INTRODUCTION
Intraspinal hematoma is a relatively rare condition resulting from a variety of causes. Traumatic causes include lumbar puncture and neuraxial anesthesia. It is more likely to occur in anticoagulated or thrombocytopenic patients, patients with neoplastic disease, or in those with liver disease or alcoholism. Approximately one-quarter to one-third of all cases are associated with anticoagulation therapy. The risk of intraspinal hematoma formation after administration of neuraxial anesthesia and analgesia is increased in patients who have received anticoagulant therapy or have a coagulation disorder. For this reason, neuraxial anesthesia is often contraindicated in the presence of a coagulopathy. Other risk factors for the development of epidural or spinal hematoma include technical difficulty (multiple attempts) in the performance of the neuraxial procedures due to anatomic abnormalities of the spine and multiple or bloody punctures.
The incidence of spinal hematoma was originally reported to be one in 150,000 epidurals and one in 220,000 spinal anesthetics. Recent epidemiologic studies have shown the incidence of spinal hematoma to be more frequent, ranging from one in 2700 to one in 19,505 epidurals. The most recent study showed an overall risk of 1 in 21,643 epidural injections. The elderly (one in 3800) are at increased risk due to degenerative spine abnormalities, osteoporosis, and peripheral vascular disease. Obstetric populations seem to have a lower incidence of spinal hematoma (one in 200,000), probably secondary to the hypercoagulable state of pregnancy, the wider capacity of the epidural space in younger parturients, and higher intra-epidural pressure. Based on a recent large retrospective study, the incidence of epidural hematoma in patients with abnormal coagulation may be as low as one in 315 patients.
The introduction of low-molecular-weight heparin (LMWH) was associated with a spike in the incidence of spinal hematoma, resulting in a warning by the Food and Drug Administration (FDA) and the introduction of the first consensus statement on regional anesthesia in patients on anticoagulants by the American Society of Regional Anesthesia and Pain Medicine (ASRA) in 1998. The guidelines were based on an extensive review of the literature and of the pharmacology of the different anticoagulants.
Recommendations were made on the timing of the neuraxial nerve block, removal of the epidural catheter, and the subsequent administration of anticoagulants. In particular, the use of low concentrations of local anesthetics for epidural infusion (to preserve motor strength for easier monitoring) and subsequent neurologic monitoring were recommended by the ASRA. The consensus guidelines, published in 1998 and updated in 2003 and 2010, have greatly assisted clinicians in decision making with regard to the use of neuraxial procedures in the setting of anticoagulation therapy. Two other sets of guidelines, published by the European Society of Anaesthesiology and the Scandinavian Society of Anaesthesiology and Intensive Care Medicine, are influential in Europe.
In this chapter, we discuss the significance of common anticoagulants and hope to offer the reader a guide in decision making about the use of neuraxial anesthesia and peripheral nerve blocks (PNBs) in clinical practice. We will also discuss the new anticoagulants, drugs that were not adequately covered in the latest ASRA guidelines and only partly covered by the European and Scandinavian guidelines. Follow the link to learn more about Diagnosis and Management of Spinal and Peripheral Nerve Hematoma.
ANTIPLATELET THERAPY
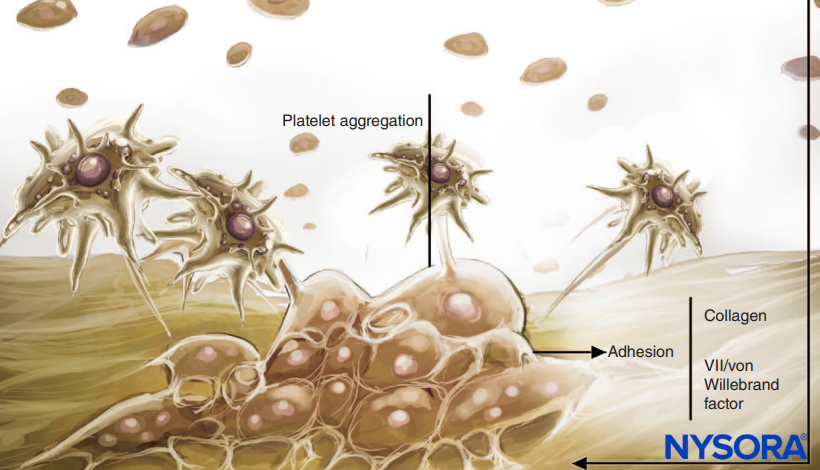
Antiplatelet medications inhibit the platelet cyclooxygenase enzyme and prevent the synthesis of thromboxane A2. Thromboxane A2 is a potent vasoconstrictor that facilitates secondary platelet aggregation and release reactions. The role of platelets in coagulation and hemostasis is shown in Figures 1 and 2. Platelets from patients on these medications have normal platelet adherence to subendothelium and normal primary hemostatic plug formation. An adequate, although potentially fragile, clot may form. Although such plugs may be satisfactory hemostatic barriers for smaller vascular lesions, they may not ensure adequate perioperative hemostatic clot formation. Platelet function in patients receiving antiplatelet medications should be assumed to be decreased for 1 week with aspirin and 1–6 days with nonsteroidal anti-inflammatory drugs (NSAIDs). This assumption does not take into consideration the continuous formation of new, functional platelets. The continuous production of fresh, normally functioning platelets, combined with the residual function of already circulating platelets, may explain the relative safety of performing neuraxial procedures in these patients.
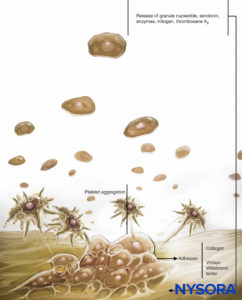
FIGURE 1. Role of platelets in coagulation. Platelets carry out their role in hemostasis through three basic reactions: adhesion, activation (and secretion), and aggregation. When vascular endothelium is damaged, platelets rapidly bind to the subendothelium by a process termed adhesion.
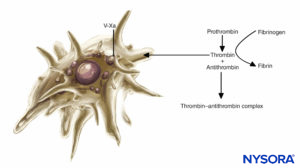
FIGURE 2. Role of platelets in coagulation: Platelets support plasma coagulation reactions. When activated, platelets bind several plasma protein complexes and secrete an activated form of factor V (factor Va), which binds to the platelet surface and binds factor Xa. Platelet-bound factor Xa then accelerates the conversion of prothrombin to thrombin.
The risk of epidural and spinal hematoma in patients on antiplatelet therapy has been raised by a case report of spontaneous epidural hematoma formation in the absence of spinal or epidural anesthesia in a patient with a history of aspirin ingestion. Vandermeulen and colleagues implicated antiplatelet therapy in 3 of the 61 cases of spinal hematoma occurring after spinal or epidural anesthesia. Other studies have shown a relatively low risk of spinal hematoma in patients on aspirin or NSAIDs undergoing a neuraxial procedure. The Collaborative Low-dose Aspirin Study in Pregnancy (CLASP) Group included 1422 high-risk obstetric patients who were administered 60 mg of aspirin daily and underwent epidural anesthesia without any neurologic sequelae. In a retrospective study of 1013 spinal and epidural anesthetics in which antiplatelet drugs were taken by 39% of the patients, including 11% of patients who were on multiple antiplatelet medications, no patient developed signs of spinal hematoma.
The patients on antiplatelet medications, however, showed a higher incidence of blood aspiration through the spinal or epidural needle or the catheter. A subsequent prospective study in 1000 patients, 39% of whom reported preoperative antiplatelet therapy, noted the absence of hemorrhagic complications. Therefore, preoperative antiplatelet therapy was not a risk factor for bloody needle or catheter placement. Female gender, increased age, history of excessive bruising or bleeding, continuous catheter technique, large needle gauge, multiple attempts, and difficult needle placement were noted to be significant risk factors. Clinical studies in pain clinic patients are similar to those undergoing surgery. Patients on aspirin or NSAIDs who underwent epidural steroid injections did not develop signs or symptoms of intraspinal hematoma.
