Intravenous nonopioid anesthetics have an important role in modern anesthesia practice. They are widely used to facilitate a rapid induction of general anesthesia and provide sedation during monitored anesthesia care (MAC) and for patients in intensive care settings.
1. INTRAVENOUS ANESTHETICS
Intravenous nonopioid anesthetics have an important role in modern anesthesia practice. (1-7) They are widely used to facilitate a rapid induction of general anesthesia and provide sedation during monitored anesthesia care (MAC) and for patients in intensive care settings. With the introduction of propofol, intravenous techniques are increasingly being used for maintenance of anesthesia. However, similar to inhaled anesthetics, the currently available intravenous drugs do not produce only desirable effects (hypnosis, amnesia, analgesia, immobility). Therefore, the concept of “balanced anesthesia” evolved by using smaller doses of multiple drugs rather than using larger doses with one or two drugs. The fundamental drugs used with “balanced anesthesia” include inhaled anesthetics, sedative/hypnotics, opioids, and neuromuscular blocking drugs.
The intravenous anesthetics used for induction of general anesthesia are lipophilic and preferentially partition into highly perfused lipid-rich tissues (brain, spinal cord), which accounts for their rapid onset of action. Regardless of the extent and speed of their metabolism, termination of the effect of a single bolus dose is a result of redistribution of the drug into less perfused and inactive tissues such as skeletal muscles and fat. Thus, all drugs used for induction of anesthesia have a similar duration of action when administered as a single dose despite significant differences in their metabolism.
2. PROPOFOL
Propofol is the most frequently administered anesthetic drug for induction of anesthesia. (2,3,6) In addition, propofol is used during maintenance of anesthesia and is a common selection for sedation in the operating room as well as in the intensive care unit (ICU). Increasingly, propofol is also utilized for sedation and short-duration general anesthesia in locations outside the operating room such as interventional radiology suites and the emergency room.
Physicochemical Characteristics
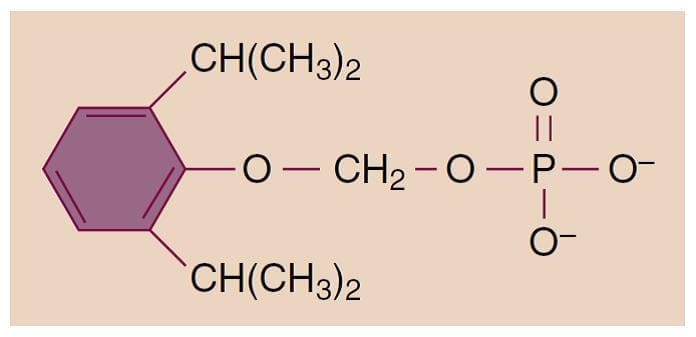
Propofol (2,6-diisopropylphenol) is an alkylphenol with hypnotic properties that is chemically distinct from other groups of intravenous anesthetics (Fig.1). It is insoluble in aqueous solutions and formulated as an emulsion containing 10% soybean oil, 2.25% glycerol, and 1.2% lecithin (the major component of the egg yolk phosphatide fraction). Sterile technique is important because the available formulations support bacterial growth. Although either ethylenediaminetetraacetic acid (0.05 mg/mL), metabisulfite (0.25 mg/mL), or benzyl alcohol (1 mg/mL) is added to the emulsions by the different manufacturers as retardants of bacterial growth, solutions should be used as soon as possible or at least within 12 hours after opening the propofol vial. The solutions appear milky white and slightly viscous, their pH is approximately 7, and the propofol concentration is 1% (10 mg/mL). In some countries, a 2% formulation is available. Allergic reactions to propofol are rare, and there is no evidence for cross-reactivity in patients with immunoglobulin E confirmed allergy to egg, soy, or peanut.(8) The addition of metabisulfite in one of the formulations is of concern for patients with reactive airways (asthma) or sulfite allergies.
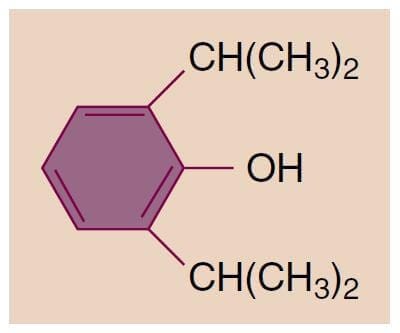
- Fig.1 Chemical structure of 2,6-diisopropylphenol (propofol).
Pharmacokinetics
Propofol is rapidly metabolized in the liver, and the resulting water-soluble compounds are presumed to be inactive and excreted through the kidneys. Plasma clearance is rapid and exceeds hepatic blood flow, thus indicating the importance of extrahepatic metabolism, which has been confirmed during the anhepatic phase of liver transplantation. The lungs probably play a major role in this extrahepatic metabolism and likely account for the elimination of up to 30% of a bolus dose of propofol. The rapid plasma clearance explains the more complete recovery from propofol with less “hangover” than observed with thiopental. As with other intravenous drugs, the effects of propofol are terminated by redistribution from the plasma and highly perfused compartments (such as brain) to poorly perfused compartments (such as skeletal muscle). A patient usually awakens within 8 to 10 minutes after an induction dose of propofol, similar to the period of decline in plasma concentration after a single bolus dose (Fig.2).(2,6)
Continuous Intravenous Infusion
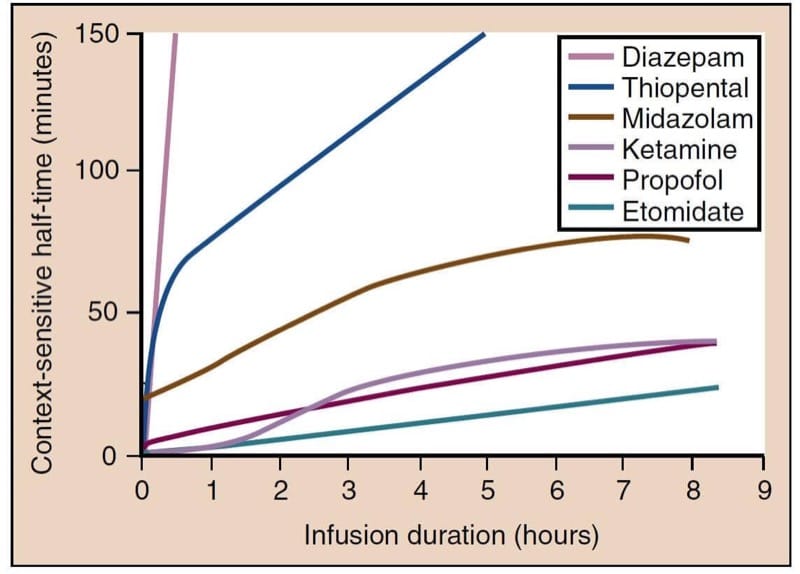
Propofol has two pharmacokinetic properties that make it ideal for use as a continuous intravenous infusion: (1) rapid metabolism and efficient clearance from plasma, and (2) slow redistribution from poorly perfused compartments back into the central compartment. One way to characterize an anesthetic infusion is the “context-sensitive half-time,” a parameter that describes the time needed for the plasma levels of a drug to drop by 50% after stopping the infusion (Fig.3).(9,10) This time depends on the duration for which an infusion has been run. The context-sensitive half-time of propofol is brief, even after a prolonged infusion, and recovery remains relatively prompt.

- Fig.3 Context-sensitive half-time for the most commonly used intravenous anesthetics. Propofol, etomidate, and ketamine have the smallest increase in context-sensitive half-times, with prolonged infusions making these drugs more suitable for use as continuous infusions. (From Vuyk J, Sitsen E, Reekers M. Intravenous anesthetics. In: Miller RD, ed. Miller’s Anesthesia. 8th ed. Philadelphia:Elsevier; 2015:821-863.)
Compartmental Model
The kinetics of propofol (and other intravenous anesthetics) after a single bolus and after continuous infusion is best described by a three-compartment model. These mathematical models have been used as the basis for the development of systems for target-controlled infusions.(11)
Pharmacodynamics
The presumed mechanism of action of propofol is through potentiation of the chloride current mediated through the γ-aminobutyric acid type A (GABAA) receptor complex.(12)
Central Nervous System
In the central nervous system (CNS), propofol primarily acts as a hypnotic and does not have any analgesic properties. It reduces the cerebral metabolic rate for oxygen (CMRO2), which leads to decreased cerebral blood flow (CBF) through preserved flow-metabolism coupling. This results in decreases in cerebral blood volume, intracranial pressure (ICP), and intraocular pressure. The magnitude of these changes is comparable to those produced by thiopental. Although propofol can produce a desired decrease in ICP, the reduced CBF combined with the reduced mean arterial pressure caused by peripheral vasodilation can critically compromise cerebral perfusion.
