Stavros Prineas
INTRODUCTION
Ophthalmic surgery is one of the most common surgical procedures requiring anesthesia in developed countries. Ophthalmic anesthesia offers insights into some fundamental principles of good anesthetic practice, especially in the conduct of local and regional nerve blocks.
LOCAL ANESTHESIA IN EYE SURGERY
Clinical strategies to minimize patient movement during eye surgery are essential. Historically, eye surgeons favored general anesthesia (GA), which usually provided akinesia (through neuromuscular block) and low intraocular pressure. However, these conditions are not always achieved under GA. A closed claim analysis by Gild and coworkers found that 30% of eye injury claims associated with anesthesia involved the patient moving during ophthalmic surgery, with most incidents occurring under GA. While perioperative morbidity and mortality rates associated with eye surgery (eg, cataract extraction) are low, patients having cataract surgery tend to be older and to have significant comorbidities. For this reason, systematic preoperative evaluation should be performed to consider whether a patient is eligible for a GA and surgery.
Appropriate anesthetic management contributes to the success or failure of ophthalmic surgery. Quicker patient rehabilitation and fewer complications in this patient population are the main reasons why many ophthalmic surgeons are now choosing local anesthesia (LA) over GA.
Traditionally, the gold standard of eye nerve blocks was retrobulbar anesthesia (RBA), with the surgeon performing the nerve block.
However, advances in technology and surgical technique, particularly in cataract surgery, have led to the replacement of the older wide-incision techniques (eg, extra-capsular cataract extraction) with minimally invasive phacoemulsification (PhE) techniques. Consequently, for new generation of cataract surgeons, total akinesia is no longer necessary for PhE.
NYSORA TIPS
• Twenty-first century innovations and trends have revolutionized eye surgery.
• Local and regional anesthetic techniques has largely replaced general anesthesia.
• Understanding functional anatomy and surgical techniques is essential for selection of regional techniques.
• Sub-Tenon’s nerve block is one of the most common choices for anesthesia as it can generally achieve akinesia with the favorable safety profile.
• Topical anesthesia, is increasingly becoming most prevalent for cataract surgery.
• Complications of eye nerve blocks are uncommon but can be life- or sight-threatening, emphasizing the need of adequate training.
Innovation has also broadened the anesthetic options for eye surgery. As the conventional RBA carries a greater risk of complications, less invasive techniques have increasingly been used, with a substantial diversity in practice styles around the world. For instance, one Australian study from 2002 reported that peribulbar nerve block was the most popular among an international sample of ophthalmic surgeons. However, a 2006 survey found that 64% of a sample of UK anesthetists favored the sub-Tenon’s technique, and, by 2008, a survey of the British Ophthalmic Anaesthesia Society found that over 87% of anesthesiologist members regularly performed sub-Tenon’s nerve blocks.
On the other hand, an annual survey of the American Society of Cataract and Refractive Surgery reported that its members’ preference for using topical anesthesia had risen steadily from 11% in 1995 to 76% in 2012. In the same survey, the use of sub-Tenon’s nerve blocks appeared to be consistently low over the years (around 1%–3%) despite its growing popularity elsewhere, with the use of retrobulbar and peribulbar nerve blocks seemingly in gradual but sustained decline.
Each anesthetic technique has its strengths and limitations.
Knowledge of the relevant anatomy for the various anesthetic techniques is essential to determine the appropriate nerve block for specific clinical situations and to best avoid life- and sight-threatening complications. In this section, we review the relevant anatomy of the eye, classical needle and non-needle nerve block techniques, and choice of LA and adjuvant agents. With a wider range of options comes a greater burden on the anesthesiologist to discuss individual anesthetic requirements with the surgeon, to be adaptable, and to have excellent communication and team-working skills.
FUNCTIONAL ANATOMY
Orbit
The orbits (Figures 1 and 2) are two symmetrical bony enclosures in the front of the skull, each containing an eyeball (or globe) and its associated structures. The cavity of each orbit is a truncated pyramid, with a flattened apex posteriorly and a trapezoidal base facing anterolaterally. The medial (nasal) walls of each orbit are parallel to each other, while the lateral (temporal) walls are perpendicular to each other. The volume of each adult orbit is approximately 30 mL.
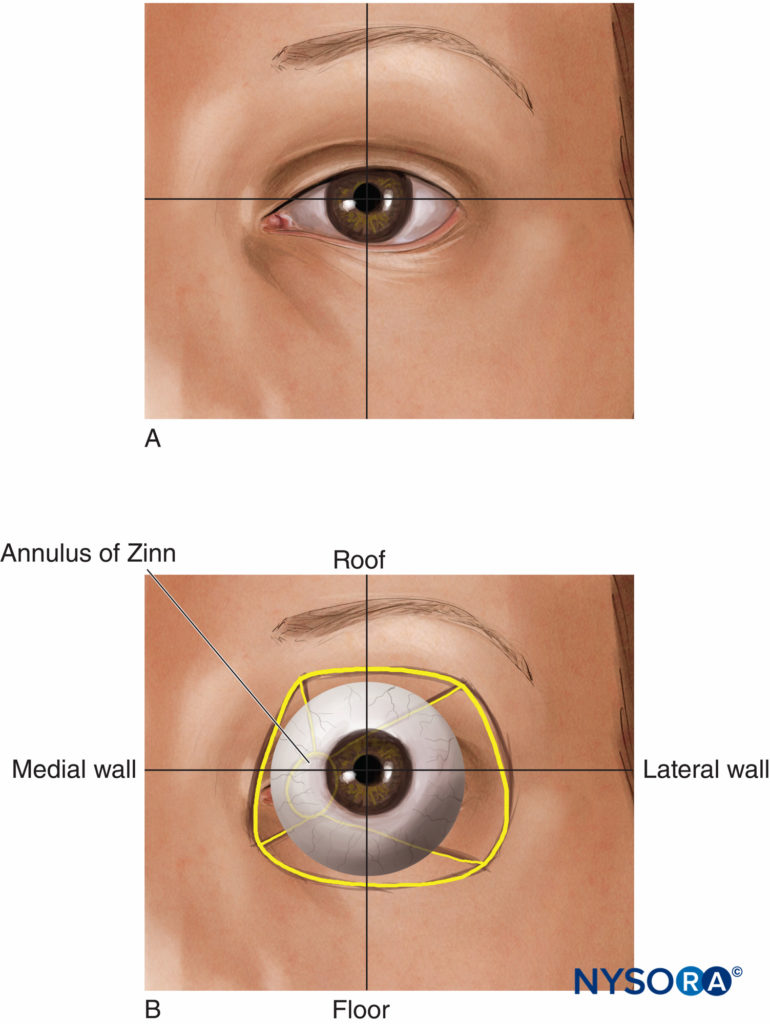
FIGURE 1. (A) The eye. (B) Surface anatomy.
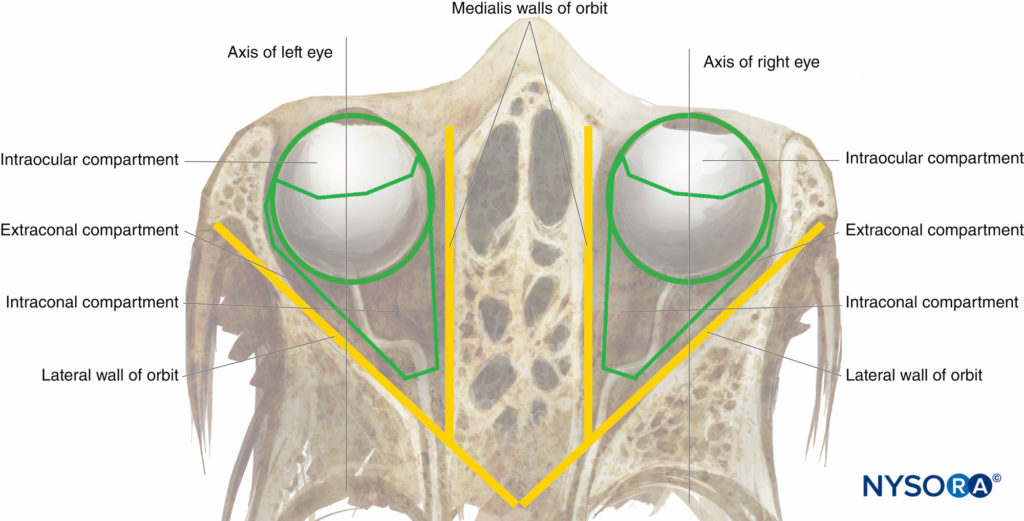
FIGURE 2. The orbit: diagrammatic superior view.