The lack of correlation between antiplatelet medication and bloody needle or catheter placement provides some evidence that preoperative antiplatelet therapy does not represent a significant risk factor for the development of neurologic dysfunction from spinal hematoma in patients on antiplatelet therapy. It should be noted that there have been case reports of intraspinal hematoma in patients on aspirin or NSAIDs, although there were complicating factors in these case reports. These included concomitant heparin administration, coexisting epidural venous angioma, and technical difficulty in performing the procedure. More recently, more case reports of spinal hematoma have been published in relation to pain interventional procedures, specifically spinal cord stimulation placements.
Based on the available evidence, the ASRA has made several recommendations concerning antiplatelet medications. Preoperative antiplatelet therapy does not represent a significant risk factor for the development of neurologic dysfunction from spinal hematoma in patients on antiplatelet therapy. The risk of bleeding complications, however, may be increased in patients on several antiplatelet medications and concurrent use of other medications affecting clotting mechanisms, such as oral anticoagulants, standard heparin, and LMWH.
NYSORA Tips
- It is probably safe to do neuraxial and regional anesthesia procedures in patients on aspirin and NSAIDs.
- The risk factors for increased bleeding and spinal hematoma include the patient’s intake of several antiplatelet drugs and making multiple attempts.
Aspirin and Interventional Pain Procedures
There have been several case reports of spinal hematoma after epidural steroid injection or placement or removal of a spinal cord stimulator in patients who were on aspirin alone, NSAIDs alone, or when the ASRA guidelines were followed. These occurrences may be related to the frequent spine abnormalities found in these patients, the presence of fibrosis following spine surgery, larger needles used in spinal cord stimulator placement, or the frequent manipulations (advancements and retractions) of the electrodes.
Medications used in pain managements also cause bleeding; these include oxcarbazepine and the selective serotonin reuptake inhibitors. For these reasons, the ASRA liaised with the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain to formulate specific guidelines for interventional pain procedures. In contrast to the ASRA regional anesthesia guidelines the multisociety guidelines recommended that aspirin be stopped for 4–6 days before interventional pain procedures.
NYSORA Tips
The guidelines on interventional pain procedures in patients on antiplatelet and anticoagulant medications are more restrictive than the ones on regional anesthesia.
COX-2 Inhibitors and P2Y12 Inhibitors
Cyclooxygenase-2 (COX-2) inhibitors gained popularity because of their analgesic properties and lack of platelet and gastrointestinal effects, and studies have shown their analgesic property in a variety of perioperative settings.
The drugs have minimal gastrointestinal (GI) toxicity and are ideal for patients who are at increased risk for serious upper GI adverse events. Compared with aspirin or NSAIDs, the effect of COX-2 inhibitors on platelet aggregation and bleeding times were not different from placebo. Blood loss does not increase during spinal fusion surgery when COX-2 inhibitors are given preoperatively. The platelet properties of these drugs make them ideal for perioperative use when neuraxial anesthetic is planned. Unfortunately, rofecoxib and valdecoxib have been withdrawn from the market because of their cardiovascular side effects, leaving only celecoxib available, but at dosages lower than previously recommended.
The thienopyridine drugs ticlopidine and clopidogrel have no direct effect on arachidonic acid metabolism. These drugs prevent platelet aggregation by inhibiting adenosine diphosphate (ADP) receptor–mediated platelet activation. They also modulate vascular smooth muscle, reducing vascular contraction. Ticlopidine is rarely used because it causes neutropenia, thrombocytopenic purpura, and hypercholesterolemia.
Clopidogrel is preferred because of its improved safety profile and proven efficacy. It was noted to be better than aspirin in patients with peripheral vascular disease. The maximal inhibition of ADP-induced platelet aggregation with clopidogrel occurs 3–5 days after initiation of a standard dose (75 mg) but within 4 to 6 hours after the administration of a large loading dose of 300–600 mg. A large loading dose is usually given to patients before they undergo percutaneous coronary intervention (PCI). There has been a case report of spinal hematoma in a patient on ticlopidine. Although there has been no case of intraspinal hematoma in a patient on clopidogrel alone, a case of quadriplegia in a patient on clopidogrel, diclofenac, and aspirin has been reported.
For the thienopyridine drugs, it is recommended that ticlopidine be discontinued for 10–14 days and clopidogrel for 7 days before neuraxial injection. There have been case reports on the safety of spinal anesthesia 5 days after stoppage of clopidogrel. A study showed that most patients had minimal platelet inhibition 5 days after clopidogrel was stopped. If a neuraxial procedure must be performed 5–6 days after discontinuation of clopidogrel,then a P2Y12 assay, or another appropriate test, should be performed to ensure minimal or no inhibition of platelet activity.
NYSORA Tips
- The ASRA guidelines recommend a 7-day interval between discontinuation of clopidogrel and a neuraxial procedure.
- If a spinal or epidural must be performed is indicated, a test of platelet function is recommended to ensure that the residual platelet inhibition is gone or negligible.
For a spinal cord stimulation trial, a 5-day discontinuation may be observed since the patient will be off clopidogrel during the trial. A test of platelet function (eg, the VerifyNow P2Y12 assay or the platelet mapping portion of the Thrombelastograph [TEG]) must be performed to ensure adequate platelet activity.
Newer Antiplatelet Drugs
Clopidogrel is the commonly used antiplatelet drug in dual antiplatelet therapy, wherein aspirin is combined with a P2Y12 receptor inhibitor, in patients with acute coronary syndromes. Prasugrel is a pro-drug similar to clopidogrel but with salutary features over clopidogrel, and ticagrelor is a direct-acting P2Y12 receptor inhibitor. The median time to peak effect is 1 hour with prasugrel and 4 hours with clopidogrel. The mean time to peak plasma concentration with prasugrel is 30 minutes, and its median half-life is 3.7 hours. These values do not reflect the duration of platelet inhibition because the inhibition of the P2Y12 receptor is irreversible. It takes 7 days for platelet activity to normalize after stopping prasugrel.
Prasugrel and ticagrelor cause 90% inhibition of platelet function compared to 60%–70% for clopidogrel. Patients with a low body mass index (BMI), those over 75 years of age, and those with a history of stroke are at risk for bleeding. Unlike clopidogrel, prasugrel is reliably converted to its active metabolite and has no drug interactions. Also, it is not susceptible to genetic polymorphisms.
Ticagrelor reversibly binds to the P2Y12 receptor, blocking ADP-mediated receptor activation. In contrast to the thienopyridines, the active metabolite and the parent drug exhibit antiplatelet activity with the parent drug responsible for the majority of the in vivo platelet inhibition. The antiplatelet effect of ticagrelor is rapid; peak platelet inhibition occurs at 2 to 4 hours after intake compared to 24 hours with clopidogrel. Mean platelet inhibition by ticagrelor is 93% compared to 58% for clopidogrel. Platelet recovery is rapid with ticagrelor; platelet activity is normal at 5 days after the last dose.
Time Between Discontinuation/Resumption of an Antiplatelet Drug and Neuraxial Injection
The interval between discontinuation of an antiplatelet drug and neuraxial injection is based on the percent platelet inhibition and percent platelet turnover. Prasugrel and ticagrelor cause 90% inhibition. Ten to 15 percent of the circulating platelet pool is formed every day, resulting in new platelets comprising 50–75% of the circulating platelet pool 5–7 days after discontinuation of the drug. An interval of 5–7 days has been recommended for ticagrelor and an interval of 7–10 days for prasugrel. These recommendations are appropriate as platelet aggregation normalizes 7 days after stopping prasugrel and 5 days after ticagrelor.
