blind pilot study to determine the effect of anaesthetic technique on troponin T release after off-pump coronary artery surgery. Anaesthesia 2004;59:545–549.
- Loick HM et al: High thoracic epidural anesthesia, but not clonidine, attenuates the perioperative stress response via sympatholysis and reduces the release of troponin T in patients undergoing coronary artery bypass grafting. Anesth Analg 1999;88:701–709.
- Fillinger MP et al: Epidural anesthesia and analgesia: effects on recovery from cardiac surgery. J Cardiothorac Vasc Anesth 2002;16:15–20.
- Scott NB et al: A prospective randomized study of the potential benefits of thoracic epidural anesthesia and analgesia in patients undergoing coronary artery bypass grafting. Anesth Analg 2001;93:528–535.
- Turfrey DJ et al: Thoracic epidural anaesthesia for coronary artery bypass graft surgery. Effects on postoperative complications. Anaesthesia 1997;52:1090–1095.
- Karagoz HY et al: Coronary artery bypass grafting in the awake patient: three years’ experience in 137 patients. J Thorac Cardiovasc Surg 2003; 125:1401–1404.
- Liu SS, Block BM, Wu CL: Effects of perioperative central neuraxial analgesia on outcome after coronary artery bypass surgery: a meta-analysis. Anesthesiology 2004;101:153–161.
- Hemmerling TM et al: Immediate extubation after aortic valve surgery using high thoracic epidural anesthesia. Heart Surg Forum 2004; 7:16–20.
- Klokocovnik T et al: Minimally invasive aortic valve replacement under thoracic epidural anesthesia in a conscious patient: case report. Heart Surg Forum 2004;7:E196–E197.
- Slin’ko SK: [State of the sympathoadrenal system and hemodynamics in children during congenital heart defect surgery with high thoracic epidural anesthesia using lidocaine-clofelin]. Anesteziol Reanimatol 2000; 1:10–13.
- Peterson KL et al: A report of two hundred twenty cases of regional anesthesia in pediatric cardiac surgery. Anesth Analg 2000;90:1014–1019.
- Hammer GB, Ngo K, Macario A: A retrospective examination of regional plus general anesthesia in children undergoing open heart surgery. Anesth Analg 2000;90:1020–1024.
- Royse C et al: Prospective randomized trial of high thoracic epidural analgesia for coronary artery bypass surgery. Ann Thorac Surg 2003;75: 93–100.
- Liem TH et al: Coronary artery bypass grafting using two different anesthetic techniques: Part 2: Postoperative outcome. J Cardiothorac Vasc Anesth 1992;6:156–161.
- Hemmerling TM et al: Ultra-fast-track anesthesia in off-pump coronary artery bypass grafting: a prospective audit comparing opioid-based anesthesia vs thoracic epidural-based anesthesia. Can J Anaesth 2004;51: 163–168.
- Bois S et al: Epidural analgesia and intravenous patient-controlled analgesia result in similar rates of postoperative myocardial ischemia after aortic surgery. Anesth Analg 1997;85:1233–1239.
- Ho SC et al: Persistent pain after cardiac surgery: an audit of high thoracic epidural and primary opioid analgesia therapies. Anesth Analg 2002;95:820–823, table of contents.
- Jensen, MK, Andersen C: Can chronic poststernotomy pain after cardiac valve replacement be reduced using thoracic epidural analgesia? Acta Anaesthesiol Scand 2004;48:871–874.
- Sanchez R, Nygard E: Epidural anesthesia in cardiac surgery: is there an increased risk? J Cardiothorac Vasc Anesth 1998;12:170–173.
- Vanstrum GS, Bjornson KM, Ilko R: Postoperative effects of intrathecal morphine in coronary artery bypass surgery. Anesth Analg 1988;67: 261–267.
- Bettex DA et al: Intrathecal sufentanil-morphine shortens the duration of intubation and improves analgesia in fast-track cardiac surgery. Can J Anaesth 2002;49:711–717.
- Mehta Y et al: Spinal (subarachnoid) morphine for off-pump coronary artery bypass surgery. Heart Surg Forum 2004;7:E205–E210.
- Fitzpatrick GJ, Moriarty DC: Intrathecal morphine in the management of pain following cardiac surgery. A comparison with morphine i.v. Br J Anaesth 1988;60:639–644
- Latham P et al: Fast-track cardiac anesthesia: a comparison of remifentanil plus intrathecal morphine with sufentanil in a desflurane-based anesthetic. J Cardiothorac Vasc Anesth 2000;14:645–651.
- Alhashemi JA et al: Effect of subarachnoid morphine administration on extubation time after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 2000;14:639–644.
- Chaney MA et al: Intrathecal morphine for coronary artery bypass grafting and early extubation. Anesth Analg 1997;84:241–248.
- Finkel JC, Boltz MG, Conran AM: Haemodynamic changes during high spinal anaesthesia in children having open heart surgery. Paediatr Anaesth 2003;13:48–52.
- Pirat A, Akpek E, Arslan G: Intrathecal versus IV fentanyl in pediatric cardiac anesthesia. Anesth Analg 2002;95:1207–1214, table of contents.
- Williams RK, Abajian JC: High spinal anaesthesia for repair of patent ductus arteriosus in neonates. Paediatr Anaesth 1997;7:205–209.
- Zarate E et al: Fast-track cardiac anesthesia: use of remifentanil combined with intrathecal morphine as an alternative to sufentanil during desflurane anesthesia. Anesth Analg 2000;91:283–287.
- Boulanger A et al: Intrathecal morphine after cardiac surgery. Ann Pharmacother 2002;36:1337–1343.
- Canto M et al: Bilateral paravertebral block for conventional cardiac surgery. Anaesthesia 2003;58:365–370.
- Exadaktylos AK et al: Pre-operative intercostal nerve block for minimally invasive coronary bypass surgery: a standardised anaesthetic regimen for rapid emergence and early extubation. Cardiovasc J S Afr 2004;15:178–181.
- McDonald SB et al: Parasternal block and local anesthetic infiltration with levobupivacaine after cardiac surgery with desflurane: the effect on postoperative pain, pulmonary function, and tracheal extubation times. Anesth Analg 2005;100:25–32.
- Behnke H et al: [Postoperative pain therapy in minimally invasive direct coronary arterial bypass surgery. I.v. opioid patient-controlled analgesia versus intercostal block]. Anaesthesist 2002;51:175–179.
- Dowling R et al: Improved pain control after cardiac surgery: results of a randomized, double-blind, clinical trial. J Thorac Cardiovasc Surg 2003; 126:1271–1278.
- Dhole S et al: Comparison of continuous thoracic epidural and paravertebral blocks for postoperative analgesia after minimally invasive direct coronary artery bypass surgery. J Cardiothorac Vasc Anesth 2001; 15:288–292