Globe
The globe (Figure 3) is suspended in the anterosuperior part of the orbit. It is roughly spherical, its contents contained within three outer layers or tunics:
- A fibrous tunic comprising the translucent cornea in front and the opaque sclera peripherally and behind
- Deep to the fibrous tunic, a vascular, pigmented tunic comprising the iris and ciliary body in front and the choroid peripherally and behind
- Deep to this again, a neural tunic overlying the posterior part of the other two tunics internally, comprising the retina.
The globe has a large posterior segment (comprising the vitreous humor, the retina, the macula, and the root of the optic nerve) and a small convex anterior segment comprising two chambers. The anterior chamber immediately behind the cornea is filled with aqueous humor produced by the ciliary body. The posterior chamber contains the lens. The two chambers are separated by the iris and communicate via the pupil of the eye. Externally, the circumferential junction of cornea and sclera (with its overlying conjunctiva) is called the limbus.
The volume of the globe is approximately 7 mL. The axial (anteroposterior) length of the adult globe is on average about 24 mm; however, this can be considerably longer in myopic individuals (> 26 mm) and shorter in hypermetropia (down to 20 mm). As a rule of thumb, the distance from the front of the globe to its equator is about 12–15 mm, but, where possible, it is better to know the measured axial length of the eye before attempting to inject behind the equator (eg, for patients undergoing cataract surgery, biometric data are routinely found in the surgeon’s clinical notes).
The sclera is thinnest at the equator and at the insertion points of the extraocular muscles. However, more myopic eyes (with a longer axial length) have a markedly increased prevalence of posterior staphyloma, an otherwise rare “blow-out” weakness in the fibrous tunic, which poses a major risk for globe perforation with blind needle techniques (see “Complications of Eye Nerve Blocks” later).
Muscles of the Eye
The four rectus and two oblique muscles of the eye insert anteriorly near the equator of the globe (see Figure 3). Posteriorly, they originate together at the apex from the tendinous annulus communis of Zinn. The four rectus muscles, running posteriorly from the equator to the annulus of Zinn, delineate the retrobulbar cone. The optic nerve traverses the cone from the posterior part of the globe and enters the orbit through the annulus of Zinn.
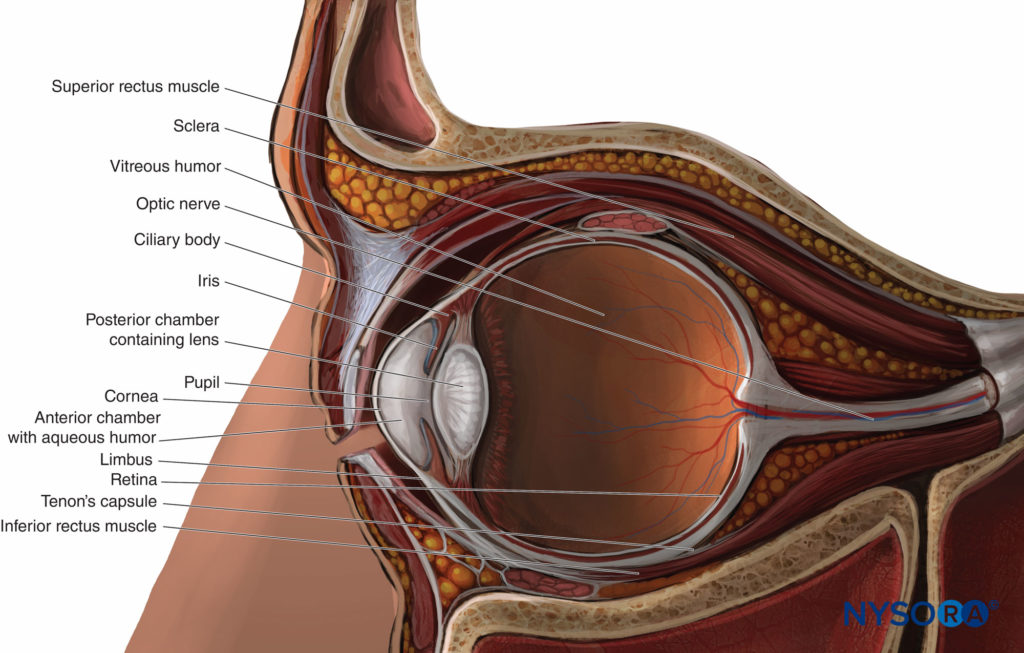
FIGURE 3. The globe: sagittal section.
Three Intraorbital Compartments
The globe and muscular retrobulbar cone define the three classical anatomical compartments of the orbital cavity: the intraocular, intraconal, and extraconal (see Figure 2). However the retrobulbar cone is not sealed by any intermuscular membrane, and, in fact, there is free communication between the intraconal and extraconal spaces. Thus, a large-volume peribulbar (extraconal) nerve block can theoretically provide an effective anesthesia and akinesia as a targeted small-volume retrobulbar nerve block.
Innervation of the Orbit
Sensory innervation of the orbit and globe is provided mostly by the frontal and nasociliary branches of the ophthalmic nerve (first branch of the trigeminal nerve, V), which pass through the muscular cone (Figures 4 and 5), while some of the floor of the orbit is supplied by the infraorbital branch of the maxillary nerve (the second branch of the trigeminal nerve).
The trochlear nerve (IV) provides motor control to the superior oblique muscles, the abducens nerve (VI) to the lateral rectus muscle, and the oculomotor nerve (III) to all other extraocular muscles, including the levator muscle. All except the trochlear nerve pass through the muscular conus.
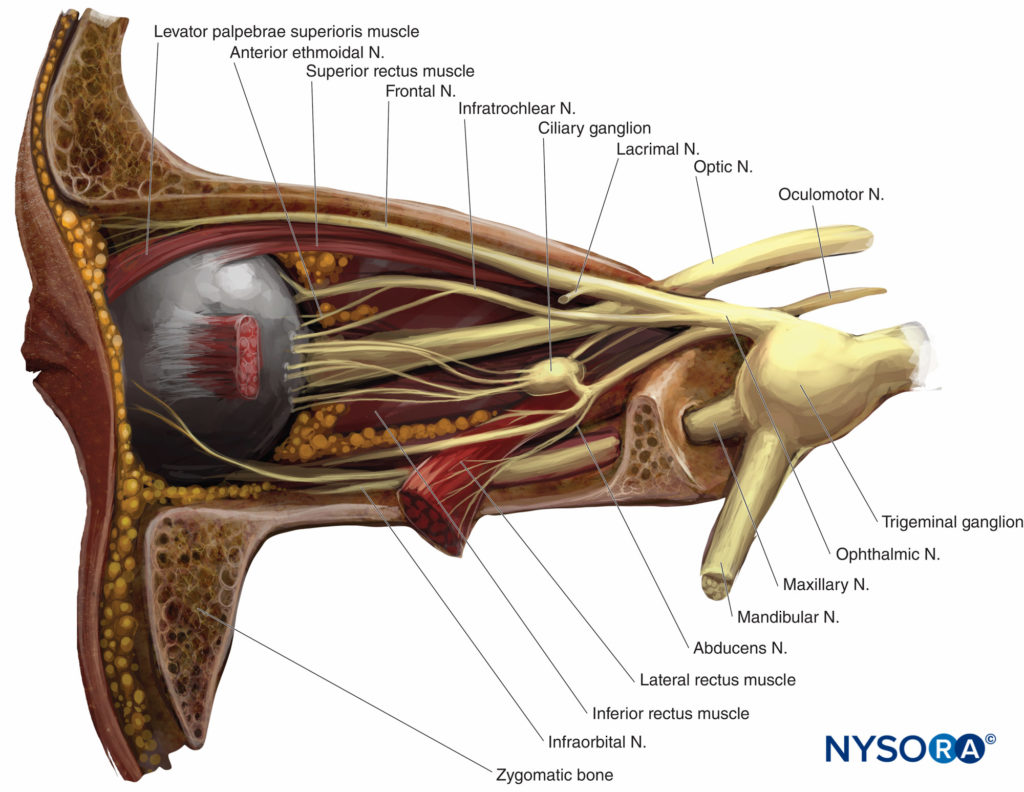
FIGURE 4. Left orbit: lateral view. Lateral wall and lacrimal gland removed.