The Scandinavian guidelines note that it is acceptable to restart antiplatelet drugs at the time of catheter removal, whereas the European Society of Anaesthesiology guidelines recommended an interval of 6 hours between removal of epidural catheter and resumption of prasugrel or ticagrelor. Other reviews have recommended caution in restarting prasugrel and ticagrelor because of their rapid effect and potent antiplatelet inhibition. A 24-hour interval may be more appropriate for these agents.
NYSORA Tips
- Prasugrel and ticagrelor should be stopped 7 and 5 days, respectively, before a spinal or epidural.
- Antiplatelet drugs can be restarted 6–24 hours after a neuraxial procedure or catheter removal.
Monitoring Platelet Function
The Ivy bleeding time was considered to be a reliable predictor of abnormal bleeding in patients receiving antiplatelet drugs. However, the post-aspirin bleeding time is not a reliable indicator of platelet function. There is large intra- and interpatient variability in the results of the test, and there is no evidence to suggest that bleeding time can predict hemostatic function, as studies have failed to show a correlation between aspirin-induced prolongation of bleeding time and surgical blood loss.
Special platelet function assays are now available to monitor platelet aggregation and degranulation. The platelet function analyzer (PFA) is a test of in vitro platelet function. It is a good screening test for von Willebrand disease and monitors the effect of desmopressin administration. The PFA is prolonged after antiplatelet therapy. Unfortunately, the PFA-100 is not a sensitive test for monitoring the antiplatelet function of the P2Y12 inhibitors clopidogrel, prasugrel, and ticagrelor. However, the PFA-200, a recent update of the PFA-100, appears to be sensitive to the effects of the P2Y12 inhibitors. However, point-of-care studies on this new PFA test are still lacking.
Newer tests that monitor P2Y12 receptor activity include the vasodilator-stimulated phosphoprotein (VASP) assay, the VerifyNow assay, the multiple-platelet aggregometry test (Multiplate), and the platelet mapping component of the Thromboelastograph (TEG). The VerifyNow assay can monitor the antiplatelet effects of aspirin and the P2Y12 inhibitors. The platelet mapping component of the TEG is commonly used in surgery and anesthesiology, whereas VerifyNow is the predominant assay in clinical cardiology. A review on the monitoring of platelet function has been discussed by several reviews and is beyond the scope of this chapter.
ORAL ANTICOAGULANTS
Warfarin exerts its anticoagulant effect by interfering with the synthesis of the vitamin K–dependent clotting factors (VII, IX, X, and thrombin) (Figure 3). It also inhibits anticoagulant proteins C and S. Factor VII has a relatively short half-life (6–8 h), and the prothrombin time (PT) may be prolonged into the therapeutic range (1.5–2 times normal) within 24–36 hours. Anticoagulant protein C also has a short half-life (6–7 h). The initial prolongation of the international normalized ratio (INR) is the result of competing effects of reduced factor VII and protein C and the washout of existing clotting factors. Because of this, the INR is unpredictable during the initial stage of treatment with warfarin. Factor VII participates only in the extrinsic pathway, and adequate anticoagulation is not achieved until the levels of biologically active factors II (half-life of 50 h) and X are sufficiently depressed. This requires 4–5 days. High loading doses of warfarin (15 mg) are occasionally employed for the first 2–3 days of therapy, and the desired anticoagulant effect is achieved within 48–72 hours. The anticoagulant effect of warfarin persists for 4–6 days after termination of therapy while new biologically active vitamin K factors are synthesized. The drawbacks of warfarin therapy include the necessity of monitoring its effect with serial monitoring of INR, its interaction with other drugs, and the need for it to be discontinued a few days before surgery. The effect of warfarin can be reversed by the transfusion of fresh frozen plasma and vitamin K injections. The 3-factor or 4-factor prothrombin complex concentrate (PCC) can be used to antagonize warfarin in emergency situations.
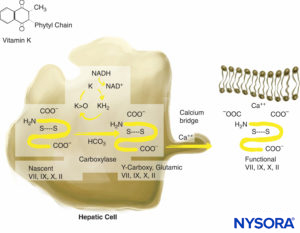
FIGURE 3. Vitamin K–dependent coagulation factor synthesis. Vitamin K is necessary for posttranslational modification of prothrombin; proteins C and S; and factors VII, IX, and X. Vitamin K is stored in hepatocytes.
Few data exist regarding the risk of spinal hematoma in patients with indwelling spinal or epidural catheters who are subsequently anticoagulated with warfarin. Odoom and Sih performed 1000 continuous lumbar epidural anesthetics in 950 patients who underwent vascular procedures and received preoperative oral anticoagulants. The thrombotest (a test of factor IX activity) was decreased and the activated partial thromboplastin time (aPTT) was prolonged in all the patients prior to epidural placement. Heparin was also administered intraoperatively. The epidural catheters remained in place for 48 hours postoperatively, and there were no neurologic complications. Unfortunately, the coagulation status of the patients at the time of catheter removal was not described. Although the results of this study are reassuring, the antiquated nature of the thrombotest as a measure of anticoagulation combined with the unknown coagulation status of the patients at the time of catheter removal limits the usefulness of the study.
The use of an indwelling epidural or intrathecal catheter and the timing of its removal in an anticoagulated patient are controversial. Although the trauma of needle placement occurs with both single-dose and continuous catheter techniques, the presence of an indwelling catheter may result additional injury to tissues and vascular structures.
Since intraspinal hematomas have occurred after catheter removal, it is recommended that the same laboratory values apply to placement and removal of the epidural catheter. No spinal hematomas were reported in 192 patients receiving postoperative epidural analgesia in conjunction with low-dose warfarin after total knee arthroplasty.
In this study, the patients received warfarin to prolong their PT to 15.0–17.3 seconds. The epidural catheters were left indwelling for 37 ± 15 hours (range 13–96 h). The mean PT at the time of epidural catheter removal was 13.4 ± 2 seconds (range 10.6–25.8 s). This and several subsequent studies have documented the relative safety of low-dose warfarin anticoagulation in patients with an indwelling epidural catheter. Another study showed higher INR levels (up to 1.9) are acceptable for the removal of epidural catheters as long as it is done within 12–14 hours after warfarin intake. A study showed the absence of spinal hematoma when the epidural catheters were removed 2–3 days after warfarin intake, even with markedly elevated INRs. The practice of pulling the epidural catheter out 3 days after initiation of warfarin, when the levels of clotting factors VII, IX, and X are low (factor II levels may still be acceptable) needs to be studied. This is especially the case since patients vary greatly in their response to warfarin, prompting some authors to recommend close monitoring of coagulation status to avoid excessive PT prolongation. Factors responsible for a prolonged PT and PTT are illustrated in Figures 4 and 5.
The ASRA recommended an INR value of 1.4 or less as acceptable for the performance of neuraxial nerve blocks. This value is based on studies that showed excellent perioperative hemostasis when the INR value was ≤1.5. Studies on the levels of clotting factors at different INR values have shown that the decline of these factors may not be significant at an INR of 1.5. At INR values of 1.5–2.0, the concentrations of factor II were noted to be 74%–82% of baseline, whereas factor VII levels were 27%–54% of baseline values.
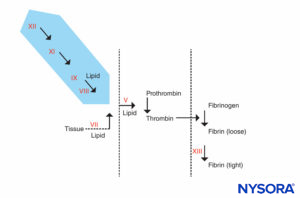
FIGURE 4. Coagulation reaction. Factors responsible for a prolonged PTT are in the shaded area. Patients who have an abnormal PTT but whose PT and other tests are normal can be divided into 2 groups: those who are prone to bleeding and those who are not. The patients who do not bleed may have an extremely prolonged PTT (90 seconds or more) but do not have a history of bleeding. They will have a deficiency in factor XII, prekalikrein, or high-molecular-weight kininogen. These patients should not be denied surgery or epidural anesthesia. The other group, patients who bleed, have both prolonged PTT and a history of bleeding. They will have a deficiency of factor VIII (hemophilia A), factor IX (hemophilia B or Christmas disease), or factor XI.