Propofol is probably neuroprotective during focal ischemia to the same extent as thiopental or isoflurane. When administered in large doses, propofol produces burst suppression in the electroencephalogram (EEG),(13) an end point that has been used for the administration of intravenous anesthetics for neuroprotection during neurosurgical procedures. Occasionally, excitatory effects such as twitching or spontaneous movement can be observed during induction of anesthesia with propofol. Although these effects may resemble seizure activity, propofol is actually an anticonvulsant and may be safely administered to patients with seizure disorders.(6) Although propofol may be toxic to developing neurons in animals and cell culture, no human study has demonstrated long-term cognitive or memory problems in children who have received propofol anesthesia.(14)
Cardiovascular System
Propofol produces a larger decrease in systemic arterial blood pressure than any other drug used for induction of anesthesia. Propofol causes profound vasodilation, whereas its direct myocardial depressant effect is not clear. Vasodilation occurs in both the arterial and venous circulation and leads to reductions in preload and afterload. The effect is worse with rapid injection, and is more pronounced in elderly patients, especially those with reduced intravascular fluid volume. The degree of vasodilation may also be altered in patients with diabetes, hypertension, or obesity.(15) Propofol markedly inhibits the normal baroreflex response and produces only a small or no increase in heart rate, thus further exacerbating hypotension. Profound bradycardia and asystole after the administration of propofol can occur in healthy adults despite administration of prophylactic anticholinergic drugs.(16)
Respiratory System
Propofol is a respiratory depressant and often produces apnea following a dose used to induce anesthesia. A maintenance infusion of propofol decreases minute ventilation via reductions in tidal volume and respiratory rate, with the effect on tidal volume being more pronounced. The ventilatory response to hypoxia and hypercapnia is also reduced. Propofol causes a more intense reduction in upper airway reflexes than does thiopental, which makes it well suited for instrumentation of the airway, such as placement of a laryngeal mask airway. Propofol increases collapsibility of the upper airway by inhibiting genioglossus and other muscles,(17) and airway obstruction may occur with sedative doses or during emergence from propofol anesthesia. When compared with thiopental, propofol decreases the incidence of wheezing after induction of anesthesia and tracheal intubation in healthy and asthmatic patients.(18)
Other Effects
Unlike many other anesthetics, propofol has antiemetic activity. Similar to thiopental and unlike volatile anesthetics, propofol probably does not enhance neuromuscular block from neuromuscular blocking drugs. Yet, propofol often provides excellent clinical conditions for endotracheal intubation without the use of neuromuscular blocking drugs. Unexpected arrhythmias or electro-cardiogram changes occurring during propofol anesthesia should prompt laboratory evaluation for possible metabolic acidosis, rhabdomyolysis, or hyperkalemia (propofol infusion syndrome).(19)
Clinical Uses
Pain from injection of propofol is a common complaint that can lead to patient distress or dissatisfaction. The most effective and expedient means to reduce injection pain is selecting an antecubital vein (larger, faster venous flow rate) for injection.(20) Alternatively, if a hand vein is chosen, injecting a small dose of lidocaine (20 to 40 mg intravenously [IV]) and applying proximal venous occlusion for 15 to 60 seconds before injecting propofol is similarly effective. Other helpful and expedient techniques are premedication with a small dose of opioid,(20) pretreatment with larger doses of lidocaine (40 to 100 mg IV) without venous occlusion, and coadministration of lidocaine and propofol as an admixture.(21)
Induction and Maintenance of General Anesthesia
Propofol (1 to 2.5 mg/kg IV) is the drug most commonly administered for induction of general anesthesia. The dose should be decreased in the elderly, especially those who have a reduced cardiovascular reserve, or after p remedication with benzodiazepines or opioids. Children generally require larger doses (2.5 to 3.5 mg/kg IV). Obese patients require a larger total dose compared with non-obese patients of similar height and age, but boluses for morbidly obese patients should be calculated per kilogram of lean body weight rather than total body weight to avoid excess hypotension.(22) Generally, titration of the induction dose of propofol (i.e., rather than an arbitrary bolus dose) helps prevent severe hemodynamic changes. Propofol is also often used to maintain anesthesia as part of a balanced regimen in combination with volatile anesthetics, nitrous oxide, sedative-hypnotics, or opioids; or as part of a total intravenous anesthetic (TIVA) technique, usually in combination with opioids. Some clinical trials suggest a reduction in postoperative pain scores and opioid consumption for patients receiving propofol-based TIVA as compared with volatile anesthesia, but it is difficult to draw firm conclusions owing to small trial size and significant patient heterogeneity.(23) Therapeutic plasma concentrations for maintenance of anesthesia normally range between 3 and 8 μg/mL (typically requiring a continuous infusion rate between 100 and 200 μg/kg/min) when combined with nitrous oxide or opioids.
Sedation
Propofol is a popular choice for sedation of mechanically ventilated patients in the ICU and for sedation during procedures in or outside the operating room. The required plasma concentration is 1 to 2 μg/mL, which normally requires a continuous infusion rate between 25 and 75 μg/kg/min. Because of its pronounced respiratory depressant effect and its narrow therapeutic range, propofol should be administered only by individuals trained in airway management. Spontaneous ventilation is usually preserved in children at quite rapid propofol infusion rates (200 to 250 μg/kg/min), making it a good choice for pediatric procedures such as magnetic resonance imaging scans(24).
Antiemetic
Subanesthetic bolus doses of propofol or a subanesthetic infusion can be used to treat postoperative nausea and vomiting (PONV) (10 to 20 mg IV, or 10 to 20 μg/kg/min as an infusion).25,26 Propofol TIVA, as compared with volatile anesthetics, reduces PONV27 but may not reduce unplanned admissions, postdischarge nausea and vomiting, or cost of anesthesia in the ambulatory setting.(28)
3. FOSPROPOFOL
Propofol is the most commonly used intravenous anesthetic for induction and maintenance of anesthesia and probably also during MAC and conscious sedation. As mentioned earlier, the lipid emulsion formulation of propofol has several disadvantages including pain on injection, risk of bacterial contamination, and hypertriglyceridemia with prolonged infusion. Intense research has therefore focused on finding alternative formulations or related drugs to address some of these problems. Fospropofol, a water-soluble prodrug of propofol, was developed as an alternative and in 2008 was licensed by the Food and Drug Administration (FDA) as a sedating anesthetic for use during MAC.(29)
Physicochemical Characteristics
Fospropofol, initially known under the name GPI 15715, is a water-soluble phosphate ester prodrug of propofol and is chemically described as 2,6-diisopropylphenoxymethyl phosphate disodium salt (Fig.4). It is metabolized by alkaline phosphatase in a reaction producing propofol, phosphate, and formaldehyde. Aldehyde dehydrogenase in the liver and in erythrocytes rapidly metabolizes form-aldehyde to produce formate, which is further metabolized by 10-formyltetrahydrofolate dehydrogenase.(29) The available fospropofol formulation is a sterile, aqueous, colorless, and clear solution that is supplied in a single-dose vial at a concentration of 35 mg/mL under the trade name Lusedra.

- Fig.4 Structure of fospropofol.
Pharmacokinetics
Because fospropofol is a prodrug that requires metabolism to form the active compound propofol, the pharmacokinetics are complex. Onset and recovery are prolonged compared to propofol. Multicompartment models with two compartments for fospropofol and three for propofol have been used to describe the kinetics. In theory, a fospropofol bolus should yield lower peak plasma levels and a delayed time-to-peak compared with propofol. However, previous studies on pharmacokinetics/pharmacodynamics and tolerability of fospropofol were based on an inaccurate analytical assay, and, therefore, reliable data on the kinetics are lacking.(29) Six previously published studies were retracted in 2010.(30)
Pharmacodynamics
The effect profile is similar to that of propofol. Given the kinetics previously described, fospropofol in theory should cause less hypotension(31) and less respiratory depression than propofol. However, these benefits have not yet been demonstrated in human studies.
Clinical Uses
Fospropofol is currently approved for sedation during MAC. Given as a bolus (6.5 mg/kg IV), onset of sedation is slower (4 to 8 min) and duration of action is longer (5 to 18 min) than an equivalent dose of propofol.(32) Sedation can be maintained by redosing with 25% of the initial bolus as needed. These dosing guidelines are recommended only for patients weighing 60 to 90 kg, and dose reduction by 25% is recommended for elderly patients (age > 65) or those with American Society of Anesthesiologists (ASA) physical classification III or IV. Common side effects of fospropofol include a perineal burning sensation and pruritus.