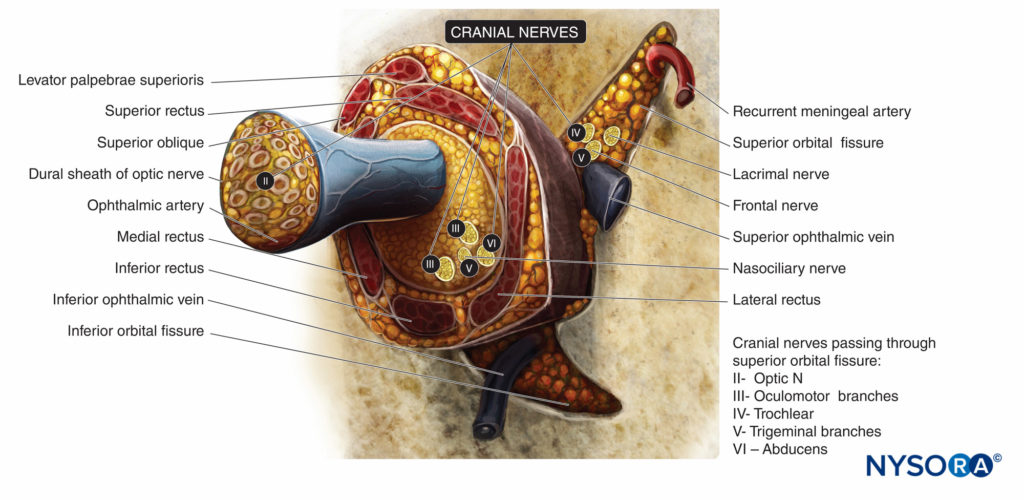
FIGURE 5. Extraocular muscles and innervation of the orbit at the level of the annulus of Zinn. Cranial nerves indicated by Roman numerals.
Anatomical Quadrants of the Globe and Orbit
The sphere of the globe can be divided in the three standard perpendicular anatomical planes into eight “quadrants”: anterior superomedial, posterior superomedial, and so on. Looking from the front, the corresponding anterior extraocular quadrants of the orbit are often referred to as the superonasal, suprotemporal, inferonasal, and inferotemporal, where nasal has the same meaning as medial, and temporal has the same meaning as lateral (Figure 6). The inferotemporal (or inferolateral) space is usually the largest and least vascular and the preferred quadrant of approach for modern retrobulbar and single-shot peribulbar nerve blocks. The inferonasal (or inferomedial) quadrant is most popular for sub-Tenon’s nerve blocks. The superonasal (or superomedial) quadrant is quite vascular but contains the anterior ethmoidal nerve, a useful nerve to nerve block for some oculoplastic procedures (see “Oculoplastic Nerve Blocks” below).
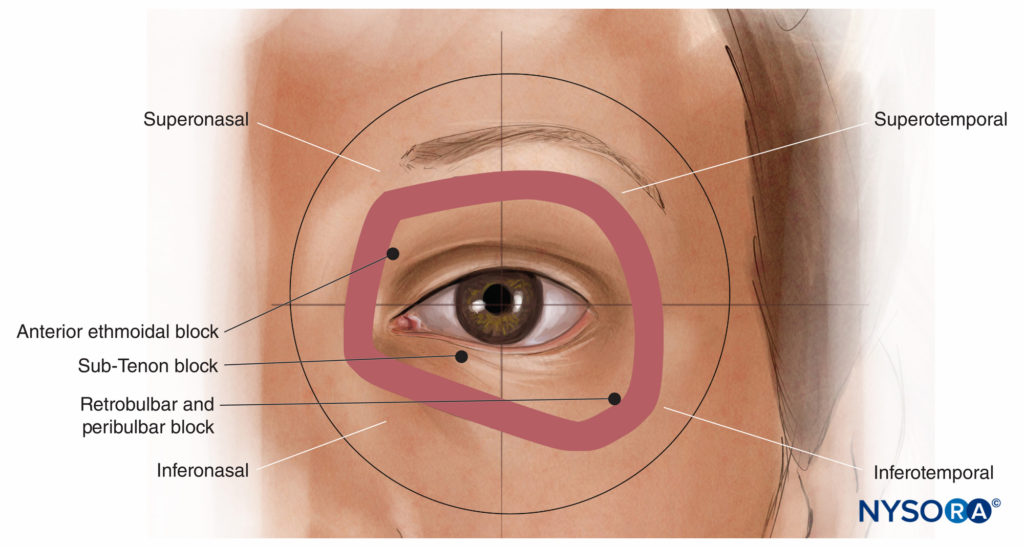
FIGURE 6. The anterior orbital quadrants.
Tenon’s Capsule and Sub-Tenon’s Space
The scleral portion of the globe is surrounded by Tenon’s capsule (also known as the fascial sheath of the eyeball), a fibroelastic layer stretching from the corneal limbus anteriorly to the optic nerve posteriorly. Tenon’s capsule generally becomes thinner and less adherent to the sclera with age. It delimits a potential space referred to as the episcleral space (sub-Tenon’s space), which expands when fluid is injected into it. The conjunctiva overlies the sclera in the anterior part of the eye until it is reflected at the fornices of the eye to continue as the mucosal lining of the underside of the eyelids. Note that Tenon’s capsule fuses with the sclera about 2 mm from the limbus.
Where the sclera is visible, the two layers are easier to pick up as one; closer to the fornix, the conjunctivum becomes fleshier and more distinct from the sub-Tenon’s layer. This helps identify the ideal breach point for the sub-Tenon’s technique (q.v.)
Spread of Local Anesthetic Within the Orbit
Injection of LA solution inside the cone will provide anesthesia and akinesia of the globe and (usually) all extraocular muscles. Only the motor nerve to the orbicularis muscle of the eyelids has an extraorbital course, coming from the superior branch of the facial nerve (VII). Many major structures are located within the muscular conus and are therefore at risk of needle and injection injury. These include the optic nerve with its meningeal coverings, the blood vessels of the orbit, and the nerves supplying the globe. For this reason, some authors advise that introduction of the needle into the muscular cone be avoided and suggest that needle insertion be limited to the extraconal space.
KEY SURGICAL CONSIDERATIONS
It is important to get to know your surgeon. Choosing an appropriate local anesthetic technique requires an understanding not only of surgical procedures but also the surgeon’s personal preferences.
Akinesia
The requirement for akinesia varies with the procedure (see below) and the surgeon. With nerve blocks behind the equator of the eye, improving the likelihood of akinesia generally requires more volume, more time, or the addition of hyaluronidase 30 (see “Choice of Local Anesthetic and Adjuvant Agents” below).
Primary Position Versus “On-Axis” Position
Complete paresis of all extraocular muscles renders the eye in the “primary” or “neutral” position. Usually, this corresponds with the surgeon having the pupil aligned with the axis of the surgical microscope (ie, the surgeon has an ideal “on-axis” view of the operative field). However, in cases of incomplete motor nerve block, or in patients who have significant spinal curvature or who are unable to lie flat, the “resting” position of the blocked eye may not correspond to an on-axis view. For this reason, many surgeons actually prefer to have a fully mobile eye for certain patients or certain procedures (eg, trabeculectomy, pterygium removal) so that they can ask the patient to look at the light of the microscope, thereby bringing the eye “on axis,” or to look away, allowing greater access to more peripheral parts of the globe.