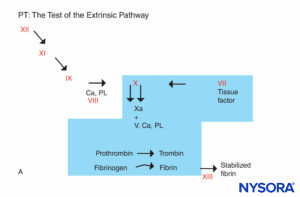
FIGURE 5A. Coagulation reaction. Factors involved in PT are in the shaded area. The PT is carried out by adding a source of tissue factor to the patient’s plasma along with calcium or phospholipid. Tissue factor forms a complex with and activates factor VII. (Ca, calcium; PL, phospholipid.)
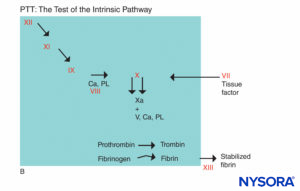
FIGURE 5B. Coagulation reaction. Factors involved in PTT are in the shaded area. In assessing PTT, coagulation is initiated by an agent that activates the Hageman factor–kininogen–prekalikrein complex. Most coagulation factors are screened by PTT, except factors VII and XIII, the protein that stabilizes fibrin clots by cross-linking them, and components of the fibrinolytic system. (Ca, calcium; PL, phospholipid.)
At INR values of 2.1 ± 1 during the initial phase of warfarin administration, factors II and VII were 65 ± 28% and 25 ± 20% of control values. Activities of 40% are considered adequate for normal hemostasis at the time of major surgery. Another study found that at INRs of 1.3–2, under stable anticoagulation with warfarin, the concentrations of clotting factors VII, IX, and X were within normal limits.
The clinician should be aware of the effect of warfarin on the coagulation cascade and the role of the INR in monitoring this effect. To minimize the risk of complications, the ASRA recommends several precautions. Chronic oral warfarin therapy should be stopped and the INR measured before a neuraxial nerve block is performed. The concurrent use of other medications, such as aspirin, NSAIDs, and heparins, which affect the clotting mechanism, increases the risk of bleeding complications without affecting the INR. If an initial dose of warfarin is given prior to surgery, the INR should be checked if the dose was given more than 24 hours earlier. If patients are on low-dose warfarin treatment (mean daily dose approximately 5 mg) during epidural analgesia, the INR should be checked daily and before catheter removal if the initial dose was given more than 36 hours previously. Higher daily doses may need more intensive monitoring. The warfarin dose should be held or reduced when the INR is >3 in patients with indwelling neuraxial catheters to prevent epidural hematoma and hemarthroma. While on warfarin therapy, the patient’s neurologic status should be checked routinely during epidural analgesic infusion, as well as 24 hours after the catheter has been removed. Dilute concentrations of local anesthetic should be utilized to minimize the degree of sensory and motor block. Clinical judgment must be exercised in making decisions about removing or maintaining neuraxial catheters in patients with therapeutic levels of anticoagulation during neuraxial catheter infusion. The warfarin dose should be reduced for patients who are likely to have an enhanced response to the drug, especially the elderly. For patients on chronic oral anticoagulation, the warfarin must be stopped and the INR measured.
NYSORA Tips
- An INR of 1.4, in the absence of easy bruisability and normal liver function, is acceptable before neuraxial injection in patients planned for neuraxial nerve blocks.
- The INR should be checked if the patient took warfarin more than 24 hours earlier.
- The INR should be normal 5 days after warfarin is stopped before a neuraxial procedure
HEPARIN
Intravenous Heparin
Heparin is a complex polysaccharide that exerts its anticoagulant effect by binding to antithrombin. The conformational change in antithrombin accelerates its ability to inactivate thrombin factors Xa and IXa.
Unfractionated heparin releases tissue factor pathway inhibitor from endothelium, enhancing its activity against factor Xa. The anticoagulant effect of subcutaneous heparin takes 1–2 hours, but the effect of intravenous heparin is immediate. In fact, the coagulation time is prolonged 2–4 times the baseline level 5 minutes after the intravenous injection of 10,000 units of heparin. Heparin has a half-life is 1.5–2 hours. The therapeutic dose of heparin ceases 4–6 hours after its administration. The aPTT is used to monitor the effect of heparin; therapeutic anticoagulation is achieved with a prolongation of the aPTT greater than 1.5 times the baseline value or a heparin level of 0.2–0.4 U/mL. The aPTT is usually not prolonged by the subcutaneous administration of low doses of heparin and is not monitored. Protamine neutralizes the effect of intravenously administered heparin.
Heparin is not the ideal anticoagulant since it is a mixture of molecules, only a fraction of which have anticoagulant activity. It binds to platelet factor 4 and to the von Willebrand factor. The heparin-antithrombin complex is also not very effective in neutralizing clot-bound thrombin. Finally, heparin is associated with immunologic thrombocytopenia and immune-mediated thrombosis. For patients receiving standard heparin therapy, the risk of bleeding complications is increased in the presence of other medications that affect other clotting mechanisms, including aspirin, NSAIDs, LMWHs, and oral anticoagulants. Several studies have demonstrated the safety of spinal or epidural anesthesia followed by systemic heparinization if certain precautions are observed. Rao and El-Etr reported no spinal hematomas in over 4000 patients who underwent lower extremity vascular surgery under continuous spinal or epidural anesthesia. In their study, patients with preexisting coagulation disorders were excluded, heparinization occurred at least 60 minutes after catheter placement, the level of anticoagulation was carefully monitored, and the indwelling catheters were removed at a time when heparin activity was low. Surgery was canceled in patients when frank blood was noted in the needle and performed the following day under general anesthesia. The same findings were noted in a subsequent report in the neurologic literature. Ruff and Dougherty noted spinal hematomas in 7 of 342 (2%) patients who underwent lumbar puncture and subsequent heparinization for evaluation of cerebral ischemia.
The presence of blood during the procedure, concomitant aspirin therapy, and heparinization within 1 hour were identified as risk factors in the development of spinal hematoma. The ASRA has made several recommendations for when a neuraxial technique is used in the presence of intraoperative anticoagulation. The ASRA advises that technique should be avoided in patients with other coagulopathies. There should be at least a 1-hour delay between needle placement and heparin administration. The catheter should be removed 2–4 hours after the last heparin dose and 1 hour before subsequent heparin administration. The aPTT or activated clotting time (ACT) should be monitored to avoid excessive heparin effect. The patient should be followed postoperatively for early detection of reoccurrence of motor block. Dilute concentrations of local anesthetics are recommended to minimize motor block.
Although there may be an increased risk in the event of a traumatic (bloody) or difficult needle placement, there are no data to support mandatory cancellation of surgery. The decision to proceed should be based on appropriate clinical judgment and full discussion with the surgeon and patient.
NYSORA Tips
- There should be at least a 1-hour delay between a neuraxial procedure and heparin re-administration.
- The catheter can be removed 2–4 hours after the last heparin dose.
Neuraxial procedures are occasionally performed in patients who undergo cardiopulmonary bypass. The following precautions have been recommended to prevent the development of intraspinal hematoma in these patients:
- Neuraxial procedures should be avoided in patients with known coagulopathy.
- Surgery should be delayed 24 hours in patients with a traumatic tap.
- The time from the neuraxial procedure to the systemic heparinization should exceed 1 hour.
- Heparinization and reversal should be monitored and controlled tightly.
- The epidural catheter should be removed when normal coagulation is restored, and the patient should be monitored closely for signs of spinal hematoma.