Small trials have demonstrated safety and efficacy of fospropofol for sedation during colonoscopy, bronchoscopy, and minor surgical procedures.(32) Single studies have investigated fospropofol for TIVA during coronary artery bypass grafting(33) and for ICU sedation of mechanically ventilated patients.(34) Similar to propofol, airway compromise remains a major concern. Hence, fospropofol should be administered only by personnel trained in airway management.
4. BARBITURATES
Before the introduction of propofol, the intravenous anesthetics most commonly used for induction of anesthesia were barbiturates (thiopental, methohexital).(2,4)
Physicochemical Characteristics
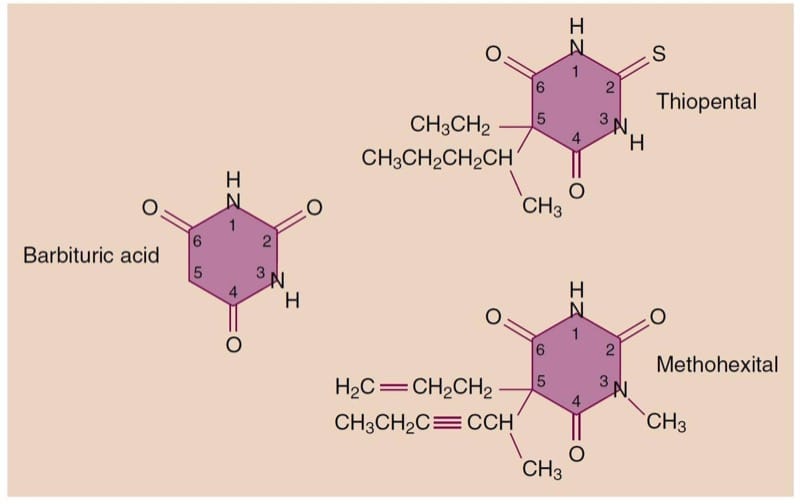
Barbiturates are derived from barbituric acid (lacks hypnotic properties) through substitutions at the N1, C2, and C5 positions (Fig.5). Based on their substitution at position 2, barbiturates can be grouped into thiobarbiturates, substituted with a sulfur (thiopental), or oxybarbiturates, substituted with an oxygen (methohexital). Hypnotic, sedative, and anticonvulsant effects, as well as lipid solubility and onset time, are determined by the type and position of substitution.

- Fig.5 Structure of barbituric acid and its derivatives.
Thiopental and methohexital are formulated as sodium salts mixed with anhydrous sodium carbonate. After reconstitution with water or normal saline, the solutions (2.5% thiopental and 1% methohexital) are alkaline with a pH higher than 10. Although this property prevents bacterial growth and helps increase the shelf life of the solution after reconstitution, it will lead to precipitation when mixed with acidic drug preparations such as neuromuscular blocking drugs. These precipitates can irreversibly block intravenous delivery lines if mixing occurs during administration. Furthermore, accidental injection into an artery or infiltration into paravenous tissue will cause extreme pain and may lead to severe tissue injury.
Several barbiturates, including thiopental and methohexital, have optical isomers with different potencies. However, the available formulations are racemic mixtures, and their potencies reflect the summation of the potencies of the individual isomers.
Pharmacokinetics
Barbiturates, except for phenobarbital, undergo hepatic metabolism most importantly by oxidation, but also by N-dealkylation, desulfuration, and destruction of the barbituric acid ring structure. The resulting metabolites are inactive and excreted through urine and, after conjugation, through bile. In contrast, phenobarbital is mainly eliminated unchanged via renal excretion. Chronic administration of barbiturates or the administration of other drugs that induce oxidative microsomal enzymes (enzyme induction) enhances barbiturate metabolism. Through stimulation of aminolevulinic acid synthetase, the production of porphyrins is increased. Therefore, barbiturates should not be administered to patients with acute intermittent porphyria.
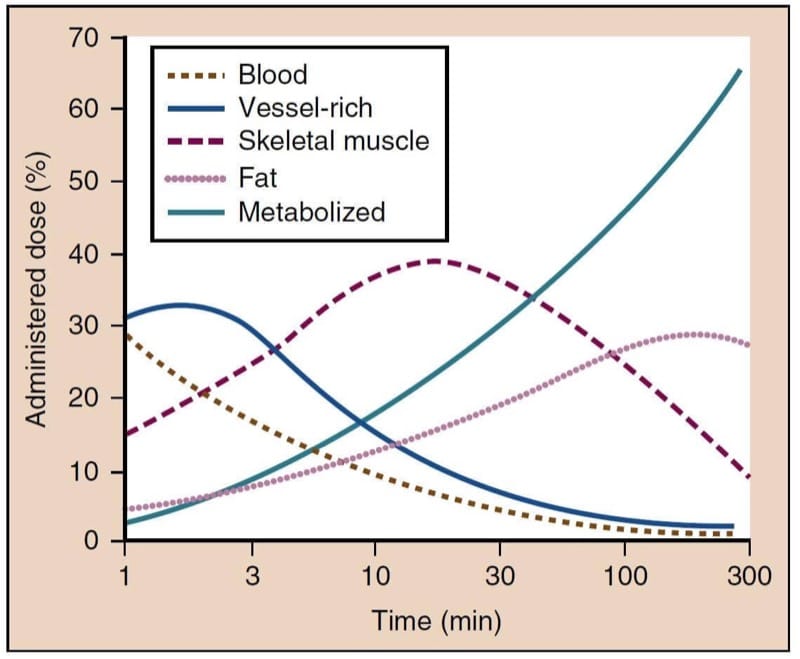
Methohexital is cleared more rapidly by the liver than thiopental and thus has a shorter elimination half-time. This accounts for faster and more complete recovery after methohexital administration. Although thiopental is metabolized slowly and has a long elimination half-time, recovery after a single bolus administration is comparable to methohexital and propofol because it depends on redistribution to inactive tissue sites rather than metabolism (Fig.6).(35) However, even single-bolus induction doses of thiopental can, in some cases, lead to psychomotor impairment that lasts up to several hours. If administered as repeated boluses or as a continuous infusion, especially when using larger doses to produce burst suppression on the EEG, recovery from the effects of thiopental will be markedly prolonged because of the long context-sensitive half-time (see Fig.3).

- Fig.6 After rapid intravenous injection of thiopental, the percentage of the administered dose remaining in blood (brown line) rapidly decreases as the drug moves from blood to highly perfused vessel-rich tissues (blue line), especially the brain. Subsequently, thiopental is redistributed to skeletal muscles (red line) and, to a lesser extent, to fat (pink line). Ultimately, most of the administered dose of thiopental undergoes metabolism (green line). (From Saidman LJ. Uptake, distribution, and elimination of barbiturates. In Eger EI, ed. Anesthetic Uptake and Action. Baltimore: Williams & Wilkins; 1974:264-284, used with permission.)
Pharmacodynamics
The mechanism of action for the effect of barbiturates in the CNS presumably involves both enhancement of inhibitory neurotransmission and inhibition of excitatory transmission. Although the effects on inhibitory transmission probably result from activation of the GABAA receptor complex, the effects on excitatory transmission are less well understood. The pentameric GABAA receptor can be composed of different combinations of subunits (see Benzodiazepines section), and the receptors may be found both at synapses and at extrasynaptic sites.(36) This diversity of location and composition may explain why several different types of GABAA-mediated currents are found in the CNS (fast versus slow, phasic versus tonic). Different intravenous anesthetics may have some selectivity for certain types of GABAA receptors.(37)
Central Nervous System
Barbiturates produce dose-dependent CNS depression ranging from sedation to general anesthesia when administered in induction doses.(4) They do not have analgesic properties and may even reduce the pain threshold, and thus could be classified as anti-analgesics. Barbiturates are potent cerebral vasoconstrictors and produce predictable decreases in CBF, cerebral blood volume, and ICP. As a result, they decrease CMRO2 in a dose-dependent manner up to the point where the EEG becomes a flat line. The ability of barbiturates to decrease ICP and CMRO2 makes these drugs useful in the management of patients with space-occupying intracranial lesions(38). Furthermore, they may provide neuroprotection from focal cerebral ischemia (stroke, surgical retraction, temporary clips during aneurysm surgery) but probably not from global cerebral ischemia (cardiac arrest). Yet, the use of barbiturates to lower ICP after traumatic brain injury cannot be justified, as the associated hypotension may compromise cerebral perfusion pressure and worsen outcomes.(39) Most barbiturates decrease electrical activity on the EEG, and intravenous infusions may be useful in the critical care setting to treat refractory status epilepticus (typically third-line therapy after midazolam and propofol have failed).(40) An exception to this rule is methohexital, which activates epileptic foci, thus facilitating their identification during surgery targeted to ablate these sites. For the same reason, methohexital is also a popular choice for anesthesia to facilitate electroconvulsive therapy.