The Volume Effect of Injections
Behind the Eye
Any substantial injection (> 3 mL) behind the equator of the eye (a peribulbar, retrobulbar, or posterior sub-Tenon injection) can significantly raise intraocular pressure and push the posterior segment forward, resulting in a crescent-shaped anterior chamber with decreased volume (Figure 7). This can make surgical conditions more challenging, and while this can mostly be offset by counter-pressure (through gentle digital pressure or application of a weight or balloon), some surgeons prefer techniques that retain a “physiological” anterior segment, and most surgeons prefer techniques that avoid large volumes of injectate (> 10 mL) in the orbit. On the other hand, in patients with significant enophthalmos, bringing the globe forward can improve surgical exposure.
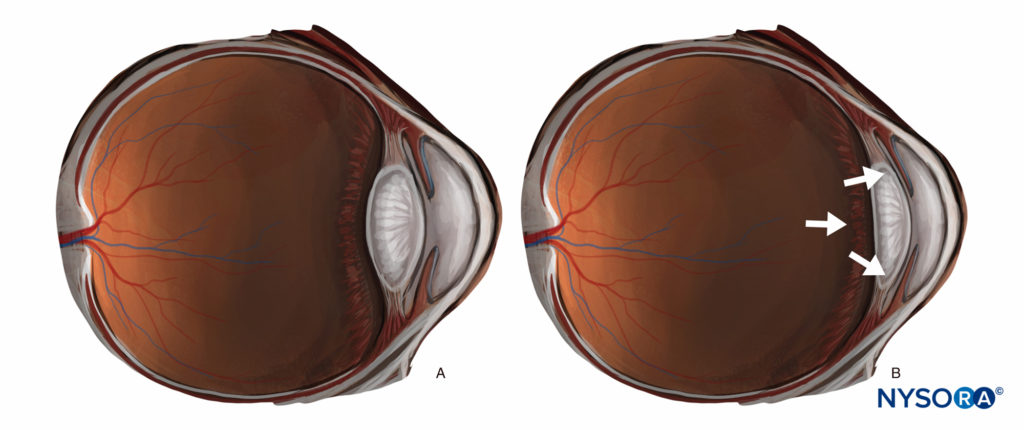
FIGURE 7. (A) “Physiological” anterior segment. (B) The effect of increased retrobulbar volume.
“The Only Eye”
In patients who have vision in only one eye—the eye being operated upon—surgeons and anesthetists alike tend to be risk averse and steer away from sharp needle nerve blocks. Techniques that do not involve loss of vision (ie, topical, subconjunctival, “superficial” sub-Tenon’s) may be preferable. Techniques that better guarantee akinesia usually cause temporary loss of vision due to anesthesia of the optic nerve. In day-case settings, it may be preferable to use a shorter-duration LA (eg, lidocaine, articaine) over longer-acting agents.
Cataract Surgery
The minimally invasive PhE technique was popularized in the 1990s. The PhE probe is inserted via a small superior, threeplane (self-sealing) incision. The contents of the posterior chamber are manipulated using a needle-width stay probe inserted laterally. After the posterior chamber is voided and cleaned, a foldable or injectable artificial intraocular lens is inserted via the same incision. Anesthesia of the anterior segment is all that is required. Some surgeons require akinesia; others (notably high-turnover surgeons) do not.
Corneal Surgery
The most common operations on the cornea involve trauma, removal of foreign bodies, conjunctival flap and pterygium surgery, corneal transplant surgery, and, increasingly, keratoprosthesis. For these procedures, anesthesia of the anterior segment is typically all that is required. Most surgeons require akinesia for penetrating corneal surgery (eg, trauma, transplant, prosthetic surgery, re-do procedures), while many do not for pterygium surgery, where the fibrous tunic remains essentially intact. Some surgeons find subconjunctival anesthesia useful for separating the conjunctiva from the sclera in pterygium surgery.
Refractive Surgery
Corneal surface ablation, incisional refractive surgery, and intracorneal ring insertion are usually performed under topical anesthesia. Refractive procedures involving the anterior chamber (eg, phakic intraocular lens insertion) are done in a manner similar to cataract surgery.
Glaucoma Surgery
Glaucoma filtration surgery and trabeculectomy both involve creating a fistula between the anterior chamber and the subconjunctival space. Again, anesthesia of only the anterior segment is needed. Depending on the surgeon, there may or may not be a need for akinesia. Some surgeons do not like the disruption of integrity of the conjunctiva that often accompanies the surgical sub-Tenon’s (or “snip”) technique, particularly in inexperienced hands. Furthermore, conjunctival hematoma can introduce reticuloendothelial cells that interfere with trabeculectomy flaps; consequently, some glaucoma surgeons prefer peribulbar anesthesia for these procedures. Topical anesthesia (with or without intracameral lidocaine) and subconjunctival anesthesia both avoid the potential effect of volume injections behind or around the orbit on pulsatile ocular blood flow in patients with advanced glaucoma. Paradoxically, nonpenetrative glaucoma surgery (eg, deep sclerotomy), being a longer and more difficult procedure, generally requires a technique that guarantees a longer nerve block with akinesia. Procedures involving aqueous tube shunts can be done under topical anesthesia but tend to be more uncomfortable for the patient.
Cyclophotocoagulation is the circumferential ablation of the ciliary body deep to the limbus. Akinesia is not essential for this procedure but may be preferred by some surgeons; however, good analgesia is essential, as the procedure can be very painful. Subconjunctival anesthesia works but needs time; sub-Tenon’s or needle nerve blocks usually provide acceptable anesthesia more quickly.
Oculoplastic Surgery
Procedures of the soft tissues of the eye include correction of eyelid malpositions such as entropion and ectropion, eyelash malpositions (districhiasis), ptosis surgery, eyelid tumor surgery, eyelid reconstruction, blepharoplasty, tear duct surgery, orbital decompression (eg, for Grave’s disease), and enucleation and evisceration. While many of these procedures can be performed under local infiltration, specific anesthetic techniques are described later in this chapter.
Extraocular Muscle Surgery
Strabismus surgery is most commonly performed in children under general anesthesia. In adults, squint surgery is usually performed in persons under the age of 30, a group which can often be less than stoic, so again GA is usually preferred. However, this surgery can be performed under regional anesthesia. While akinesia is helpful, the main requirement is for deep anesthesia of the muscle cone, as pulling on extraocular muscles is usually quite painful and can induce an oculocardic reflex. Simple suture adjustments can be done under topical anesthesia.
Vitreoretinal Surgery
Vitrectomy and retinal detachment repairs (including scleral buckling) require both anesthesia of the posterior segment and akinesia of the eye; topical and subconjunctival techniques are inadequate for this. Thus, a sub-Tenon’s, retrobulbar, or peribulbar nerve block is more appropriate. Prior buckling surgery is a relative contraindication to any regional technique, as both the buckle and scar tissue around it will hamper the spread of local anesthetic. However, if the position of the buckle is known, a deep sub-Tenon’s nerve block may be possible via one of the unaffected quadrants. The “tap-and-inject” treatment for endophthalmitis is usually performed using a retrobulbar or peribulbar nerve block.
Open-Globe Injuries
Patients with a known or suspected open-globe injury are most frequently managed under general anesthesia to avoid the risks of infection, retrobulbar hemorrhage, and raised intraocular pressure, which can cause further damage. There are authors who advocate repair under local anesthesia and sedation, arguing that this method avoids the dilemma of using succinylcholine, which can markedly increase intraocular pressure, in patients with both a penetrating eye injury and a full stomach. Rapid-onset nondepolarizing muscle relaxants (NDMRs), such as rocuronium, and the wider availability of the NDMR reversal agent, sugammadex, have largely mitigated this problem. Nevertheless, recent literature appears to suggest that the risk of vitreous extrusion remains very real, and, perhaps in carefully selected situations, local anesthesia should not be discounted as an alternative in cases of eye trauma.