Subcutaneous Heparin
The therapeutic basis of low-dose twice-daily subcutaneous heparin (5000 units every 8–12 hours) is the heparin-mediated inhibition of activated factor X. Smaller doses of heparin are required when administered as prophylaxis rather than as treatment for thromboembolic disease. Following the intramuscular or subcutaneous injection of 5000 units of heparin, maximum anticoagulation effect is observed in 40–50 minutes and returns to baseline within 4 to 6 hours. The aPTT may remain in the normal range and often is not monitored. However, wide variations in individual patient responses to subcutaneous heparin have been reported. Neuraxial techniques are not contraindicated during subcutaneous (mini-dose) prophylaxis, but the risk of bleeding may be reduced by delaying heparin administration until after the nerve block. Bleeding may be increased in debilitated patients or after prolonged therapy. The safety of major neuraxial anesthesia in the presence of anticoagulation with twice-daily subcutaneous doses of unfractionated heparin has been documented by several studies. The twice-daily subcutaneous heparin regimen has been replaced by a thrice-daily regimen in most hospitals to decrease the incidence of postoperative venous thromboembolism. However, this practice has been associated with spontaneous hematomas. For this reason, the latest ASRA guidelines recommend against neuraxial procedures in patients on a thrice-daily regimen until more data become available.
NYSORA Tips
- Neuraxial procedures can be performed in patients on twice-daily subcutaneous heparin.
- Because of the lack of adequate data, the ASRA recommends that neuraxial procedures not be performed in patients on thrice-daily subcutaneous heparin.
Low-Molecular-Weight Heparin
Unfractionated heparin is a heterogeneous mixture of polysaccharide chains that can be separated into fragments of various molecular weights. The anticoagulant effect of LMWH is similar to that for unfractionated heparin; it activates antithrombin, accelerating antithrombin’s interaction with thrombin and factor Xa. LMWH has a greater activity against factor Xa, whereas unfractionated heparin has equivalent activity against thrombin and factor Xa. The plasma half-life of the LMWHs ranges from 2–4 hours after an intravenous injection and 3–6 hours after a subcutaneous injection; the half-life of an LMWH is 2–4 times that of standard heparin. It has a low affinity for plasma protein, resulting in a greater bioavailability. The advantages of LMWH over unfractionated heparin include a higher and more predictable bioavailability after subcutaneous administration and a longer biological half-life. Also, laboratory monitoring of the anticoagulant response of LMWH is not required, and dose adjustment for weight is not necessary (although an overdose may occur in patients with a low BMI). LMWH exhibits a dose-dependent antithrombotic effect that is accurately assessed by measuring the anti-Xa activity level. The recovery of anti-factor Xa activity after a subcutaneous injection of LMWH approaches 100%, making laboratory monitoring unnecessary except in patients with renal insufficiency or those with body weight less than 50 kg or more than 80 kg. The reaction time from the thrombelastogram appears to correlate with the serum anti-Xa concentration.
The three commercially available LMWHs in the United States are enoxaparin (Lovenox), dalteparin (Fragmin), and tinzaparin (Innohep), although the latter has been discontinued because of low usage. Enoxaparin is given either once daily or every 12 hours when used as prophylaxis, and dalteparin and tinzaparin are given once daily. The three drugs appear to have comparable efficacy in the treatment and prevention of venous thromboembolism. Enoxaparin and dalteparin have comparable efficacy in the prevention of death or myocardial infarction among patients with unstable angina.
The recommended thromboprophylactic dose in the United States is 30 mg enoxaparin twice daily, although some clinicians increase the dose in patients who are obese (1.5 mg/kg daily or 1 mg/kg every 12 hours). The European dosing schedule is enoxaparin 20–40 mg once daily, and patients receive their starting dose 12 hours before surgery, a practice not observed in the United States.
Numerous cases of neuraxial hematoma occurred in the United States, prompting the FDA to issue a health advisory in December 1997 and the convening of the first ASRA consensus conference on anticoagulation and neuraxial anesthesia. The ASRA guidelines recommend the smallest effective dose of LMWH should be administered. The postoperative administration of LMWH therapy should be delayed as long as possible, for a minimum of 12 hours and ideally 24 hours postoperatively. A single-dose spinal anesthetic may be the safest neuraxial technique in patients receiving preoperative LMWH. Waiting for at least 12 hours after the prophylactic LMWH dose is recommended before performing a neuraxial technique. Patients who receive higher doses of LMWH (eg, enoxaparin 1 mg/kg twice daily) require longer delays (24 h). The catheter should be removed when anticoagulation activity is low, at least 12 hours after prophylactic LMWH administration and 4 hours before the next dose. Extreme vigilance of the patient’s neurologic status must be observed if LMWH thromboprophylaxis is implemented while an indwelling catheter is infusing. Dilute local anesthetic solution is recommended so that neurologic function can be better monitored. The use of other medications affecting hemostasis, such as antiplatelet drugs, standard heparin, dextran, or oral anticoagulants, in combination with LMWH creates an additional risk of bleeding complications.
NYSORA Tips
- In patients on a prophylactic dose of LMWH, a 12-hour interval is recommended before a neuraxial injection.
- For patients on a therapeutic dose of LMWH, a 24-hour interval is appropriate.
- A 4-hour delay following epidural catheter removal before LMWH is resumed has been recommended by the FDA.
THROMBOLYTIC THERAPY
Thrombolytic agents actively dissolve fibrin clots that have already formed. Exogenous plasminogen activators such as streptokinase and urokinase not only dissolve thrombus but also affect circulating plasminogen, leading to decreased levels of both plasminogen and fibrin. Recombinant tissue-type plasminogen activator (r-TPA), an endogenous agent, is more fibrin selective and has less effect on circulating plasminogen levels. Clot lysis leads to an elevation of fibrin degradation products, which have an anticoagulant effect by inhibiting platelet aggregation.
Although epidural or spinal needle and catheter placement with subsequent heparinization appears relatively safe, the risk of spinal hematoma in patients who receive thrombolytic therapy is not well defined. Cases of spinal hematoma in patients who had epidural or indwelling epidural catheters and who received thrombolytic agents have been reported in the literature.
The ASRA guidelines make recommendations with respect to neuraxial procedures after thrombolytic or fibrinolytic therapy. The concurrent use of heparin with fibrinolytic or thrombolytic drugs places patients at high risk of adverse neuraxial bleeding during spinal or epidural anesthesia. Except in highly unusual circumstances, patients receiving fibrinolytic or thrombolytic therapy should be cautioned against receiving spinal or epidural anesthesia. There are no available data to clearly determine the appropriate length of time after discontinuation of these drugs and the safe performance of a neuraxial technique. The European guidelines recommend leaving the epidural catheter in place when a patient is given a thrombolytic drug, removing it only once effect of the drug is gone. The Scandinavian guidelines recommend a 24-hour interval between discontinuation of the drug and neuraxial procedure. Frequent neurologic monitoring is recommended for an appropriate length of time in patients who have had neuraxial nerve blocks after fibrinolytic or thrombolytic therapy. If a patient has a continuous epidural infusion and has received fibrinolytic or thrombolytic therapy, drugs that minimize sensory and motor block should be used. There has been no definitive recommendation on the timing of removal of neuraxial catheters in patients who unexpectedly receive fibrinolytic or thrombolytic therapy. Measurement of fibrinogen levels may be helpful in guiding a decision about catheter removal or maintenance.
Herbal Therapy
The most commonly used herbal medications are garlic, ginkgo, and ginseng. Garlic inhibits platelet aggregation, and its effect on hemostasis appears to last 7 days. Ginkgo inhibits platelet-activating factor, and its effect lasts 36 hours. Ginseng has a variety of effects: it inhibits platelet aggregation in vitro and prolongs both thrombin time (TT) and aPTT in laboratory animals; its effect lasts 24 hours. In spite of their effect on platelet function, herbal drugs by themselves appear to present no added significant risk in the development of spinal hematoma in patients having epidural or spinal anesthesia. Mandatory discontinuation of these medications, or cancellation of surgery in patients in whom these medications have been continued, is not supported by available clinical data. However, the concurrent use of other medications that affect clotting mechanisms, such as oral anticoagulants or heparin, may increase the risk of bleeding complications in these patients. There is no accepted test to assess adequacy of hemostasis in the patient who has taken herbal medications. At this time, there appear to be no specific concerns as to the timing of neuraxial nerve block in relationship to the dosing of herbal therapy, postoperative monitoring, or the timing of neuraxial catheter removal.
