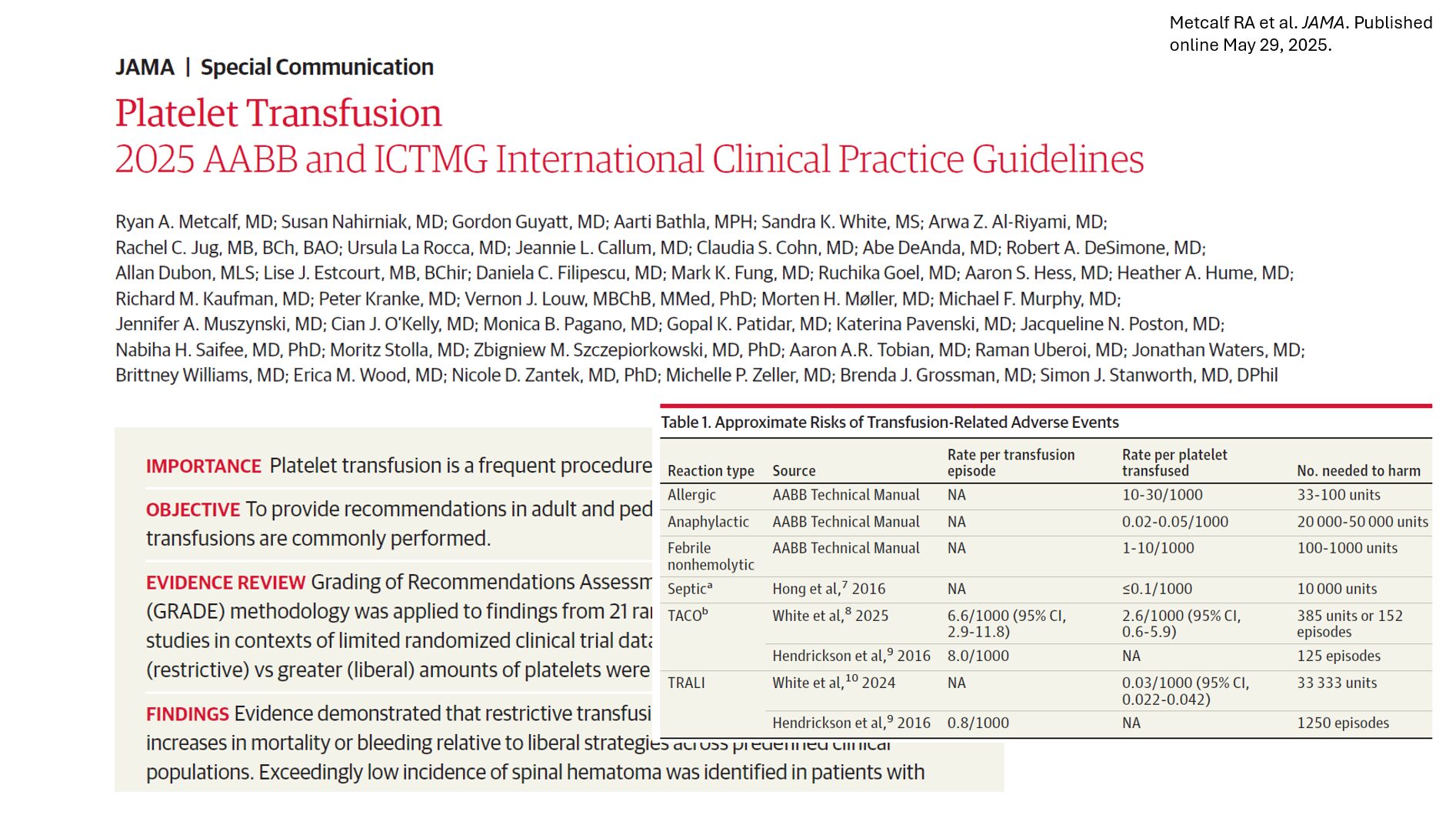
In a landmark move published in JAMA in May 2025, the Association for the Advancement of Blood and Biotherapies (AABB) and the International Collaboration for Transfusion Medicine Guidelines (ICTMG) unveiled updated international clinical guidelines on platelet transfusion. Backed by a robust panel of global experts and patient partners, these guidelines emphasize a conservative, or restrictive, approach to platelet transfusion to minimize harm, conserve resources, and enhance clinical outcomes.