Ophthalmic Oncology
Most procedures for the removal of eye tumors can be performed under local or general anesthesia, according to surgeon and/or patient preference. However, procedures that require stereotactic immobilization or deliberate hypotension should be performed under general anesthesia.
ANESTHETIC TECHNIQUES
Sub-Tenon’s (Episcleral) Nerve Block Common Principles
Sub-Tenon’s anesthesia, first described in 1884, places the local anesthetic into the potential space between Tenon’s capsule and the sclera (Figure 8). While this space can theoretically be accessed from any quadrant of the globe, the inferonasal conjunctival fornix is most popular, at approximately the 4:30 (right eye) or 7:30 (left eye) position to avoid encountering the insertions of the medial rectus and inferior oblique muscles at the equator.
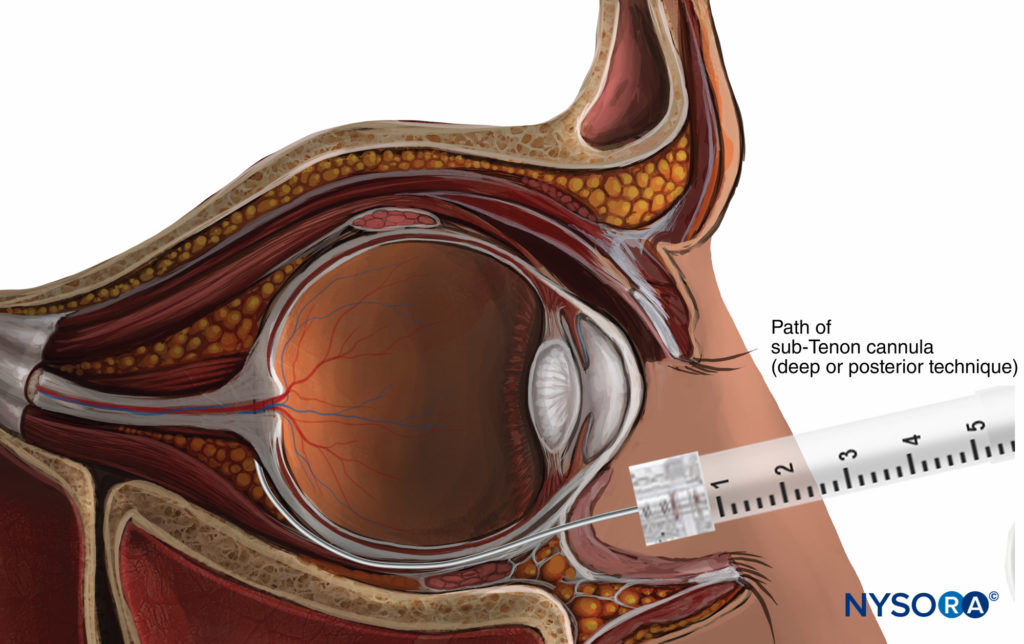
FIGURE 8. Sub-Tenon nerve block.
Using either a surgical or nonsurgical approach (see below), a purpose-specific needle or cannula is directed posteriorly following the curve of the globe. A superficial or deep injection of LA solution can then be performed. “Superficial” or more anterior injections (immediately beyond the equator) allow the LA to spread circularly around the scleral portion of the globe, ensuring high-quality analgesia of the whole globe with relatively low injection volumes (usually 3–5 mL). Injection of a larger volume (up to 8–11 mL) causes the LA to spread to the extraocular muscle sheaths, ensuring reproducible akinesia.
However, larger volumes often cause chemosis (a subconjunctival spread of LA), which requires compression to resolve, as well as a significant increase in intraocular pressure.
On the other hand, “deep” or posterior injections direct more injectate into the posterior intra- and extraconal spaces and are more likely to achieve anesthesia at lower volumes and without chemosis, without akinesia (2–3 mL) or with full akinesia (3–5 mL). While the “surgical” deep injection technique is currently the most popular, several approaches have been described.
Surgical (“Snip”) Technique with a Blunt Cannula
This technique, first proposed as a supplement to (or rescue nerve block from) retrobulbar anesthesia, is probably the most popular variation of the sub-Tenon’s approach. After topical anesthesia, the bulbar conjunctiva is grasped with small forceps in the inferonasal quadrant near the fornix. Small scissors are used to create a small opening into the conjunctiva and Tenon’s capsule to gain access to the episcleral space. The same scissors are used to blunt-dissect a passage to the posterior sub-Tenon’s space. A blunt metal (eg, Stevens) or plastic (eg, Helica) cannula is then inserted into this space to allow for the injection.
Some practitioners opt for a superficial passage and then “hydro-dissect” using the local anesthetic injectate; others (particularly for posterior segment surgery) prefer to probe-dissect as close to the intraconal space as possible using closed scissors. It is also possible, particularly in the elderly, to gently push the cannula posteriorly without dissection. The usual LA injection volume is 3–5 mL. Increasing the injection volume (up to 11 mL) results in an increased likelihood of akinesia but is rarely necessary. The main advantage of this technique is its safety, as it avoids the blind introduction of a sharp needle into the orbit. In 6000 cases, no serious complications were reported, with only a 7% rate of subconjunctival hematoma and a 6% rate of subconjunctival edema. Surgery was canceled because of subconjunctival hematoma in only one patient out of the 6000.
Nonsurgical Technique with a Blunt Cannula
Cutting the conjunctiva can result in long-term scarring and creates a portal for bacteria and occasionally other foreign bodies to enter. With practice, a round-tipped blunt cannula (such as a 21-gauge Eagle Laboratories “Tri-Port” sub-Tenon cannula or even a Stevens cannula) can be introduced through the conjunctiva and Tenon’s layers without a prior incision.
This causes less trauma, reduces bleeding, and is preferred particularly where conjunctival damage needs to be minimized (eg, glaucoma surgery).
Nonsurgical Technique with a Sharp Needle
A 25-gauge, 25-mm, short-bevel sharp needle is introduced into the fornix between the semilunaris fold of the conjunctiva and the globe, tangentially to the globe. The needle is shifted slightly medially and advanced posteriorly. After a small loss of resistance (click) at 10–15 mm, LA is injected. Using a large volume with this technique (6–11 mL) results in good globe and eyelid akinesia that is more reproducible than classic peribulbar anesthesia; however, note that these are large volumes compared to other techniques, and, as for all sharp-needle techniques, the risk of needle misplacement and its subsequent complications must always be kept in mind. Nevertheless, this technique is associated with a low risk of complications, and it is simple to learn and use. In a series of 2000 cases, no serious complications occurred.
Improvised Cannula Technique
This historical technique was briefly popular before specialized cannulae became widely available. A short intravenous (IV) cannula (18- or 20-gauge) was typically used to inject low volumes of LA (3–5 mL). It provides good globe analgesia but only partial akinesia of the globe and lids. The injection causes only a minor increase in intraocular pressure, and preoperative compression of the globe is typically unnecessary. However, given that it requires introduction of the cannula by piercing the Tenon’s capsule, not unlike the technique of IV cannulation, the risk of needle misplacement has reduced the popularity of this technique.
Limitations of the Sub-Tenon’s Technique
The sub-Tenon’s technique may not be possible where the conjunctival fornices have been obliterated, such as in chronic ocular pemphigus. It should also be avoided where the sclera is known to be thin or frail (eg, the “blue sclerae” of osteogenesis imperfecta or an anterior staphyloma).
Access may be challenging in patients whose inferonasal space is already scarred by multiple previous nerve blocks or in those with a scleral buckle in place, particularly one that is circumferential. In most of these situations, access is possible via an alternative quadrant (most commonly the inferotemporal). Chronic eye inflammation can cause the conjunctiva to become fleshy and painful to touch and to bleed easily. Access through a pterygium should be avoided for the same reasons.