Thrombin Inhibitors
Recombinant hirudin derivatives, including desirudin, and bivalirudin, inhibit both free and clot-bound thrombin. Argatroban, an l-arginine derivative, has a similar mechanism of action. These drugs are primarily used in the treatment of heparin-induced thrombocytopenia. There is no pharmacologic reversal to the effect of these drugs. There have been no case reports of spinal hematoma related to neuraxial anesthesia in patients who have received a thrombin inhibitor. However, spontaneous intracranial bleeding has been reported. According to the ASRA guidelines, no statement regarding risk assessment and patient management can be made.
Fondaparinux
Fondaparinux is a synthetic anticoagulant that produces its antithrombotic effect through selective inhibition of factor Xa. The drug exhibits consistency in its anticoagulant effect since it is chemically synthesized. It is 100% bioavailable. Rapidly absorbed, it attains maximum concentration within 1.7 hours of administration. Its half-life is 17–21 hours.
Fondaparinux is recommended as an antithrombotic agent after major orthopedic surgery and as initial treatment for pulmonary embolism. The extended half-life (approximately 20 hours) allows once-daily dosing. The FDA has issued a black box warning for fondaparinux similar to that for the LMWHs and heparin.
The actual risk of spinal hematoma with fondaparinux is unknown. A study showed no complications in patients who received neuraxial injections. In this study, the catheters were removed 36 hours after the last dose of fondaparinux, and dosing was delayed for 12 hours after catheter removal. Patients were excluded from the study if difficulties were encountered in performing the neuraxial procedure (more than 3 attempts required), the procedure was complicated by bleeding, they required antiplatelet drugs, or the plan was to withdraw the epidural catheter the day after surgery. Because of the unrealistic requirements in clinical practice, the ASRA recommends against the use of fondaparinux in the presence of an indwelling epidural catheter. Their recommendations are based on the sustained and irreversible antithrombotic effect of fondaparinux, early postoperative dosing, and the spinal hematoma reported during the initial clinical trials of the drug. Close monitoring of the literature for risk factors associated with surgical bleeding may be helpful in risk assessment and patient treatment. Performance of neuraxial techniques should occur under conditions used in clinical trials (single needle pass, atraumatic needle placement, avoidance of indwelling neuraxial catheters). If this is not feasible, an alternative method of prophylaxis should be considered.
Summary
The time interval between discontinuation of the anticoagulant and neuraxial procedure and between epidural catheter removal and resumption of the drug are summarized in Table 1.
TABLE 1. Recommended time intervals before or after neuraxial procedure and epidural catheter removal.
| Drug | Time Before Neuraxial Procedure or Catheter Removal | Time After Neuraxial Procedure or Catheter Removal | Comments |
|---|---|---|---|
| Aspirin | None | None | |
| NSAIDs | None | None | |
| Clopidogrel | 7 days* | After catheter removal | Per European & & Scandinavian guidelines |
| Prasugrel | 7-10 days | 6h | Per European Guidelines |
| Ticagrelor | 5 days | 6h | (As above) |
| Warfarin | 5 days (normal INR) | After cateter removal | |
| Heparin (IV) | 4-6 h | 1-2h | |
| Heparin | |||
| -(Sc, BID) | None | None | |
| -(Sc, TID) | Not applicable | Neuraxial procedure | |
| LMWH | |||
| Prophylactic | 12 hours | 4 hours | FDA recommendation |
| Therapeutic | 24 hours | 4 hours | |
| Fondaparinux | 36-42 hours | 6-12 hours | Per European guidelines. ASRA recommended against neuraxial procedures in patients on the drug. *If neuraxial procedure has to be performed at 5 days, a test of platelet function is recommended (see text |
NEW ANTICOAGULANTS
A discussion of the new anticoagulants dabigatran, rivaroxaban, and apixaban involves a review of certain background information.
The Interval Between Anticoagulant Discontinuation and Neuraxial Injection and Between Neuraxial Procedure or Epidural Catheter Removal and Resumption of Anticoagulant
It has been recommended that two half-lives is an adequate compromise between the risk of venous thromboembolism (VTE) and the prevention of spinal hematoma. The European and Scandinavian guidelines recommended a 2-half-life interval between discontinuation of anticoagulant and neuraxial injection.
This decision was like made because subclinical VTE occurs in a fair percentage of patients immediately after surgery and having residual anticoagulation might prevent this occurrence. The presence of residual anticoagulation facilitates the transition to full anticoagulation after a neuraxial procedure. After 1, 2, 3, 4, 5, and 6 half-lives, the following percentages of drug remain in the circulation: 50%, 25%, 12.5%, 6.25%,
3.125%, and 1.5625%, respectively (Table 2).However, these findings are based on studies in young healthy volunteers in single-dosing pharmacokinetic studies in the absence of other anticoagulants. By contrast, in clinical practice, patients are usually older and have concomitant comorbidities.
TABLE 2. Recommended time intervals before or after neuraxial procedure and epidural catheter for the new anticoagulants.
| Drug | Half-life | European Guidelines | Scandinavian Guidelines | Five Half-lives |
|---|---|---|---|---|
| Dabigatran | 12-17h 28 hours (renal disease) | (Contraindicated per manufacturer) | Data not available | 85h (4d) 6d (renal patients) |
| Rivaroxaban | 9-13h | 22-26h | 18h | 65h (3d) |
| Apixaban | 15.2 +/-8.5h | 26-30h | Data not available | 75h (3-4d) |
In patients who are at risk for VTE, such as those with a previous history of stroke, a 2- or 3-half-life interval might be appropriate, recognizing that adequate hemostasis is not assured. For patients without thrombotic risk factors, an interval of 4–6 half-lives between the last dose of anticoagulant and neuraxial injection ensures a more complete elimination of the drug and less risk of bleeding. A compromise between the conservative recommendations of 4–6 half-lives and 2–3 half-lives is an interval of 5 half-lives with LMWH bridge therapy.
Regarding the resumption of the anticoagulant after neuraxial injection or removal of epidural catheter, the Scandinavian guidelines are based on the recommendation of Rosencher et al. of 8 hours minus the time it takes for the anticoagulant to reach peak effect.
Eight hours was presumed to be adequate for the clot to stabilize, a presumption supported by the efficacy of thrombolytic agents to lyze a clot if given within 6 hours of clot formation. Tertri and colleagues also noted that giving enoxaparin within 24–48 hours after intracerebral hemorrhage did not enlarge the size of hematoma, so a 24-hour interval is probably safer. Other authors recommend a more conservative approach because the reinstitution of antithrombotic therapy within 24 hours after a major procedure might increase the risk of periprocedural bleeding. Liew and Douketis recommend a minimum of 24 hours in patients with low bleeding risk and 48 hours in those with a high bleeding risk before resuming dabigatran, rivaroxaban, or apixaban. The options therefore are either 8 hours or 24 hours minus the peak effect of the drug.
There is probably little difference between these two options since the risks of VTE, stroke, or acute coronary syndrome are probably the same. In addition, the onset and times to peak effect of the new anticoagulants are short.
Dabigatran
Dabigatran etexilate is a prodrug that is hydrolyzed by esterases in the stomach to the active drug. Dabigatran etexilate has a bioavailability of 7.2%. Dabigatran is a direct thrombin inhibitor that nerve blocks the interaction of thrombin with various substrates. Peak plasma concentrations are attained 1.5–3 hours after intake of the prodrug. It has a half-life of 14–17 hours. Renal clearance accounts for 80% of the total clearance of dabigatran. In cases of endstage renal disease, the elimination half-life doubles from 14 hours to 28 hours.
