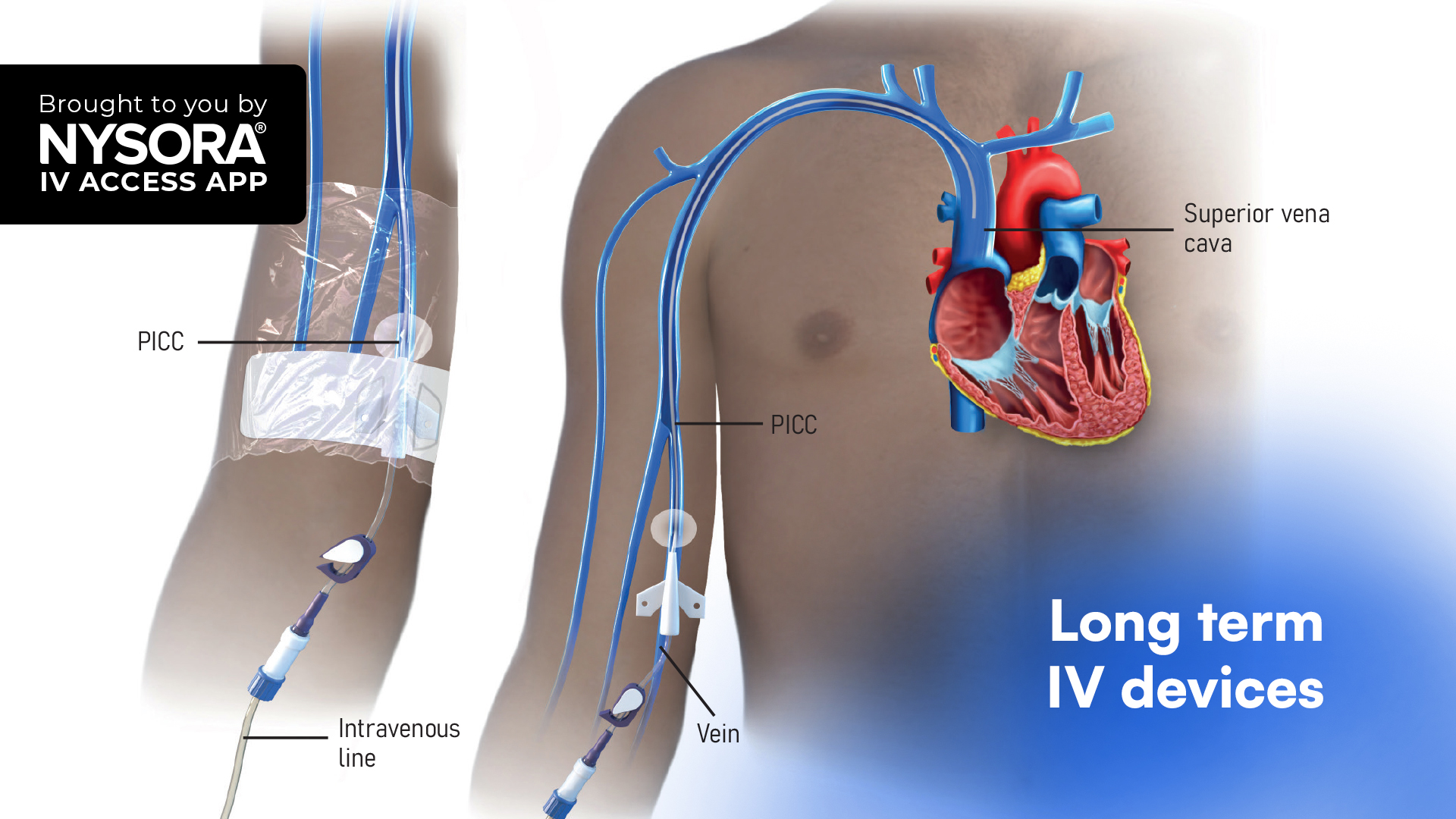
On May 13, 2025, the CDC issued a nationwide alert about bloodstream infections traced back to contaminated, non-sterile, multi-use ultrasound gel, a real-world safety breach that puts everyday procedures at risk.