NYSORA TIPS
The Surgical “Snip” Technique of the Sub-Tenon’s Nerve Block According to Guise
• Use half-strength iodine: A substantial body of evidence suggests that this reduces the risk of bacterial endophthalmitis.
• Stay in the “line of longitude” from pole to pole: Keep equally between the medial and inferior recti.
• Keep the Westcott scissors closed: Only one snip at the start is needed.
• Keep the tip of the cannula tight against the sclera: It’s easy to overshoot.
• Inject in different directions: Rotating the syringe slightly will aid spread behind the eye.
• Avoid the pterygia: They make access to the sub-Tenon’s space more difficult, and they bleed.
• Keep the local anesthetic posterior to the equator: Keep the channel created by the Westcott scissors as narrow as possible to minimize reflux.
• Reduce the rate and force of injection: Too fast and hard is painful for the patient and more likely to cause chemosis.
• Use less than 5mL to minimize chemosis and the increase in intraocular pressure.
• Avoid ocular massage, which can cause dramatic peaks in intraocular pressure and may even cause anterior chamber bleeding. Gentle, steady pressure, if anything, is better.
• Take care with scleral buckles: The inferotemporal quadrant is usually clear, but ask your surgeon. If it is not clear, and you are not comfortable trying a different quadrant, or if you expect or encounter significant scarring, try a different technique.
RETROBULBAR ANESTHESIA
Historically, retrobulbar anesthesia (RBA) was the gold standard for anesthesia of the eye and orbit. This technique generally consists of injecting a small volume of LA solution (3–5 mL) inside the muscular cone (see Figure 3). A facial nerve block is occasionally required to prevent blinking (see “Oculoplastic Nerve Blocks” below). Because of its extraconal motor control, the superior oblique muscle frequently may remain functional, precluding total akinesia of the globe. The main hazard of RBA is the risk of injury to the globe or to one of the anatomical structures in the muscular cone. Near the apex, these structures are packed in a very small space and are fixed by the tendon of Zinn, which prevents them from moving away from a needle.
Classical Technique (Now Obsolete)
In Atkinson’s classical 1936 description, the patient is asked to look in the “up-and-in” direction. The needle is introduced through the skin below the inferior lid at the junction between the lateral third and the medial two-thirds of the inferior orbital edge. The needle is directed toward the apex of the orbit (slightly medially and cephalad) and advanced to a depth of 25–35 mm. A volume of 2–4 mL of LA solution is then injected. An additional facial nerve block may be needed to prevent blinking; the technique most frequently used is the Van Lint block (see “Oculoplastic Nerve Blocks”).
Modern Techniques
Modern retrobulbar nerve blocks are performed with the eye in the neutral position (Figure 9). The Atkinson “up-and-in” gaze position was abandoned when Liu et al. and Unsöld et al. warned that it increased the risk of optic nerve injury since this position places the optic nerve near the path of the needle. Moreover, the optic nerve is stretched and can be injured easily by the needle, rather than being pushed aside. Alternative puncture sites and specially designed bent or curved needles have been proposed but have not gained popularity.
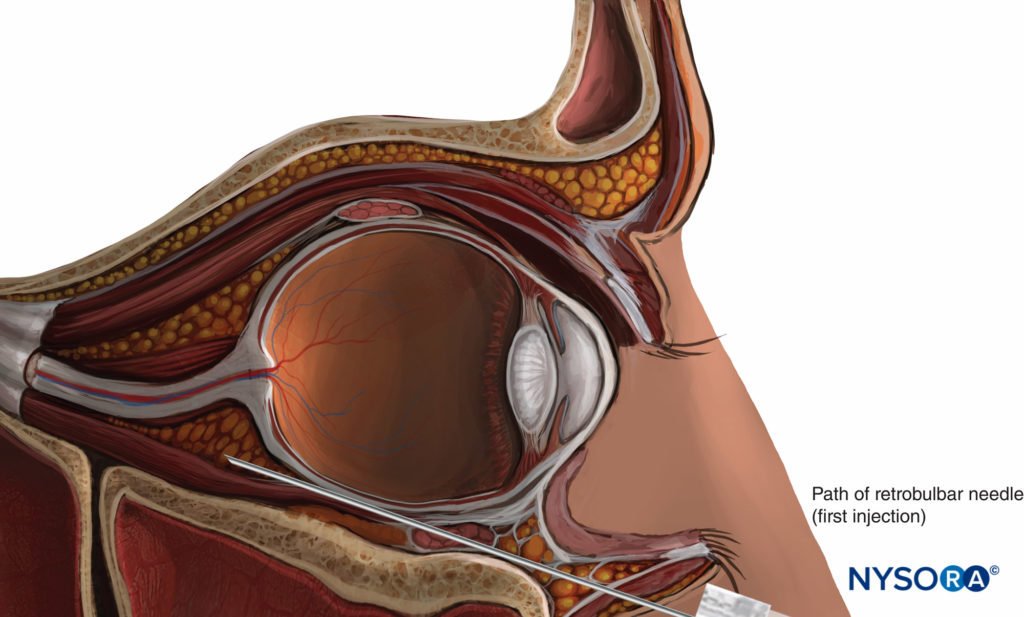
FIGURE 9. Retrobulbar nerve block.
RBA: Is There a Future?
Given the versatility and rising popularity of sub-Tenon’s Nerve blocks, and given that there is no situation in which a retrobulbar nerve block would be preferred over a peribulbar nerve block, it seems unlikely that retrobulbar nerve blocks will remain part of the repertoire of the modern anesthesiologist.
PERIBULBAR ANESTHESIA
With peribulbar anesthesia (PBA), the needle is introduced into the extraconal space (Figure 10). The classical technique involves two injections. The first injection is inferior and temporal, the needle being introduced at the same site as for an RBA injection, but with a lesser up-and-in angle.
The second injection is superior and nasal, between the medial third and lateral two-thirds of the orbital roof edge (see Figure 10).
The injected volume of LA (6–12 mL) is larger than that for a retrobulbar injection. This larger volume allows the LA to spread into the whole corpus adiposum of the orbit, including the intraconal space, where the nerves to be blocked are located. Additionally, such a large volume allows for the anterior spread of LA to the lids to provide a nerve block of the orbicularis muscle and to avoid the need for an additional lid nerve block.
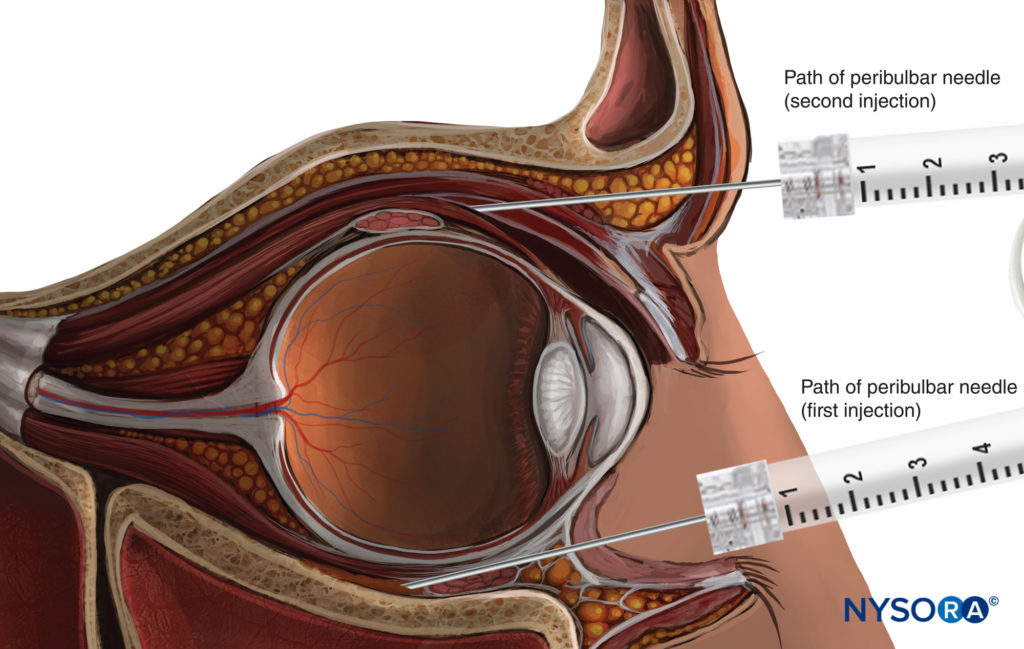
FIGURE 10. Peribulbar nerve block: superior second injection technique.