Why restrictive transfusion strategies matter
Platelet transfusions are lifesaving in specific clinical contexts, particularly for patients with thrombocytopenia (low platelet counts) or platelet dysfunction. However, the procedure is not without risks. Platelets are biologically active and prone to causing immune and non-immune adverse events more frequently than red blood cell transfusions.
Key considerations:
- Short shelf life: Platelets have a shelf life of 5–7 days, making supply chains highly vulnerable.
- High demand: Unlike red blood cell use, platelet use has not declined in recent years.
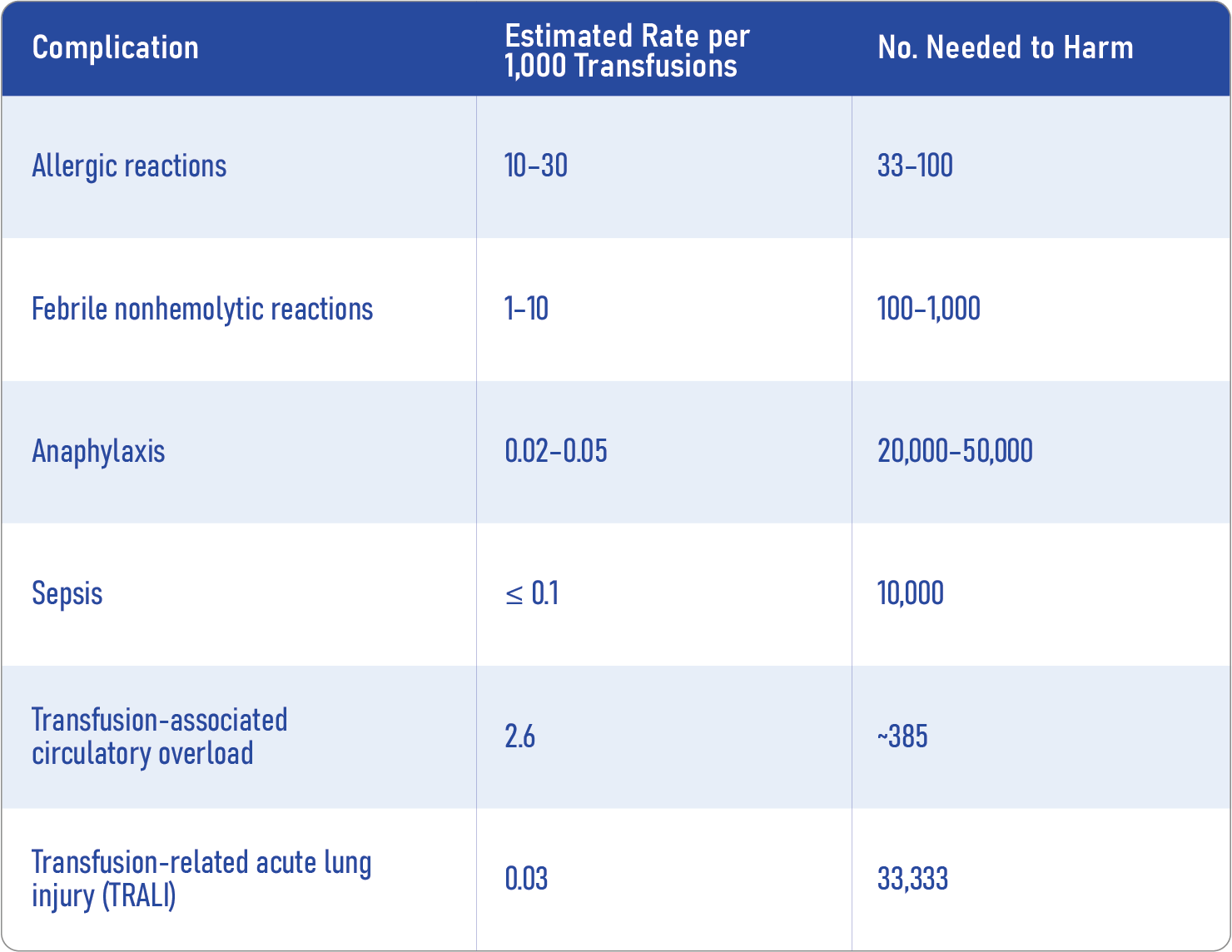
- Adverse effects: These include allergic reactions, febrile nonhemolytic reactions, and life-threatening events like TRALI and TACO.