Cardiovascular System
Administration of barbiturates for induction of anesthesia typically produces modest decreases in systemic arterial blood pressure that are smaller than those produced by propofol. This decrease in systemic arterial blood pressure is principally due to peripheral vasodilation and reflects barbiturate-induced depression of the medullary vasomotor center and decreased sympathetic nervous system outflow from the CNS. Although barbiturates blunt the baroreceptor reflex, compensatory increases in heart rate limit the magnitude and duration of hypotension. Moreover, dilation of peripheral capacitance vessels leads to pooling of blood and decreased venous return, thus resulting in reduced cardiac output and systemic arterial blood pressure. Indeed, exaggerated decreases in blood pressure are likely to follow the administration of barbiturates to patients with hypovolemia, cardiac tamponade, cardiomyopathy, coronary artery disease, or cardiac valvular disease because these groups are less able to compensate for the effects of peripheral vasodilation. Hemodynamic effects are also more pronounced with larger doses and rapid injection. The negative inotropic effects of barbiturates, which are readily demonstrated in isolated heart preparations, are usually masked in vivo by baroreceptor reflex-mediated responses.
Respiratory System
Barbiturates are respiratory depressants and lead to decreased minute ventilation via smaller tidal volumes and respiratory rates. Anesthetic induction doses of thiopental and methohexital typically induce transient apnea, which is more pronounced in the presence of other respiratory depressants. Barbiturates also decrease the ventilatory responses to hypercapnia and hypoxia. Resumption of spontaneous breathing after an anesthetic induction dose of a barbiturate is characterized by a slow breathing rate and decreased tidal volume. Suppression of laryngeal reflexes and cough reflexes is not as profound as after propofol administration, which makes barbiturates an inferior choice for airway instrumentation in the absence of neuromuscular blocking drugs. Furthermore, stimulation of the upper airway or trachea (secretions, laryngeal mask airway, direct laryngoscopy, tracheal intubation) during inadequate depression of airway reflexes may result in laryngospasm or bronchospasm. This phenomenon is not unique to barbiturates but is true in general when the dose of anesthetic is inadequate to suppress the airway reflexes.
Side Effects
Accidental intra-arterial injection of barbiturates results in excruciating pain and intense vasoconstriction, often leading to severe tissue injury involving gangrene.(4) Aggressive therapy is directed at reversing the vasoconstriction to maintain perfusion and reduce the drug concentration by dilution. One approach to treatment is block of the sympathetic nervous system in the involved extremity (stellate ganglion block). Barbiturate crystal formation probably results in the occlusion of distal, small-diameter arterioles. Crystal formation in veins is less hazardous because of the ever-increasing diameter of veins. Accidental subcutaneous injection (extravasation) of barbiturates results in local tissue irritation, thus emphasizing the importance of using dilute concentrations (2.5% thiopental, 1% methohexital). If extravasation occurs, some recommend local injection of the tissues with 0.5% lidocaine (5 to 10 mL) in an attempt to dilute the bar-biturate concentration.
Life-threatening allergic reactions to barbiturates are rare, with an estimated occurrence of 1 in 30,000 patients. However, barbiturate-induced histamine release is occasionally seen.
Clinical Uses
The principal clinical uses of barbiturates are rapid intravenous induction of anesthesia, treatment of increased ICP, or to provide neuroprotection from focal cerebral ischemia.(4) A continuous intravenous infusion of a bar-biturate such as thiopental is rarely used to maintain anesthesia because of its long context-sensitive half-time and prolonged recovery period (see Fig.3).(10) Prolonged infusions to achieve “barbiturate coma” for neuroprotection may cause immune suppression, hypo- and hyperkalemia, and hypothermia.(38)
Induction of Anesthesia
Administration of thiopental (3 to 5 mg/kg IV) or methohexital (1 to 1.5 mg/kg IV) produces unconsciousness in less than 30 seconds. Patients may experience a garlic or onion taste during induction of anesthesia. When used as an anesthetic for electroconvulsive therapy, methohexital may allow for longer seizure duration when compared with propofol.(41) With the introduction of sugammadex as a reversal drug for rocuronium neuromuscular block, methohexital plus rocuronium may become a more common anesthetic cocktail for electroconvulsive therapy.
The utility of barbiturates can be illustrated by describing several different techniques for anesthesia induction. After administering a barbiturate, it is common to give succinylcholine or a nondepolarizing neuromuscular blocking drug to produce skeletal muscle relaxation and facilitate tracheal intubation. In some situations, the anesthesia provider may choose a “rapid-sequence induction” of anesthesia, typically when a patient is at increased risk for aspiration of gastric contents. The classic drug regimen for rapid-sequence induction is a barbiturate, usually thiopental, followed by succinylcholine in rapid succession. Important advantages of this technique are avoidance of bag-mask ventilation and early tracheal intubation with a cuffed tube. Although thiopental was the traditional drug used for rapid-sequence induction, propofol is currently the more frequent choice.
For patients who are not at increased risk of aspirating gastric contents, intravenous barbiturates may be used to initiate a gradual induction. Small doses of thiopental (0.5 to 1.0 mg/kg IV) may improve patient acceptance of a face mask and negate any unpleasant memory of pungent volatile anesthetics. The induction can then be completed by delivering an inhaled agent such as sevoflurane. This type of slow induction helps titrate the anesthetic effect more carefully and avoids exaggerated hemodynamic responses. A gradual induction can also be accomplished by careful titration of intravenous anesthetics alone, but propofol is probably a more logical choice for this application because it has a shorter context-sensitive half-time (see Fig.3).(10) Rectal administration of a barbiturate such as methohexital (20 to 30 mg/kg) may be used to facilitate induction of anesthesia in mentally challenged and uncooperative pediatric patients.

- Fig.3 Context-sensitive half-time for the most commonly used intravenous anesthetics. Propofol, etomidate, and ketamine have the smallest increase in context-sensitive half-times, with prolonged infusions making these drugs more suitable for use as continuous infusions. (From Vuyk J, Sitsen E, Reekers M. Intravenous anesthetics. In: Miller RD, ed. Miller’s Anesthesia. 8th ed. Philadelphia:Elsevier; 2015:821-863.)
Neuroprotection
When used for neuroprotection, barbiturates have traditionally been titrated to achieve an isoelectric EEG, an end point that indicates maximal reduction of CMRO2. More recent data demonstrating equal protection after smaller doses have challenged this practice.(4) One risk of using high-dose barbiturate therapy to decrease ICP or to provide protection against focal cerebral i schemia (cardiopulmonary bypass, carotid endarterectomy, thoracic aneurysm resection) is the associated hypotension, which can critically reduce cerebral perfusion pressure and may require the administration of vasoconstrictors to maintain systemic arterial blood pressure. In a large registry of patients undergoing repair of acute type A aortic dissection, there was no benefit from barbiturates for preventing permanent neurologic dysfunction,(42) and these drugs are unlikely to be of benefit during cardiac surgery.
5. BENZODIAZEPINES
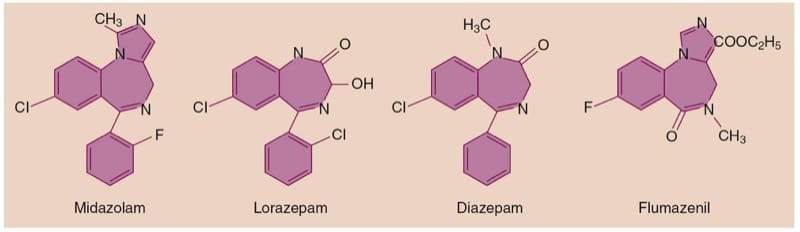
Benzodiazepines commonly used in the perioperative period include diazepam, midazolam, and lorazepam, as well as the selective benzodiazepine antagonist flumazenil.(1,5)
Benzodiazepines are unique among intravenous anesthetics in that their action can readily be terminated by administration of their selective antagonist flumazenil. Their most desired effects are anxiolysis and anterograde amnesia, which are extremely useful for premedication.