Dabigatran is effective in the treatment of acute VTE and in the prevention of recurrent VTE. In patients with atrial fibrillation, dabigatran reduces the rates of stroke and systemic embolism to a degree similar to that of warfarin. Dabigatran was not shown to be consistent in preventing VTE after total joint surgery. Studies have shown it to be either more effective, noninferior, or inferior to enoxaparin. A meta-analysis of the trials noted no differences between dabigatran and enoxaparin in any of the endpoints analyzed. The manufacturer states that epidural catheters should not be placed in patients receiving dabigatran. A 2-hour minimum interval between indwelling catheter removal and dabigatran administration has been recommended by Levy and collegues. This interval appears to be shorter than 6 hours, that is, the difference between 8 hours minus the 2-hour time to reach peak effect of the drug. There have been reports of increased bleeding after taking dabigatran. The Haematology Society of Australia and New Zealand identified 78 bleeding episodes in approximately 7000 patients over a 2-month period. An audit by the FDA, however, concluded that there was not an absolute increase of bleeding with dabigatran compared to warfarin.
The aPTT is prolonged after dabigatran, but the relationship is curvilinear. The thrombin time (TT), also known as thrombin clotting time (TCT), is highly sensitive to the effects of dabigatran and is more appropriately used for detecting the presence of the anticoagulant effect of dabigatran than quantifying the effect of the drug. A dilute TT has inearity across pharmacologically relevant plasma dabigatran concentrations. The ecarin clotting time (ECT), which directly measures thrombin generation, is dose-dependent prolonged by dabigatran. It is the most sensitive assay for dabigatran, but few institutions have the test available. The prothrombin time (PT) is the least sensitive test. The dilute TT and the ECT are the tests of choice for dabigatran.
To date, there is no antidote to reverse the effect of dabigatran or the other new oral anticoagulants. Activated charcoal prevents absorption of the drug, but it needs to be given within 2 hours of dabigatran ingestion. Dialysis might speed drug elimination. Plasma complex concentrates (PCCs) that contain either 3 (factors II, IX, and X) or 4 (factors II, VII, IX, and X) clotting factors have been suggested, but their efficacy has not been proven. Idarucizumab, a monoclonal antibody fragment that binds with free and thrombin-bound dabigatran, was recently approved by the FDA.
NYSORA Tips
- Dabigatran is primarily dependent on the kidneys for elimination, and its half-life is doubled in patients with kidney disease.
- A longer interval between stoppage of the drug and neuraxial procedure, probably 6 days, is recommended in these patients.
Rivaroxaban
Rivaroxaban is a direct factor Xa inhibitor. Peak plasma concentrations are observed within 2.5–4 hours, and the maximum inhibition of factor Xa (up to 68%) occurs 3 hours after dosing and is maintained for at least 12 hours, or 24–48 hours when higher doses are given in elderly patients. Rivaroxaban has a terminal half-life of 5.7–9.2 hours, but this can be as long as 11–13 hours in elderly patients due to agerelated decline in renal function. One-third of the drug is eliminated by the kidneys, one-third by the fecal/biliary route, and one-third is changed to inactive metabolites. The maximum concentration is not affected by obesity (patients weighing ≥120 kg) but is increased by 24% in patients weighing ≤50 kg. The renal clearance of rivaroxaban decreases with increasing renal impairment.
Rivaroxaban is effective in the treatment of symptomatic VTE and noninferior to warfarin in the prevention of embolic stroke during atrial fibrillation. The addition of rivaroxaban to standard antiplatelet therapy reduces the composite endpoint of death from cardiovascular causes, myocardial infarction, or stroke in patients with a recent acute coronary syndrome. Rivaroxaban was reported to be as effective or superior to enoxaparin in preventing VTE after total joint surgery. In the RECORD 1, 2, 3, and 4 studies, rivaroxaban was a more effective thromboprophylactic agent than enoxaparin, with a similar safety profile. Rosencher et al. stated that epidural catheters were not removed until at least 2 half-lives after the last dose of rivaroxaban, and the next rivaroxaban dose was given 4-6 hours after catheter removal. None of the 1141 patients who were given rivaroxaban and had neuraxial anesthesia developed spinal hematoma. However, this small number of patients is not adequate to make a firm conclusion on the perioperative safety of this regimen.
The European and Scandinavian guidelines recommend a 2-half-life interval between rivaroxaban discontinuation and epidural catheter placement or removal (18 hours in the Scandinavian guidelines and 22–26 hours in the European guidelines). These guidelines also recommend a 4–6-hour interval before resumption of the next dose, as rivaroxaban takes 2.5–4 hours to reach peak effect.
A linear correlation was observed between the effects of rivaroxaban and PT. However, there is marked variability in the sensitivity of PT reagents to rivaroxaban, so it is recommended that each laboratory should calibrate their PT specifically for rivaroxaban. The aPTT lacks sufficient sensitivity to determine the effect of rivaroxaban. The inhibition of factor Xa may also be a surrogate for the plasma concentrations of rivaroxaban. The PT and anti-Xa are the recommended tests for monitoring the effects of rivaroxaban.
The use of activated charcoal has been recommended to remove rivaroxaban, but it must be given within 8 hours of rivaroxaban intake. A 4-factor PCC has been shown to reverse the in vitro anticoagulant activity of rivaroxaban in healthy volunteers. Because of their high protein binding, rivaroxaban and apixaban may not be dialyzable.
Apixaban
Apixaban is a highly specific factor Xa inhibitor. It is rapidly absorbed and attains peak concentrations in 1–2 hours. Studies have shown the terminal half-life of apixaban to be 13.5 +/− 9.9 hours, or 15.2 +/− 8.5 hours after a single 5 mg dose and 11.7 +/− 3.3 after multiple 5 mg doses. Maximum plasma concentration is affected by body weight, with higher concentrations of apixaban in subjects with low body weight. Plasma anti–factor Xa activity has shown a direct linear relationship with apixaban plasma concentration.
Apixaban has an oral bioavailability of more than 45%. After oral administration, it is eliminated via multiple elimination pathways as well as direct renal and intestinal excretion. Twenty-four to 29 percent of the dose is excreted via the kidneys, and 56% of the dose is recovered in the feces. More than half of apixaban is excreted unchanged, lessening the risk of metabolic drug–drug interactions Apixaban is effective in reducing stroke or systemic embolism without increasing the risk of bleeding. Apixaban is superior to warfarin in preventing stroke or systemic embolism in patients with atrial fibrillation. Apixaban provides effective thromboprophylaxis in total knee arthroplasty, comparable to enoxaparin or warfarin. Apixaban is equally efficacious with enoxaparin in preventing VTE after total knee replacement (TKR) while having a lower or similar rate of major bleeding. Apixaban is more effective than enoxaparin in preventing VTE after total hip replacement (THR) without increased bleeding. In this trial, “devices in connection with intrathecal or epidural anesthesia were removed at least 5 hours before the first dose” of apixaban. In all studies of apixaban, the drug was started 12–24 hours after surgery.
Compared to rivaroxaban, apixaban has little effect on PT when given in approved doses. The dilute PT assay has improved sensitivity over conventional PT. There appears to be a linear correlation between anti-Xa activity and the plasma concentrations of apixaban. The anti-Xa assay was noted to be more sensitive than the PT and as sensitive as the dilute PT assay and appears to be the best choice for clinical monitoring of the anticoagulant effect of apixaban. Activated charcoal, given within 3 hours of ingestion, reduces the absorption of apixaban.