- Resource strain: Platelet collection and storage are costly, requiring tight coordination.
By advocating for a restrictive strategy, the 2025 guidelines aim to reduce unnecessary transfusions, improve safety, and optimize the use of limited blood products.
Major findings from the evidence review
The guideline development was grounded in the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) methodology, which assessed 21 randomized clinical trials (RCTs) and 13 observational studies spanning several decades and diverse patient populations.
Overall results show:
- No increase in mortality with restrictive strategies.
- Minimal difference in grade 3–4 bleeding.
- Reduced exposure to transfusion-related harms.
Who should get transfused and when?
These are backed by moderate to high-certainty evidence.
1. Non-bleeding cancer patients or stem cell recipients
- Threshold: < 10,000/μL.
- Rationale: Evidence shows no benefit from transfusing at higher counts.
2. Neonates without bleeding
- Threshold: < 25,000/μL.
- Rationale: Liberal transfusion increases mortality in this vulnerable population.
3. Lumbar puncture patients
- Threshold: < 20,000/μL.
- Rationale: Spinal hematoma risk is exceedingly low; transfusion offers little benefit at higher levels.
4. Dengue patients without bleeding
- Recommendation: Do not transfuse.
- Rationale: No mortality benefit, potential for harm.
Tailored based on individual risk
Supported by lower-certainty evidence, these recommendations offer flexibility in clinical decision-making.
1. Autologous stem cell transplant or aplastic anemia (non-bleeding)
- Recommendation: No routine prophylaxis.
- Rationale: Benefits are unclear; quality of life considerations are important.
2. Critical illness-related thrombocytopenia
- Threshold: < 10,000/μL.
- Rationale: Limited trial data, but practical to minimize exposure.
3. Central venous catheter placement (compressible sites)
- Threshold: < 10,000/μL.
- Rationale: Bleeding risk is low in compressible sites like the internal jugular.
4. Interventional radiology procedures
- Threshold:
- Low-risk: < 20,000/μL
- High-risk: < 50,000/μL
- Rationale: Bleeding risk varies by procedure.
5. Major non-neuraxial surgery
- Threshold: < 50,000/μL.
- Rationale: Threshold balances bleeding risk and resource use.
6. Cardiovascular surgery (no thrombocytopenia or hemorrhage)
- Recommendation: No transfusion.
- Rationale: No benefit demonstrated.
7. Intracranial hemorrhage (platelet count > 100,000/μL or on antiplatelets)
- Recommendation: No transfusion.
- Rationale: Data does not support efficacy, and there are possible harms.
Risks of platelet transfusion: know the numbers
How to implement the new guidelines: step-by-step
1. Confirm the clinical indication
Is the transfusion for prophylaxis, bleeding, or an invasive procedure?
2. Assess platelet count and risk category
Use the appropriate threshold based on the population (e.g., cancer, neonate, LP, etc.).
3. Consider alternatives
Antifibrinolytics, factor replacement, and procedural modifications may reduce bleeding risk without transfusion.
4. Engage in shared decision-making
Discuss risks and benefits with patients or caregivers, especially when the evidence is of low certainty.
Implications for hospitals and blood banks
Adopting these guidelines not only benefits patients but also enhances sustainability for blood services:
- Reduced waste: Platelets have a short shelf life; fewer unnecessary transfusions result in increased product availability.
- Improved safety: Lower rates of transfusion reactions.
- Financial impact: Potential cost savings in transfusion services and patient care.
Conclusion
The 2025 JAMA platelet transfusion guidelines mark a significant shift toward evidence-based, patient-centered care. Embracing restrictive strategies ensures better safety, preserves critical resources, and aligns with a global trend toward more judicious blood product use.
Reference: Metcalf RA et al. Platelet Transfusion: 2025 AABB and ICTMG International Clinical Practice Guidelines. JAMA. Published online May 29, 2025.
For more information on transfusion guidelines, check out Anesthesia Updates on the NYSORA Anesthesia Manual App.
Get access to step-by-step management algorithms, the latest research, and peer-reviewed insights—all in one place. Download the app today and experience the future of anesthesia education and decision-making.
We asked Anesthesia Manual’s MAIA (Medical AI Assistant)
When to transfuse platelets?