Physicochemical Characteristics
The chemical structure of the benzodiazepines contains a benzene ring fused to a seven-member diazepine ring, hence their name (Fig.7). The three benzodiazepines commonly used in the perioperative setting are all highly lipophilic, with midazolam having the highest lipid solubility. All three drugs are highly protein bound, mainly to serum albumin. Although they are used as parenteral formulations, all three drugs are absorbed after oral administration. Other possible routes of administration include intramuscular, intranasal, and sublingual. Exposure of the acidic midazolam preparation to the physiologic pH of blood causes a change in the ring structure that renders the drug more lipid soluble, thus speeding its passage across the blood-brain barrier and its onset of action.

- Fig.7 Chemical structure of the most commonly used benzodiazepines and their antagonist flumazenil.
Pharmacokinetics
The highly lipidsoluble benzodiazepines rapidly enter the CNS, which accounts for their rapid onset of action, followed by redistribution to inactive tissue sites and subsequent termination of the drug effect (see Table 1). Metabolism of benzodiazepines occurs in the liver through microsomal oxidation (N-dealkylation and aliphatic hydroxylation) and glucuronide conjugation. Microsomal oxidation, the primary pathway for metabolism of midazolam and diazepam, is more susceptible to external factors such as age, diseases (hepatic cirrhosis), and the administration of other drugs that modulate the efficiency of the enzyme systems. Lorazepam is one of few benzodiazepines that does not undergo oxidative metabolism and is excreted after a single-step conjugation to glucuronic acid.
Diazepam undergoes hepatic metabolism to active metabolites (desmethyldiazepam and oxazepam) that may contribute to the prolonged effects of this drug. By contrast,midazolam is selectively metabolized by hepatic cytochrome P450 3A4 to a single dominant metabolite, 1-hydroxymid-azolam. Whereas 1-hydroxymidazolam has sedative effects similar to the parent compound, it undergoes rapid glucuronidation and clearance.(2) This metabolite does not cause significant sedation in patients with normal hepatic and renal function unless midazolam is given as a prolonged infusion. Furthermore, the short duration of action of a single dose of midazolam is due to its lipid solubility and rapid redistribution as prevously described. Despite its prompt passage into the brain, midazolam is considered to have a slower effect-site equilibration time than propofol and thiopental. In this regard, intravenous doses of midazolam should be sufficiently spaced to permit the peak clinical effect to be recognized before a repeat dose is considered.
The elimination half-time of diazepam greatly exceeds that of midazolam, thus explaining why the CNS effects of diazepam are prolonged, especially in elderly patients. Of the three commonly used intravenous benzodiazepines, midazolam has the shortest context-sensitive half-time, making it the most suitable for continuous infusion (see Fig.6).(10)

- Fig.6 After rapid intravenous injection of thiopental, the percentage of the administered dose remaining in blood (brown line) rapidly decreases as the drug moves from blood to highly perfused vessel-rich tissues (blue line), especially the brain. Subsequently, thiopental is redistributed to skeletal muscles (red line) and, to a lesser extent, to fat (pink line). Ultimately, most of the administered dose of thiopental undergoes metabolism (green line). (From Saidman LJ. Uptake, distribution, and elimination of barbiturates. In Eger EI, ed. Anesthetic Uptake and Action. Baltimore: Williams & Wilkins; 1974:264-284, used with permission.)
Recently, a new ultrashort-acting benzodiazepine named remimazolam (CNS-7056) has entered clinical trials. Remimazolam contains a carboxylic ester group that is rapidly hydrolyzed by tissue esterases, analogous to remifentanil.(32) As compared to midazolam, remimazolam has a smaller volume of distribution, faster clearance, and clearance independent of body weight.(43) The metabolite of remimazolam has extremely low GABAA affinity (>400 times lower than CNS-7056) and is unlikely to yield clinically relevant sedation. The kinetic properties of remimazolam make it a promising intravenous anes-thetic drug that may yield less prolonged sedation compared with midazolam, particularly in patients with liver disease or those taking cytochrome P450–inhibiting drugs.
Pharmacodynamics
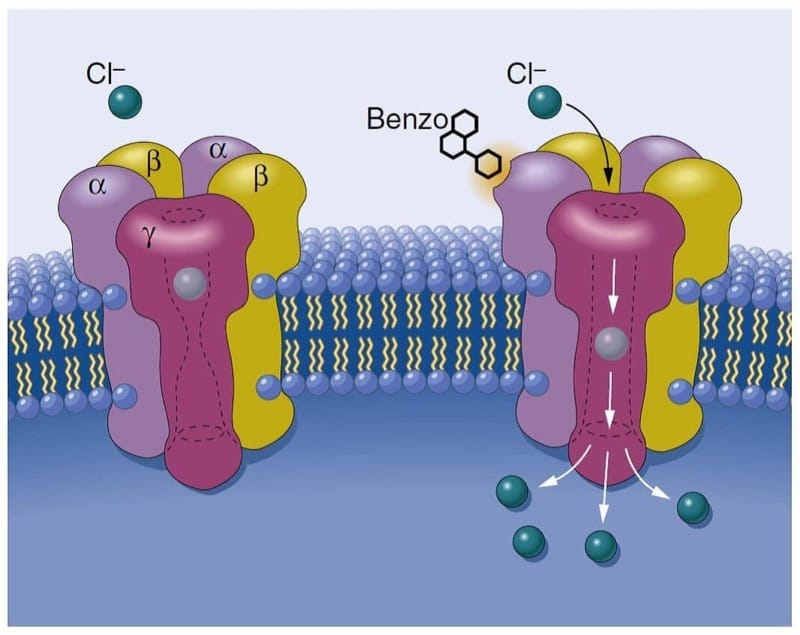
Benzodiazepines work through activation of the GABAA receptor complex and enhancement of GABA-mediated chloride currents, thereby leading to hyperpolarization of neurons and reduced excitability (Fig.8).(44) There are specific binding sites for benzodiazepines on GABAA receptors, thus explaining why they were initially termed “benzodiazepine receptors.” Consistent with its greater potency, midazolam has an affinity for GABAA receptors that is approximately twice that of diazepam.

- Fig.8 Schematic depiction of the γ-aminobutyric acid (GABA) type A receptor forming a chloride ion channel. Benzodiazepines (Benzo) attach selectively at the interface of α- and γ- subunits and are presumed to facilitate the action of the inhibitory neurotransmitter GABA. (From Mohler H, Richards JG. The benzodiazepine receptor:a pharmacological control element of brain function. Eur J Anesthesiol Suppl. 1988;2:15-24, used with permission.)
GABAA receptors that are responsive to benzodiazepines occur almost exclusively on postsynaptic nerve endings in the CNS, with the greatest density found in the cerebral cortex. The anatomic distribution of GABAA receptors (restricted to the CNS) is consistent with the minimal effects of benzodiazepines outside the CNS. Indeed, the magnitude of depression of ventilation and the development of hypotension after the administration of benzodiazepines are lower than that observed when barbiturates are used for induction of anesthesia.
Spectrum of Effects
The wide spectrum of effects of benzodiazepines is similar for all drugs in this class, although potencies for individual effects may vary between drugs.(5) The most important effects of benzodiazepines are their sedative-hypnotic action and their amnestic properties (anterograde, but not retrograde, amnesia).(45) In addition, benzodiazepines function as anticonvulsants and are used to treat seizures. These effects are mediated through the α-subunits of the GABA receptor, whereas anxiolysis and muscle relaxation are mediated through the γ-subunits. The site of action for muscle relaxation is in the spinal cord, and this effect requires much larger doses.
Safety Profile
Benzodiazepines have a very favorable side effect profile. When administered alone, these drugs cause only minimal depression of ventilation and the cardiovascular system, making them relatively safe even in larger doses. Furthermore, the CNS effects can be reversed by the selective benzodiazepine antagonist, flumazenil, thus adding to the safety margin.
Central Nervous System
Like propofol and barbiturates, benzodiazepines decrease CMRO2 and CBF, but to a lesser extent. In contrast to propofol and thiopental, midazolam is unable to produce an isoelectric EEG, thus emphasizing that there is a ceiling effect on reduction of CMRO2 by benzodiazepines. Patients with decreased intracranial compliance demonstrate little or no change in ICP after the administration of midazolam. Benzodiazepines have not been shown to possess neuroprotective properties. They are potent anticonvulsants for the treatment of status epilepticus, alcohol withdrawal, and local anesthetic-induced seizures.