Andexanet is a recombinant modified human factor Xa decoy protein that binds and sequesters factor Xa inhibitors. Studies in volunteers and in patients showed andexanet to reveres the anticoagulant activity of rivaroxaban and apixaban. As of 2016, andexanet is not yet clinically available in the United States.
Summary of Recommendations for the New Anticoagulants
While a 2–3-half-life interval may be acceptable in patients who are at high risk for VTE or stroke, an interval of 4–6 half-lives between stoppage of the drug and neuraxial injection is probably safer in most patients at low risk of thrombosis. A 5-half-life interval in conjunction with an LMWH bridge therapy is an alternative in most patients, as shown in Table 2. After a neuraxial procedure or removal of an epidural catheter, the anticoagulant can be resumed 6 hours (8 hours minus the onset/peak effect of the drug, which is usually 2 hours) later. Anticoagulants are typically resumed within 24–48 hours in most patients, but they can be resumed sooner in patients who are at higher risk for VTE or stroke; that is, 24 hours minus the time to peak effect of the drug. Others recommended a 24-houir interval (Table 3)
TABLE 3. Recommended time intervals for resumption of drug after neuraxial procedure or catheter removal.
| Drug | European Guidelines | Scandinavian Guidelines | Liew & Douketis (102); Connolly and Spyropoulos (98) |
|---|---|---|---|
| Dabigatran | 6h | 6h | 24h |
| Rivaroxaban | 4-6h | 6h | 24h |
| Apixaban | 4-6h | 6h | 24h |
Laboratory monitoring of the anticoagulant effect is appropriate in some situations, and reversal agents are suggested when there is a need to rapidly restore hemostatic function.
NYSORA Tips
- For the new anticoagulants, a 5-half-life interval between discontinuation of the drug and neuraxial procedure is recommended until there is more experience with these agents.
- An interval of either 8 or 24 hours-time to peak effect of the drug is recommended before the drug is resumed after catheter removal; a 24-hour interval is probably the safest.
CLINICAL FEATURES, DIAGNOSIS & MANAGEMENT OF EPIDURAL HEMATOMA
Patients who develop spinal hematoma usually present with sudden, severe, constant back pain with or without a radicular component. Percussion over the spine aggravates the pain as do maneuvers that increase intraspinal pressure, including coughing, sneezing, or straining. In addition, the return of the motor weakness and/or sensory deficit after the apparent resolution of the epidural or spinal block is highly suggestive of epidural or spinal hematoma formation. Motor and sensory findings depend entirely on the level and size of the hematoma but may include weakness, paresis, loss of bowel or bladder function, and virtually any sensory deficit. Magnetic resonance imaging (MRI) is the diagnostic study of choice. The differential diagnosis includes spinal abscess, epidural neoplasm, acute disk herniation, and spinal subarachnoid hemorrhage. Recovery without surgery is rare, and neurosurgical consultation for consideration of emergent decompressive laminectomy should be obtained as soon as spinal hematoma is suspected. Functional recovery is related primarily to the length of time the symptoms are present before surgery. The clinical features, diagnosis, differential diagnosis, and treatment of a patient with a spinal hematoma are discussed in more detail in Local Anesthetic Systemic Toxicity.
SUMMARY
Practitioners should periodically update their knowledge base on new anticoagulant medications, anticoagulation protocols, current guideline recommendations, and FDA alerts. Since spinal hematoma may occur even in the absence of identifiable risk factors, vigilance in monitoring is critical for early evaluation of neurologic dysfunction and prompt intervention. The decision to perform neuraxial block and the timing of catheter removal in a patient receiving anticoagulant therapy should be made on an individual basis, weighing the benefits of regional anesthesia against the small, though definite, risk of spinal hematoma.
Anticoagulation & Peripheral Nerve Blocks
If appropriate, peripheral nerve blocks can be performed in patients taking anticoagulants. In contrast to neuraxial procedures in the presence of anticoagulants, there have been no prospective studies on peripheral nerve blocks in the presence of anticoagulants. The ASRA recommends the same guidelines for peripheral nerve blocks as for neuraxial procedures. Cases of psoas and retroperitoneal hematomas have been reported after lumbar plexus nerve blocks and psoas compartment nerve blocks. These patients were either on enoxaparin, ticlopidine, or clopidogrel. In some cases, the hematoma occurred in spite of adherence to the ASRA guidelines.
The symptoms of hematoma formation after peripheral nerve block may include pain (flank or paravertebral pain, or groin pain in psoas bleeding), tenderness in the area, a steady decline in hemoglobin/hematocrit, hypotension due to hypovolemia, and sensory–motor deficit. Definite diagnosis is made by computerized tomography (CT); ultrasound can also be used to detect the presence of renal subcapsular hematoma after psoas compartment nerve block. Treatment may include surgical consultation, reversal of anticoagulation, blood transfusion as necessary, and watchful waiting versus surgical drainage.
It is probably too restrictive to adapt the ASRA guidelines on neuraxial nerve blocks to patients undergoing peripheral nerve blocks. The European Society of Anaesthesiology has noted that the guidelines for neuraxial nerve block do not routinely apply to peripheral nerve blocks. The Austrian Society for Anesthesiology, Resuscitation and Intensive Care, on the other hand, has suggested that superficial nerve blocks can be safely performed in the presence of anticoagulants. Because of the possibility of retroperitoneal hematoma, lumbar plexus and paravertebral nerve blocks merit the same recommendations as for neuraxial injections. The same guidelines should also apply to visceral sympathetic nerve blocks. The ASRA guidelines may, therefore, be applicable to nerve blocks in vascular and noncompressible areas, such as celiac plexus nerve blocks, superior hypogastric plexus nerve blocks, and lumbar plexus nerve blocks. Clinicians should individualize their decision and discuss the risks and benefits of the nerve block with the patient and the surgeon. Most importantly, the clinician should follow the patient closely after the nerve block placement.
NYSORA Tips
- The guidelines on neuraxial injections should also apply to lumbar plexus nerve blocks and visceral sympathetic nerve blocks.
- For superficial nerve blocks, ultrasound-guided regional nerve blocks can probably be performed in the presence of residual anticoagulation.
Clinical updates
The clinical review of Douketis et al. (JAMA, 2024) underscores current best practices for managing patients on anticoagulants who require neuraxial or other regional anesthesia and surgery. It highlights that continuation of direct oral anticoagulants (DOACs) and warfarin requires careful timing relative to procedure and neuraxial access, with individualized interruption based on drug half-life, renal function, and procedural bleeding risk to minimize both hematoma and thromboembolic events. The article emphasizes the importance of coordination with surgical and medical teams, the use of risk stratification tools to guide decisions on when to hold or restart agents such as apixaban, rivaroxaban, dabigatran, and warfarin, and advises that neuraxial techniques should be attempted onlyafter appropriate washout and normalization of coagulation parameters. It reinforces that urgent reversal strategies (e.g., idarucizumab for dabigatran, prothrombin complex concentrates for factor Xa inhibitors) should be available when necessary, and that clear documentation and patient education are critical to the safe use of anticoagulants during regional anesthesia and surgery.
- Read more about the study HERE.
Suleiman et al. (British Journal of Anaesthesia, 2025) compare conservative ASRA guidance for DOAC interruption before neuraxial anaesthesia with the pharmacokinetics-based PAUSE strategy, highlighting that PAUSE allows shorter DOAC holds (~60–68 h vs 72–120 h) without routine drug-level testing. Data from the PAUSE and PAUSE-2 pilot trials show that >94% of patients achieved low residual DOAC levels (<30 ng ml⁻¹) with similar bleeding signals, while reducing interruption duration by ~25%. The authors emphasize that although these findings suggest feasibility of less conservative management, the catastrophic but rare risk of spinal epidural haematoma justifies continued caution until results of the full PAUSE-2 trial are available.
- Read more about the editorial HERE.