Several variations on PBA have been described (Figure 11). The most common are (1) medial canthus injection, (2) lacrimal caruncle injection and (3) inferior and temporal injections.
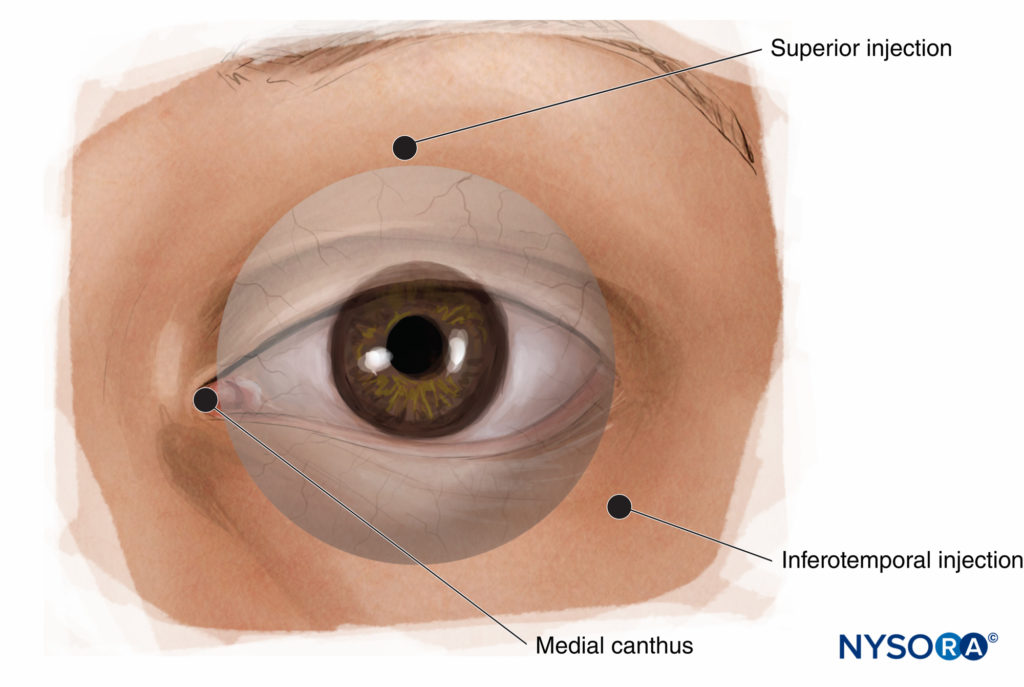
FIGURE 11. Peribulbar nerve block: alternative injection sites.
Principles of Peribulbar Anesthesia
• Single-injection versus multiple-injection technique: Increasing the injected volume of LA provides sufficient anesthesia. Additional injections are not needed. In addition, anatomic distortion following the first injection may increase the risk of complications associated with consecutive injections. As a rule of thumb, a second injection should be performed only as a supplement when the first injection has failed to provide effective anesthesia.
• Needle insertion sites: Needle insertion through the superior nasal site should be avoided. At this level, the distance between the orbital roof and the globe is reduced, theoretically increasing the risk of globe perforation.
Additionally, the needle may injure the superior oblique muscle. The inferonasal approach or an approach through the medial canthus should be used instead. The needle is introduced at the medial junction of the lids, nasal to the lacrimal caruncle, in a strictly posterior direction to a depth of 15 mm or less. At this level, the space between the orbital wall and the globe is similar in size to that of the inferior and temporal approach and is free of blood vessels. Moreover, myopic staphyloma, an anatomical anomaly that represents a risk factor for perforation, is infrequently encountered on the nasal side of the globe.
• Needle insertion depth: Limit the needle insertion depth to 25 mm. Posterior to the globe, the rectus muscles are in contact with the orbital walls, so that the extraconal space entirely disappears and becomes virtual. Increasing the needle insertion depth would be expected to cause a peribulbar injection to become a retrobulbar injection. Some posterior peribulbar nerve blocks are in fact unintentional retrobulbar injections. This is a plausible explanation for optic nerve injury after an attempted peribulbar injection. Moreover, a long needle fully introduced into the orbit may reach the apex of the orbit, another hazardous area. Inserting the needle to a depth of 40 mm has led to performing the injection directly through the optical foramen in 11% of cases.
• Fine needles (25-gauge) are suggested for reducing pain on needle insertion: The use of short-bevel needles may be safer because they may enhance the tactile perception of resistance during needle insertion (intraneural or intramuscular placement). Indeed, on cadavers, more pressure is required with short-bevel needles to perforate the sclera. Nevertheless, these are only theoretical considerations, since the complication rate with peribulbar nerve blocks is low.
• Use compression to lower intraocular pressure, which increases after injection: Compression has not been shown to enhance the quality of the nerve block. Applying a pressure of 30 mm Hg for 5–10 minutes is usually sufficient.
• In all cases, the spread of LA within the corpus adiposum of the orbit remains somewhat unpredictable, leading to the need for more anesthetic to prevent an imperfect nerve block: Depending on the surgeon’s request for akinesia, additional anesthetic is required in up to half of all cases. The poor reproducibility in nerve block efficacy is the main disadvantage of peribulbar anesthesia.
Retrobulbar Versus Peribulbar Versus Sub-Tenon’s Nerve Blocks
RBA has been traditionally assumed to be more effective than PBA. However, when a sufficient volume of LA is injected, both nerve blocks appear to have similar success rates. This is explained by the absence of an intermuscular membrane to separate extra- from intraconal compartments, resulting in a favorable spread of LA (see “Functional Anatomy”). Therefore, if the effectiveness is similar, one should prefer to use the technique with a lower risk of complications. Since RBA theoretically carries a higher risk of complications (eg, optic nerve injury, brainstem anesthesia, retrobulbar hemorrhage; see “Complications of Eye Nerve Blocks” below), PBA is deemed preferable to RBA.
However, all other requirements being equal, the sub-Tenon’s nerve block offers the best safety profile of the three nerve blocks and can reliably deliver both surgical anesthesia and akinesia if performed by an experienced practitioner with an appropriate volume (with or without adjuvants). However, where chemosis, conjunctival hematoma, or the disruption of the conjunctiva are undesirable, or where the posterior anatomy is seriously abnormal, this technique may not be appropriate.
The diversity of opinion and data suggest, to this author at least, that the individual efficacy of any of these nerve blocks is heavily operator dependent. To a significant extent, the same is true in regard to their safety profiles.
COMPLICATIONS OF EYE NERVE BLOCKS
The primary cause of serious complications is needle misplacement. Although some anatomical features may increase the risk of complications, the main risk factor is inadequate knowledge and limited experience on the part of the operator. However, it should be noted that complications such as retrobulbar hemorrhage and oculocardic reflex may occur with even the most experienced practitioners. Presenting signs, symptoms, and mechanisms of common complications are summarized in Tables 1 and 2.