Cardiovascular System
When used for induction of anesthesia, midazolam produces a larger decrease in arterial blood pressure than diazepam. These changes are most likely due to peripheral vasodilation inasmuch as cardiac output is not changed. Midazolam-induced hypotension is more likely in hypovolemic patients.
Respiratory System
Benzodiazepines produce minimal depression of ventilation, although transient apnea may follow rapid intravenous administration of midazolam for induction of anesthesia, especially in the presence of opioid premedication. Benzodiazepines decrease the ventilatory response to carbon dioxide, but this effect is not usually significant if they are administered alone. More severe respiratory depression can occur when benzodiazepines are administered together with opioids.(1,46)
Side Effects
Allergic reactions to benzodiazepines are extremely rare to nonexistent. Pain during intravenous injection and subsequent thrombophlebitis are most pronounced with diazepam and reflect the poor water solubility of this benzodiazepine. It is the organic solvent, propylene glycol, required to dissolve diazepam that is most likely responsible for pain during intramuscular or intravenous administration, as well as for the unpredictable absorption after intramuscular injection. Midazolam is more water soluble (but only at low pH), thus obviating the need for an organic solvent and decreasing the likelihood of exaggerated pain or erratic absorption after intramuscular injection or pain during intravenous administration.
Clinical Uses
Benzodiazepines are used for (1) preoperative medication, (2) intravenous sedation, (3) intravenous induction of anesthesia, and (4) suppression of seizure activity. The slow onset and prolonged duration of action of lorazepam limit its usefulness for preoperative medication or induction of anesthesia, especially when rapid and sustained awakening at the end of surgery is desirable. Flumazenil (8 to 15 μg/kg IV) may be useful for treating patients experiencing delayed awakening, but its duration of action is brief (about 20 minutes) and resedation may occur.
Preoperative Medication and Sedation
The amnestic, anxiolytic, and sedative effects of benzodiazepines are the basis for the use of these drugs for preoperative medication. Midazolam (1 to 2 mg IV) is effective for premedication, sedation during regional anesthesia, and brief therapeutic procedures.(5,47) Addition of midazolam to propofol sedation for colonoscopy may improve operating conditions without slowing recovery time or worsening cognitive impairment at discharge.(48) When compared with diazepam, midazolam produces a more rapid onset, with more intense amnesia and less postoperative sedation. Many patients who receive preoperative midazolam do not recall the operating room, and some have no memory of the preoperative holding area.(49) Both anesthesia providers and surgeons should be aware of this fact when providing information to patients and families prior to surgery. Although awareness during anesthesia is rare, benzodiazepines seem to be superior to ketamine and barbiturates for prevention of recall.(50) Midazolam is commonly used for oral premedication of children. For example, 0.5 mg/kg administered orally 30 minutes before induction of anesthesia provides reliable sedation and anxiolysis in children without producing delayed awakening.(51) Midazolam also lowers the incidence of PONV.(52) Despite these possible benefits, the routine use of premedication with benzodiazepines for elective surgery may not improve patient experience.(53)
The synergistic effects between benzodiazepines and other drugs, especially opioids and propofol, facilitate better sedation and analgesia. However, the combination of these drugs also exacerbates respiratory depression and may lead to airway obstruction or apnea.(46) These drugs may also increase the risk for aspiration of gastric contents by impairing pharyngeal function and the coordination between breathing and swallowing.(54) Benzodiazepine effects, as well as synergy with other respiratory depressants, are more pronounced in the elderly so smaller doses and careful titration may be necessary. Caution is advised when using benzodiazepines for sedation of critically ill, mechanically ventilated patients, as this drug class has been linked to longer duration of ICU stay and increased delirium compared with alternative regimens (propofol or dexmedetomidine).(55,56)
Induction of Anesthesia
Although rarely used for this purpose, general anesthesia can be induced by the administration of midazolam (0.1 to 0.3 mg/kg IV). The onset of unconsciousness, however, is slower than after the administration of thiopental, propofol, or etomidate. Onset of unconsciousness is facilitated when a small dose of opioid (fentanyl, 50 to 100 μg IV) is injected 1 to 3 minutes before midazolam is administered. Despite the possible production of lesser circulatory effects, it is unlikely that the use of midazolam or diazepam for induction of anesthesia offers any advantages over barbiturates or propofol. Delayed awakening is a potential disadvantage after an induction dose of a benzodiazepine.
Suppression of Seizure Activity
The efficacy of benzodiazepines as anticonvulsants owes to their ability to enhance the inhibitory effects of GABA, particularly in the limbic system. Indeed, d iazepam (0.1 mg/kg IV) is often effective in abolishing seizure activity produced by local anesthetics or a lcohol withdrawal. Lorazepam (0.1 mg/kg IV) is the intravenous benzodiazepine of choice for status epilepticus. Diazepam (0.2 mg/kg IV) may also be used. For prehospital treatment of status epilepticus, intramuscular (IM) administration of midazolam (10 mg IM for patients > 40 kg; 5 mg IM for patients 13 to 40 kg) is effective, can be performed more rapidly than intravenous therapy, and may decrease the need for hospitalization. (57)
6. KETAMINE
Ketamine, a phencyclidine derivative that received FDA approval for clinical use in 1970, is different from most other intravenous anesthetics in that it produces significant analgesia.(2,3) The characteristic cataleptic state observed after an induction dose of ketamine is known as “dissociative anesthesia,” wherein the patient’s eyes remain open with a slow nystagmic gaze (cataleptic state).
Physicochemical Characteristics
Ketamine is a partially water-soluble and highly lipid-soluble derivative of phencyclidine (Fig.9). It is between 5 and 10 times more lipid soluble than thiopental. Of the two stereoisomers the S(+) form is more potent than the R(−) isomer. Only the racemic mixture of ketamine (10, 50, 100 mg/mL) is available in the United States.

- Fig.9 Chemical structure of ketamine.
After its initial introduction, ketamine was for a time established as a safe anesthetic. However, the popularity of ketamine has since declined, and its unpleasant psychomimetic side effects have limited its use in anesthesia. Still, the unique features of ketamine (potent analgesia with minimal respiratory depression) make it a very valuable alternative in certain settings. More recently it has become popular as an adjunct administered at subanalgesic doses to limit or reverse opioid tolerance and in the treatment of major depression.(58,59)
Pharmacokinetics
The high lipid solubility of ketamine ensures a rapid onset of effect. Like other intravenous induction drugs, the effect of a single bolus injection is terminated by redistribution to inactive tissue sites. Metabolism occurs primarily in the liver and involves N-demethylation by the cytochrome P-450 system. Norketamine, the primary active metabolite, is less potent (one third to one fifth the potency of ketamine) and is subsequently hydroxylated and conjugated into water-soluble inactive metabolites that are excreted in urine. Ketamine is the only intravenous anesthetic that has low protein binding (12%).
Pharmacodynamics
The mechanism of action of ketamine is complex, but the major anesthetic effect is produced through inhibition of the N-methyl-D-aspartate (NMDA) receptor complex.(60) If ketamine is administered as the sole anesthetic, amnesia is not as complete as with the administration of a benzodiazepine. Reflexes are often preserved, but it cannot be assumed that patients are able to protect their upper airway. The eyes remain open, and the pupils are moderately dilated with a nystagmic gaze. Frequently, lacrimation and salivation are increased, and premedication with an anticholinergic drug may be indicated to limit this effect.
Emergence Reactions
The unpleasant emergence reactions after ketamine administration are the main factor limiting its use. Such reactions may include vivid colorful dreams, hallucinations, out-of-body experiences, and increased and distorted visual, tactile, and auditory sensitivity. These reactions can be associated with fear and confusion. A euphoric state may also be induced, which explains the potential for abuse of the drug. Children usually have a lesser incidence of severe emergence reactions. Administration of a benzodiazepine in combination with ketamine may help limit the unpleasant emergence reactions and also increase amnesia.
Central Nervous System
In contrast to other intravenous anesthetics, ketamine is a cerebral vasodilator that increases CBF as well as CMRO2. Thus, ketamine is usually avoided in patients with intracranial disease, especially increased ICP. Nevertheless, the undesirable effects on CBF may be blunted by controlled ventilation and the maintenance of normocapnia.(61) Despite the potential to produce myoclonic activity, ketamine is considered an anticonvulsant and may be considered for treatment of status epilepticus when more conventional drugs are ineffective.
