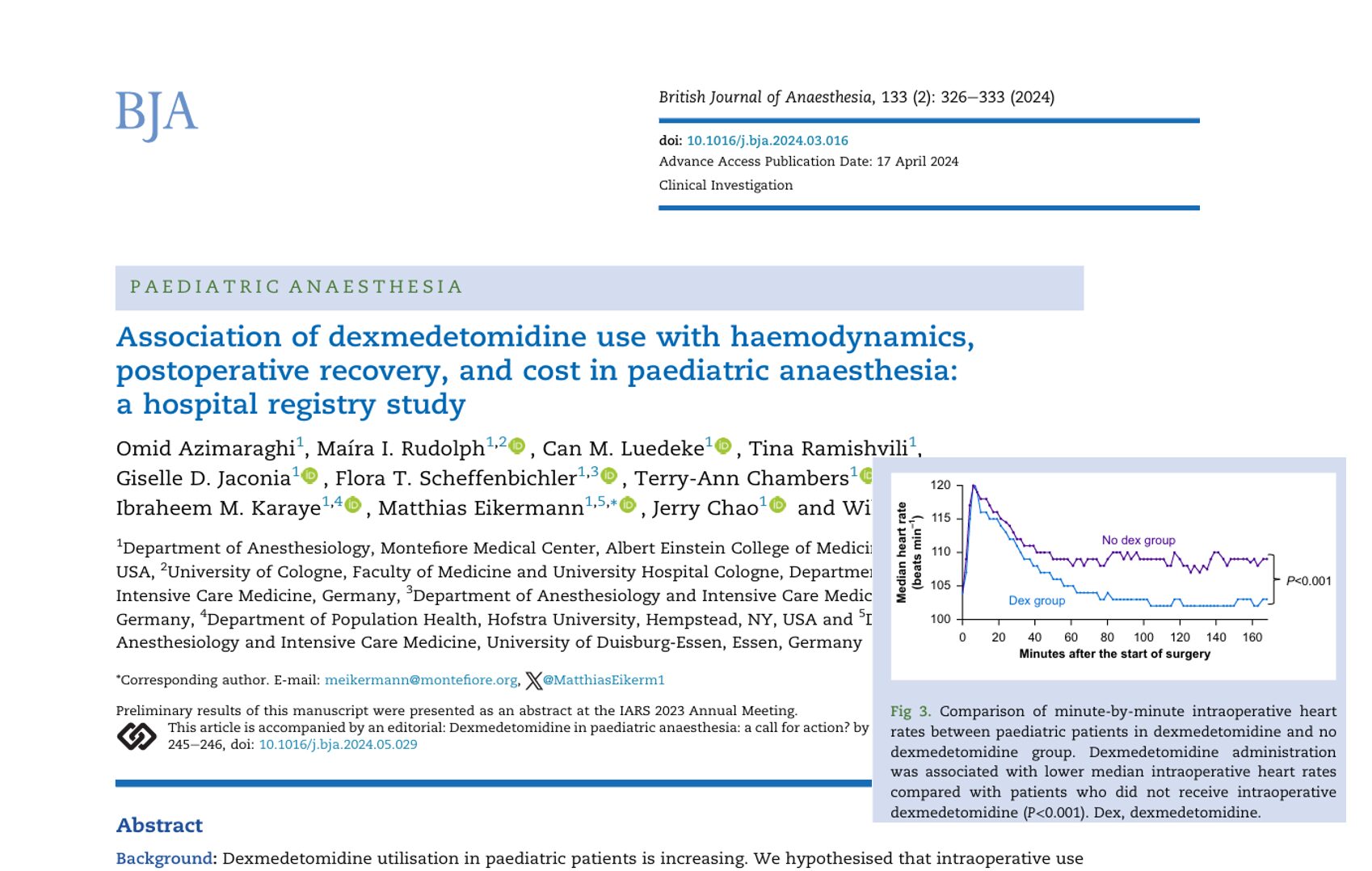
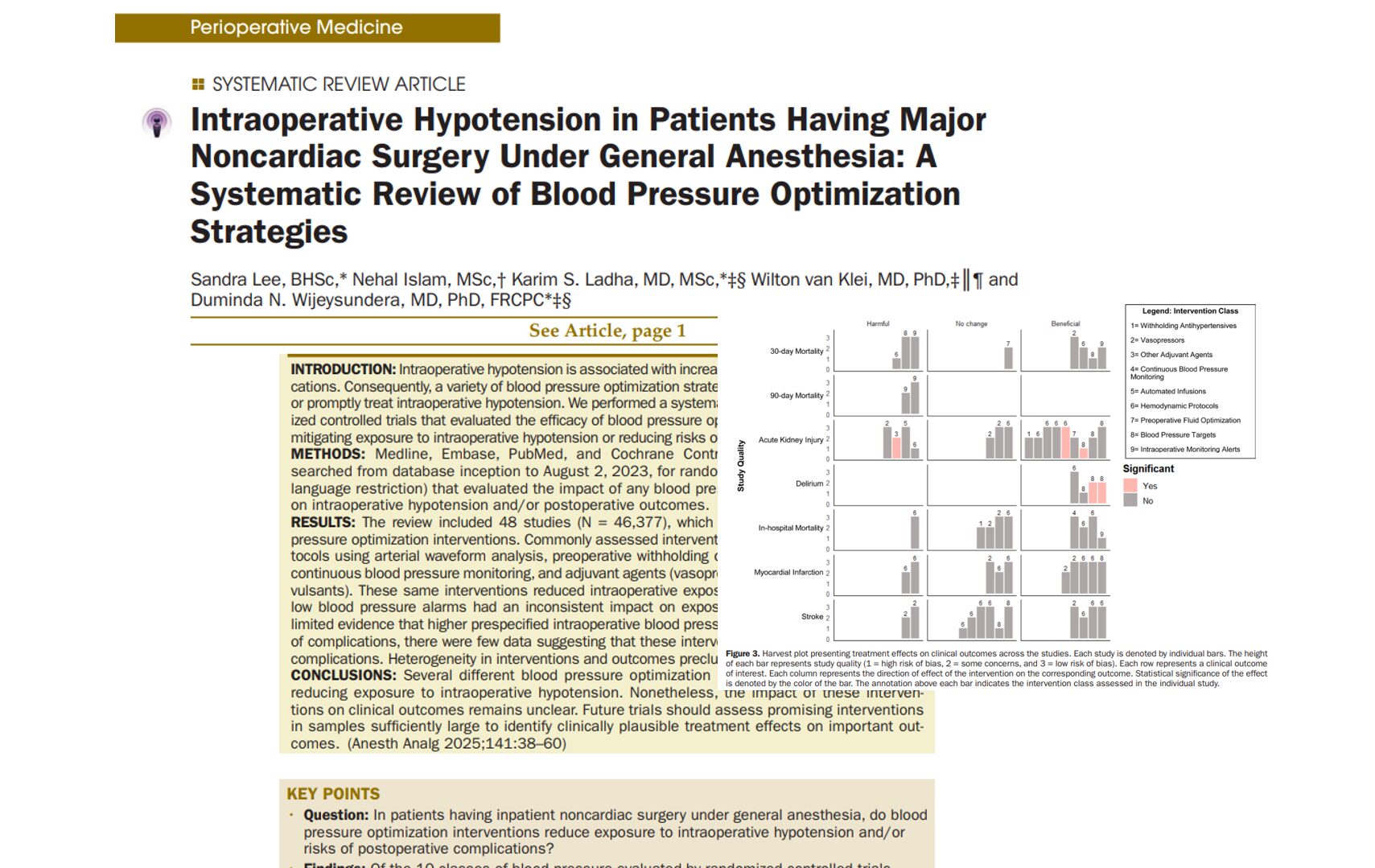
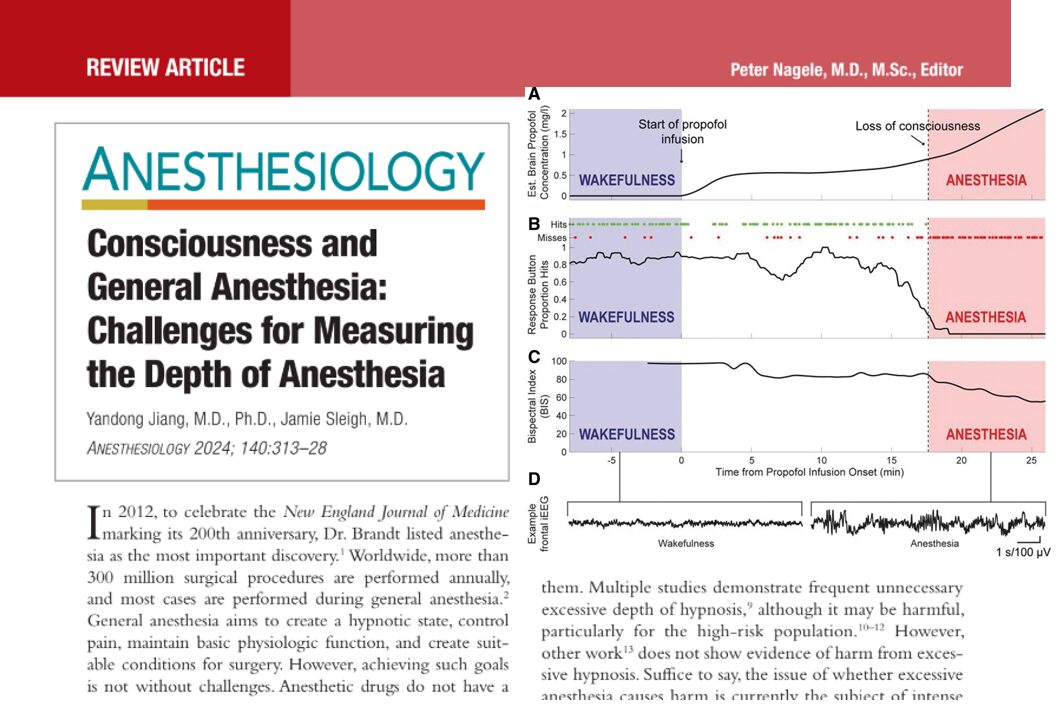
Can a modified anesthesia regimen reduce neurodevelopmental risks in young children? A 2025 randomized clinical trial by Ji et al. explores whether adding dexmedetomidine and remifentanil to sevoflurane can protect brain development during surgery in infants and toddlers.
Background: why neurodevelopment and anesthesia matter
- Preclinical animal studies show that volatile anesthetics (like sevoflurane) can cause neuronal damage and cognitive impairments when administered during critical periods of brain development.
- Human studies such as GAS, PANDA, and MASK found no substantial cognitive deficits from short-term anesthesia exposure, but concerns persist.
- Dexmedetomidine, a sedative with minimal neurotoxic effects, and remifentanil, a short-acting opioid, are proposed as safer adjuncts to reduce sevoflurane exposure.