Why is this important
The CDC has now confirmed over 40 cases of bloodstream infections, caused by Paraburkholderia fungorum, that were genetically linked to two contaminated lots of non-sterile ultrasound gel (MediChoice® and ClearImage®). These isolates were identified in four U.S. states and two other countries, resulting in significant patient harm. Ultrasound-guided procedures have become standard in modern medicine, especially in regional anesthesia and peripheral vascular access. However, they’re only as safe as the infection-control protocols we follow.
How the CDC traced the problem
Genomic sequencing linked the infection outbreaks to multi-use bottles of non-sterile ultrasound gel, which are still too commonly used for ultrasound-guided procedures that violate the skin integrity (e.g., nerve blocks, IV access, arterial lines), despite long-standing recommendations. The CDC’s updated guidance is now crystal clear: Use only single-use gel that is labeled “sterile” for any percutaneous procedure, including ultrasound-guided nerve blocks and peripheral IV access. This guidance aligns with existing ACEP (American College of Emergency Physicians), AIUM (American Institute of Ultrasound Medicine), and ASRA (American Society of Regional Anesthesia) guidelines, which also mandate sterile, single-use probe covers for ultrasound-guided procedures where the skin integrity is breached.
Sterile gel is not enough
While sterile gel addresses one contamination source, it doesn’t address another major vector: the ultrasound transducer itself. Probes easily transfer pathogens from one patient to the next or back to the operator’s hands. Unfortunately, based on social media posts, it appears that adherence to using probe covers remains variable, and clinicians continue to improvise with transparent dressings like Tegaderm®. These are not approved as probe covers, are advised against by ASRA guidelines, and may leave residue, which can even damage the probe over time
What we use at NYSORA: The EZCOVER® probe covers.
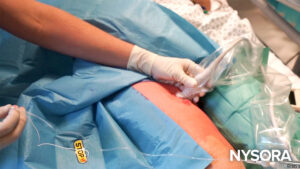
The EZCOVER® probe covers have a STOP sticker forcing the operator to do a brief 3-point safety check.
“STOP Before You BLOCK”: built-in safety timeout
The EZCOVER® probe covers we use at NYSORA centers also have a STOP sticker that physically blocks the probe face. Before scanning, the operator must peel it off, forcing a brief 3-point safety check:
- Confirm patient identity
- Confirm procedure type
- Confirm site and side
This automatic pause supports compliance with point-of-care time-out protocol, reducing the risk of wrong-site blocks and procedural errors.

The EZCOVER® probe covers have a STOP sticker forcing the operator to do a brief 3-point safety check.
The takeaway
The message from the CDC is urgent and unequivocal: Sterile technique must include both sterile gel and a sterile, single-use probe cover. Relying on workarounds, like repurposed dressings or multi-use gel bottles, is no longer an acceptable practice and never should have been.
Clinical practice recommendations
- Remove all non-sterile, multi-use ultrasound gel from procedure areas today.
- Switch to single-use, sterile gel packets for any percutaneous procedure, including routine IV placements.
- Use a validated, sterile probe cover for every invasive ultrasound-guided procedure. Yes, even for a “quick IV.”
- Avoid transparent dressings like Tegaderm® for probe protection. These are not FDA-cleared for this purpose and may compromise probe integrity and patient safety.
- Upgrade to EZCOVER®: Consider incorporating this probe cover system to streamline infection control and integrate a point-of-care safety check without disrupting workflow. (EZCOVER® | B. Braun)
For samples, pricing, or to request a live demo of the “STOP before you BLOCK” workflow, contact your local B. Braun representative.
Stay sharp, stay safe
Dr. Hadzic and the NYSORA Team
References:
- CDC Health Alert Network (HAN) No. 503 – Paraburkholderia fungorum Infections Linked to Contaminated Ultrasound Gel Products, May 13, 2025.
- ACEP, AIUM Guidelines on Infection Control for Ultrasound-Guided Procedures.
- Provenzano DA. et al. ASRA Pain Medicine consensus practice infection control guidelines for regional anesthesia and pain medicine. Regional Anesthesia & Pain Medicine; Published online: 20 January 2025.
- CIVCO Medical Solutions, Probe Care Documentation.
- EZCOVER®