Cardiovascular System
Ketamine can produce significant, but transient increases in systemic arterial blood pressure, heart rate, and cardiac output, presumably by centrally mediated sympathetic stimulation. These effects, which are associated with increased cardiac work and myocardial oxygen consumption, are not always desirable and can be blunted by coadministration of benzodiazepines, opioids, or inhaled anesthetics. Though more controversial, ketamine is a direct myocardial depressant. This property is usually masked by its stimulation of the sympathetic nervous system, but it may become apparent in critically ill patients with limited ability to increase their sympathetic nervous system activity.
Respiratory System
Ketamine does not produce significant respiratory depression. When used as a single drug, the respiratory response to hypercapnia is preserved and arterial blood gases remain stable. Transient hypoventilation and, in rare cases, a short period of apnea can follow rapid administration of large intravenous doses for induction of anesthesia. The ability to protect the upper airway in the presence of ketamine cannot be assumed despite the presence of active airway reflexes. Especially in children, the risk for laryngospasm because of increased salivation is increased and can be reduced by premedication with an anticholinergic drug. Ketamine relaxes bronchial smooth muscles and may be helpful in patients with reactive airways and in the management of patients experiencing bronchoconstriction.
Clinical Uses
The unpleasant emergence reactions after the administration of ketamine have restricted its use as a general anesthetic.(62) Nevertheless, ketamine’s unique properties, including profound analgesia, stimulation of the sympathetic nervous system, bronchodilation, and minimal respiratory depression, make it an important alternative to the other intravenous anesthetics and a desirable adjunct in many cases. Moreover, ketamine can be administered by multiple routes (intravenous, intramuscular, oral, rectal, epidural), thus making it a useful option for premedication in mentally challenged and uncooperative pediatric patients.
Induction and Maintenance of Anesthesia
Induction of anesthesia can be achieved with ketamine, 1 to 2 mg/kg IV or 4 to 6 mg/kg IM. Though not commonly used for maintenance of anesthesia, the short context-sensitive half-time makes ketamine a consideration for this purpose (see Fig.3).(10) For example, general anesthesia can be achieved with the infusion of ketamine, 15 to 45 μg/kg/min, plus 50% to 70% nitrous oxide or by ketamine alone, 30 to 90 μg/kg/min.

- Fig.3 Context-sensitive half-time for the most commonly used intravenous anesthetics. Propofol, etomidate, and ketamine have the smallest increase in context-sensitive half-times, with prolonged infusions making these drugs more suitable for use as continuous infusions. (From Vuyk J, Sitsen E, Reekers M. Intravenous anesthetics. In: Miller RD, ed. Miller’s Anesthesia. 8th ed. Philadelphia:Elsevier; 2015:821-863.)
Analgesia
Small bolus doses of ketamine (0.2 to 0.8 mg/kg IV) may be useful during regional anesthesia when additional analgesia is needed (e.g., cesarean section under neuraxial anesthesia with an insufficient regional block). Ketamine provides effective analgesia without compromise of the airway. An infusion of a subanalgesic dose of ketamine (3 to 5 μg/kg/min) during general anesthe-sia and in the early postoperative period may be useful to produce analgesia or reduce opioid tolerance and opioid-induced hyperalgesia,(63) although not all studies examining the use of ketamine as an adjunct show the desired improvement in pain scores and recovery.(64) Because of the presence of NMDA receptors on peripheral nociceptors, local and topical application of ketamine appears to be a reasonable approach that might allow achievement of higher local tissue concentrations in an attempt to avoid undesired CNS effects. However, convincing evidence from controlled trials is lacking and so far support for this modality mainly comes from case reports.(65)
Treatment of Major Depression
Ketamine has recently received increasing attention as a therapeutic option for treatment-resistant major depression. A single intravenous infusion of ketamine (0.5 mg/kg over 40 minutes) was shown to be superior to midazolam for reduction of depressive symptoms in less than 24 hours.(66) With optimization of dose, timing, and frequency of treatments, ketamine may prove useful for maintenance of antidepressant effects in treatment-resistant patients.(59)
7. ETOMIDATE
Etomidate is an intravenous anesthetic with hypnotic but not analgesic properties and with minimal hemodynamic effects.(2,3,7) The pharmacokinetics of etomidate make it suitable for use as a continuous infusion, but it is not widely used mainly because of its endocrine side effects.
Physicochemical Characteristics
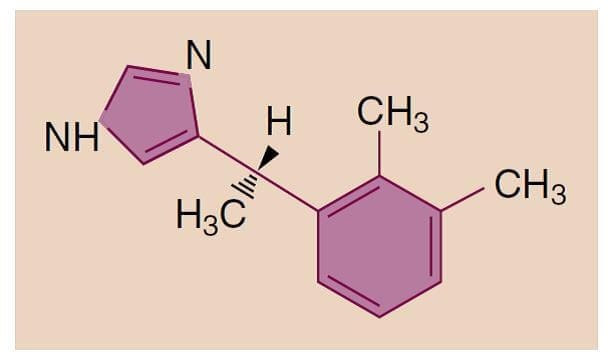
Etomidate is a carboxylated imidazole derivative that has two optical isomers (Fig.10). The available preparation contains only the active D(+) isomer, which has hypnotic properties. The drug is poorly soluble in water and is therefore supplied as a 2 mg/mL solution in 35% propylene glycol. The solution has a pH of 6.9 and thus does not cause problems with precipitation like thiopental does.

- Fig.10 Chemical structure of etomidate.
Pharmacokinetics
An induction dose of etomidate produces rapid onset of anesthesia, and recovery depends on redistribution to inactive tissue sites (comparable to thiopental and propofol). Metabolism is primarily hepatic by ester hydrolysis to inactive metabolites, which are then excreted in urine (78%) and bile (22%). Less than 3% of an administered dose of etomidate is excreted unchanged in the urine. Clearance of etomidate is about five times that for thio-pental, as reflected by a shorter elimination half-time (see Table 1). The duration of action is linearly related to the dose, with each 0.1 mg/kg providing about 100 seconds of unconsciousness. Because of its minimal effects on hemodynamics and short context-sensitive half-time, larger doses, repeated boluses, or continuous infusions can safely be administered (see Fig.3).10 Etomidate, like most other intravenous anesthetics, is highly protein bound (77%), primarily to albumin. Development of novel short-acting etomidate derivatives (e.g., ABP-700) is under way with the goal of finding an analog with limited adrenal side effects.(67)
Pharmacodynamics
Etomidate has GABA-like effects and seems to primarily act through potentiation of GABAA-mediated chloride currents, like most other intravenous anesthetics.(7)
Central Nervous System
Etomidate is a potent cerebral vasoconstrictor, as reflected by decreases in CBF and ICP. These effects of etomidate are similar to those produced by comparable doses of thiopental. Despite its reduction of CMRO2, etomidate failed to show neuroprotective properties in animal studies, and human studies are lacking. Excitatory spikes on the EEG are more frequent after etomidate than from thiopental. Similar to methohexital, etomidate may activate seizure foci, manifested as fast activity on the EEG. In addition, spontaneous movements characterized as myoclonus occur in more than 50% of patients receiving etomidate, and this myoclonic activity may be associated with seizure-like activity on the EEG.
Cardiovascular System
A characteristic and desired feature of induction of anesthesia with etomidate is cardiovascular stability after bolus injection.(7) Arterial blood pressure decreases are modest or absent and principally reflect decreases in systemic vascular resistance. Any hypotensive effects of etomidate are probably exaggerated in the presence of hypovolemia. Etomidate produces minimal changes in heart rate and cardiac output. The depressive effects of etomidate on myocardial contractility are minimal at concentrations used for induction of anesthesia.
Respiratory System
The depressant effects of etomidate on ventilation are less pronounced than those of barbiturates, although apnea may occasionally follow rapid intravenous injection. Depression of ventilation may be exaggerated when etomidate is combined with inhaled anesthetics or opioids.