Study overview: randomized, double-blind, controlled design
- Population: 400 Korean children under age 2 undergoing elective, non-repetitive surgery.
- Intervention groups:
- Control group: Sevoflurane alone.
- DEX-R group: Low-dose sevoflurane plus dexmedetomidine and remifentanil.
- Control group: Sevoflurane alone.
- Primary aim: Assess neurodevelopmental outcomes at 28–30 months using:
- Korean Leiter International Performance Scale (nonverbal IQ)
- Child Behavior Checklist (CBCL) (behavioral/emotional development)
- Korean Leiter International Performance Scale (nonverbal IQ)
- Follow-up planned: Full-Scale IQ assessment at 5 years (not yet reported).
Key results
- Sample size analyzed: 343 children (169 control, 176 DEX-R).
- Anesthesia duration: ~73–77 minutes, comparable between groups.
- Sevoflurane exposure:
- Significantly lower in the DEX-R group (1.8% vs. 2.6%; P < 0.001).
- Significantly lower in the DEX-R group (1.8% vs. 2.6%; P < 0.001).
- IQ and behavior outcomes:
- No significant differences in:
- Full-scale IQ (102.5 vs. 103.6; P = 0.442)
- Behavioral outcomes (CBCL total score: 46.8 vs. 47.6; P = 0.469)
- Full-scale IQ (102.5 vs. 103.6; P = 0.442)
- No increase in behavioral disorders (ADHD, internalizing/externalizing problems).
- No significant differences in:
Conclusion: cautious optimism with balanced anesthesia
- The dexmedetomidine–remifentanil combination reduced anesthetic exposure but did not improve developmental outcomes at 30 months.
- No evidence of harm was found from a single short-duration general anesthesia session.
- Future results at age 5 will be crucial to determine long-term cognitive effects.
Reference: Ji SH et al. Effects of Dexmedetomidine-Remifentanil on Neurodevelopment of Children after Inhalation Anesthesia: A Randomized Clinical Trial. Anesthesiology. 2025;143:827-834.
Download the AA App now to put trusted anesthesia guidance in your pocket.