Endocrine System
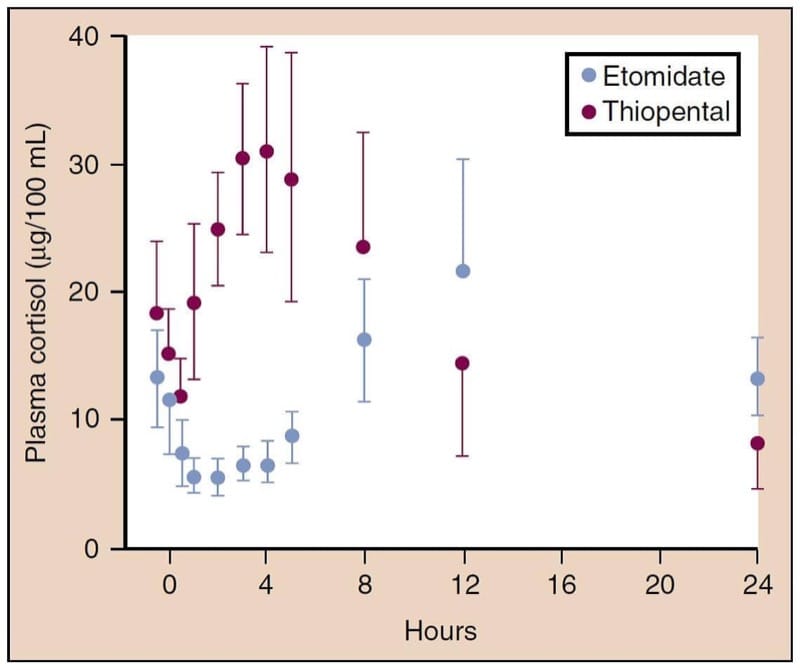
Etomidate causes adrenocortical suppression by producing a dose-dependent inhibition of 11β-hydroxylase, an enzyme necessary for the conversion of cholesterol to cortisol (Fig.11).(68) This suppression lasts at least 4 to 8 hours after a single induction dose of etomidate, and relative adrenal insufficiency may last up to 24 to 48 hours.(69) This property has generated great controversy over the safety of etomidate for intubation of critically ill patients and as an induction drug for general anesthesia.(70)

- Fig.11 Etomidate, but not thiopental, is associated with decreases in the plasma concentrations of cortisol. p < 0.005 versus thiopental, mean ± SD (standard deviation). (From Fragen RT, Shanks CA, Molteni A, et al. Effects of etomidate on hormonal responses to surgical stress. Anesthesiology. 1984;61:652-656, used with permission.)
Clinical Uses
Etomidate is an alternative to propofol and barbiturates for the rapid intravenous induction of anesthesia, especially in patients with compromised myocardial contractility, coronary artery disease, or severe aortic stenosis.(71,72) After a standard induction dose (0.2 to 0.3 mg/kg IV), the onset of unconsciousness is comparable to that achieved by thiopental and propofol. There is a frequent incidence of pain during intravenous injection of etomidate, which may be followed by venous irritation. Involuntary myoclonic movements are common but may be masked by the concomitant administration of neuromuscular blocking drugs. Awakening after a single intravenous dose of etomidate is rapid, with little evidence of any residual depressant effects. Etomidate does not produce analgesia, and PONV may be more common than after the administration of thiopental or propofol. The principal limiting factor in the clinical use of etomidate for induction of anesthesia is its ability to transiently depress adrenocortical function.(68) Theoretically, this suppression may be either desirable if it reduces neurohormonal stresses during surgery and anesthesia, or undesirable if it prevents useful protective responses against perioperative stresses. Recent meta-analyses have challenged earlier findings that etomidate increases mortality after a single dose for intubation of septic patients.(73-75) Etomidate remains a useful hypnotic for electroconvulsive therapy as it provides a longer seizure duration than propofol or methohexital.(76)
8. DEXMEDETOMIDINE
Dexmedetomidine is a highly selective α2-adrenergic agonist.(77) Recognition of the usefulness of α2-agonists was based on the observation that patients receiving chronic clonidine therapy have decreased anesthetic requirements. The effects of dexmedetomidine can be reversed with α2-antagonist drugs.
Physicochemical Characteristics
Dexmedetomidine is the active S-enantiomer of medetomidine, a highly selective α2-adrenergic agonist and imidazole derivative that is used in veterinary medicine. Dexmedetomidine is water soluble and available as a parenteral formulation (Fig.12).

- Fig.12 Chemical structure of dexmedetomidine.
Pharmacokinetics
Dexmedetomidine undergoes rapid hepatic metabolism involving conjugation, N-methylation, and hydroxylation. Metabolites are excreted through urine and bile. Clearance is high, and the elimination half-time is short. However, there is a significant increase in the context-sensitive half-time from 4 minutes after a 10-minute infusion to 250 minutes after an 8-hour infusion.
Pharmacodynamics
Dexmedetomidine produces its effects through activation of CNS α2-receptors.
Central Nervous System
Hypnosis presumably results from stimulation of α2-receptors in the locus ceruleus, and the analgesic effect originates at the level of the spinal cord. The sedative effect produced by dexmedetomidine has a different quality than that of other intravenous anesthetics in that it more resembles a physiologic sleep state through activation of endogenous sleep pathways. Dexmedetomidine decreases CBF without significant changes in ICP and CMRO2 (see Table 2). Tolerance and dependence can develop. Although changes in the EEG do occur, spikes from seizure foci are not suppressed making dexmedetomidine a useful drug for epilepsy surgery.(78) Evoked potentials monitored during spine surgery are not suppressed at usual infusion doses.(79)
Cardiovascular System
Dexmedetomidine infusion produces moderate decreases in heart rate and systemic vascular resistance and, consequently, decreases in systemic arterial blood pressure. A bolus injection may produce transient increases in systemic arterial blood pressure and pronounced decreases in heart rate, an effect that is probably due to vasoconstriction mediated by peripheral α2-adrenergic receptors. Clinically useful initial doses (0.5 to 1 μg/kg IV over 10 minutes) increase systemic vascular resistance and mean arterial pressure, but probably do not significantly increase pulmonary vascular resistance.(80) Bradycardia or hypotension associated with dexmedetomidine infusion may require treatment. Increasing age and decreased baseline arterial blood pressure (mean arterial pressure < 70 mm Hg) are risk factors for hemodynamic instability during dexmedetomidine infusion.(81) Heart block, severe bradycardia, or asystole may result from unopposed vagal stimulation. The response to anticholinergic drugs is unchanged. When used as an adjunct to general anesthesia, dexmedetomidine reduces plasma catecholamine levels and may attenuate heart rate increases during emergence.(82,83)
Respiratory System
The effects of dexmedetomidine on the respiratory system are a small to moderate decrease in tidal volume and minimal change in the respiratory rate. The ventilatory response to carbon dioxide is minimally impaired, but the response to hypoxia seems reduced to a similar degree as propofol.(84) Although the respiratory effects are mild, upper airway obstruction as a result of sedation is possible. In addition, dexmedetomidine has a synergistic sedative effect when combined with other sedative-hypnotics.
Clinical Uses
Dexmedetomidine is principally used for the short-term sedation of tracheally intubated and mechani-cally ventilated patients in an intensive care setting.(77) Although there is no evidence of benefit to mortality risk, d exmedetomidine may reduce the duration of mechanical ventilation, shorten length of ICU stay,(85) and improve sleep quality.(86) In the operating room, dex-medetomidine may be used as an adjunct to general anesthesia or to provide sedation during regional anes-thesia or awake fiberoptic tracheal intubation.87 When administered during general anesthesia, dexmedetomidine (0.5- to 1-μg/kg IV initial dose over a period of 10 to 15 minutes, followed by an infusion of 0.2 to 0.7 μg/kg/h) decreases the dose requirements for inhaled and intravenous anesthetics. Awakening and the transition to the postoperative setting may benefit from dexmedeto-midine-produced sedative and analgesic effects without respiratory depression. Dexmedetomidine seems to decrease perioperative opioid consumption and improve pain scores,(88) but analgesic benefit has not been shown in all settings.(89)
Dexmedetomidine has been used extensively in children and has demonstrated efficacy in this population.(90) Specifically, it may be beneficial for prevention of emergence delirium after pediatric anesthesia(91). At the other extreme of age, dexmedetomidine may be superior to propofol for reducing delirium in elderly patients requiring sedation after cardiac or noncardiac surgery(92,93).
9. QUESTIONS OF THE DAY
1. What are the expected cardiovascular and respiratory effects of propofol? What techniques can reduce injection pain with propofol?
2. What are the risks of high-dose barbiturate therapy to decrease intracranial pressure (ICP) or provide neuroprotection?
3. What are the respiratory effects of benzodiazepines when administered alone and when administered concurrently with opioids? How do benzodiazepines impact pharyngeal function?
4. How do the central nervous system (CNS) effects of ketamine differ from that of propofol or barbiturates? What are the potential benefits of ketamine as an analgesic drug?
5. What are the effects of dexmedetomidine on tidal volume and respiratory rate? What are the expected cardiovascular effects of dexmedetomidine infusion? What cardiovascular effects may be evident after a bolus injection of dexmedetomidine?