TABLE 1. Signs, symptoms, and mechanisms of complications of retrobulbar anesthesia.
| Complication | Signs and Symptoms | Mechanism of Complication |
|---|---|---|
| Ocular | ||
| Globe perforation | Ocular pain, intraocular hemorrhage, restlessness | Direct trauma: myopic eye, posterior staphyloma, repeated injections |
| Retrobulbar hemorrhage | Subconjunctival or eyelid ecchymosis, increasing proptosis pain, and/or increased intraocular pressure | Direct trauma (artery or vein) |
| Optic nerve damage | Visual loss, possible early optic disc welling, late optic disc pallor | Direct injury to nerve or blood vessels, vascular occlusion |
| Systemic | ||
| Intra-arterial injection | Cardiopulmonary arrest, convulsions | Retrograde flow to internal carotid and access to midbrain structures |
| Optic nerve sheath injection | Brainstem anesthesia: agitation, ptosis, mydriasis dysphagia, dizziness, confusion, contralateral ophthalmoplegia, loss of consciousness, respiratory depression/arrest, cardiac arrest | Subdural or subarachnoid injection |
| Oculocardiac reflex | Bradycardia, other arrhythmias, asystole | Trigeminal nerve (afferent, arc) to floor of fourth ventricle with efferent arc via vagus nerve |
TABLE 2. Other complications of retrobulbar anesthesia.
| Complication | Comment |
|---|---|
| Chemosis (subconjunctival edema) | Usually of minimal concern; disappears with pressure. |
| Venous hemorrhage | Usually mild and, while unsightly, is easily controlled. |
| Arterial hemorrhage | Can be dramatic, causing proptosis, extensive subconjunctival and lid hematoma, and a dramatic increase in intraocular pressure. This is a sight-threatening complication: inform surgeon immediately, as orbital decompression (eg, lateral canthotomy/cantholysis) may be necessary to prevent blindness. Usually causes postponement of surgery. |
| Globe perforation | Likely more common to occur in long, myopic eyes. A long eye has thinner sclera and may have an irregular outline (staphylomata). The needle should be inserted tangentially to the globe and move freely in the orbital fat without rotating the globe. |
| Damage to the optic nerve | A result of direct trauma, injection into the nerve sheath, or the ischemic consequences of the pressure on injection. |
| Decreased visual acuity | Usually resolves with resolution of the block, but beware “wipe-out”, especially in patients with advanced glaucoma (see “Choice of Local Anesthetic and Adjuvant Agents”). |
| Myotoxicity | May follow direct injection into a muscle or the use of high concentrations of LA (probably more common than currently recognized; usually resolves during surgical convalescence). |
| Systemic complications | Subarachnoid injection during retrobulbar block is a possible cause of respiratory arrest. |
| Grand mal seizures, loss of consciousness, and respiratory depression or cardiac arrest | These complications can result from systemic LA toxicity, injection of LA into the optic nerve sheath (and thence to the brainstem), or retrograde arterial flow. |
| Pulmonary edema | Rare; mechanism is poorly understood. |
| Reaction to epinephrine | Often inappropriately referred to as “epinephrine toxicity”; in patients with hypertension, angina, or arrhythmias, the amount of epinephrine injected with the LA should be reduced. |
| Oculocardiac reflex, vasovagal reaction | See text for presentation and management. |
| Allergic reactions | Extremely rare with amide-type LAs; more common with animal-derived hyaluronidase. |
Central Nervous System Complications
Central nervous system eye nerve block complications may occur following a needle nerve block by two different mechanisms:
1. An unintentional intra-arterial injection may reverse the blood flow in the ophthalmic artery up to the anterior cerebral or internal carotid artery,70 so that an injected volume as small as 4 mL may cause seizures. Symptomatic treatment by maintaining a patent airway; providing oxygenation; and abolishing seizure activity with small doses of benzodiazepam, propofol, or a barbiturate is usually adequate and results in a rapid recovery without sequelae.
2. An unintentional subarachnoid injection via puncture of the dura mater sheath of the optic nerve or directly through the optic foramen results in partial or total brainstem anesthesia. Katsev et al. have shown that the apex of the orbit may be reached with a 40-mm needle in up to 11% of patients. Depending on the dose and volume of LA spreading toward the brainstem, subarachnoid injection can lead to bilateral nerve block; cranial nerve palsy with sympathetic activation, confusion, and restlessness; or total spinal anesthesia with tetraparesis, arterial hypotension, bradycardia, and eventually respiratory arrest. Symptomatic treatment (oxygen, vasopressors, and, if required, tracheal intubation and ventilation) should permit complete recovery after the spinal nerve block wears off
(a few hours).
Unintentional globe perforation and rupture is the most devastating complication of eye nerve blocks. It has a poor prognosis, especially when the diagnosis is delayed. The incidence is between 1 in 350 and 7 in 50,000 cases. Main risk factors include inadequate experience of the operator and a highly myopic eye (ie, a long eyeball). In a study of 50,000 cases, Edge and Navon observed that myopic staphyloma was a significant risk factor. This suggests that isolated high myopia may not be a risk factor per se but acts as a confounding factor, as staphyloma seems to occur only in myopic eyes. Vohra and Good observed with B-mode ultrasound that the probability of staphyloma is greater in highly myopic than in slightly myopic eyes. Moreover, staphyloma was more frequently found at the posterior pole of the globe (accounting for perforations after RBA) or in the inferior area of the globe (accounting for perforations after inferior and temporal punctures, both peri and retrobulbar). As a result, at least in myopic patients, and at best in all patients, ultrasound measurement of the axial length of the globe (biometry) should be available. In cases of a highly myopic eye (axial length greater than 26 mm), a needle nerve block can carry an increased risk of globe perforation, and a sub-Tenon’s or topical nerve block is preferable. Unusual causes of globe perforation include misplaced infraorbital nerve blocks (see “Oculoplastic Nerve Blocks”) and patients sneezing under propofol sedation (see “Sedation”).
Extraocular Muscle Injury
Injury to an extraocular muscle may cause diplopia and ptosis. Several mechanisms can be involved, including direct injury by the needle resulting in intramuscular hematoma, high pressure as a result of injection into the muscle sheath, or direct LA myotoxicity.
Direct Extraocular Myotoxicity
It has been known for some time that high concentrations of amide-type local anesthetics, particularly bupivacaine, cause myotoxic changes in skeletal muscle. The extraocular muscles seem particularly susceptible, and numerous case reports of persistent diplopia after cataract surgery have been attributed to this. However, to date, no formal clinical evaluation of skeletal muscle function after eye nerve block has been performed. In monkeys, a 0.75% bupivacaine retrobulbar nerve block typically causes a myopathic response that peaks at 14 days and is largely resolved by 27 days. In rabbits, bupivacaine myotoxicity appears to be concentration dependent, with 0.75% solutions producing extensive and longer-lasting damage, whereas 0.19% produces little sign of short- or longterm damage. Lidocaine solutions containing epinephrine cause substantially more damage to skeletal muscle than does plain lidocaine, both in rats and humans. Indeed in humans, bupivacaine toxicity of eye muscles is deliberately induced to treat strabismus. The injury progresses in three steps: first, the muscle is paralyzed; second, the muscle seems to recover; and third, retractile hyperplasia develops.
Given the case reports and the impressive and consistent pathological evidence across species, and with the many thousands of eye nerve blocks being performed each year, it is surprising that the phenomenon is not more clinically apparent. It has been proposed that postoperative pain may mask myopathic changes and that any eye discomfort or dysfunction is readily attributed to the operation rather than other causes. Furthermore, the animal evidence suggests that in most cases there is likely to be rapid recovery with complete tissue regeneration. In another animal study, skeletal muscle damage induced by femoral nerve block was more severe in younger rats than older rats, so perhaps older humans, too, are less susceptible.
Based on the limited available evidence, it would seem that extraocular myotoxicity is probably a more frequent concomitant of volume nerve blocks behind the equator of the eye that is generally recognized and that it is concentration dependent and more likely to occur with bupivacaine, but that any clinically significant adverse effects in the relevant patient population usually (but not always) resolve completely within the time frame of healing of the operation.
NYSORA TIPS
Retrobulbar Hemorrhage
• Retrobulbar hemorrhage is typically caused by an inadvertent arterial puncture. It may lead to a compressive hematoma, which can threaten retinal perfusion.
• At the time of hemorrhage, it is imperative to have an ophthalmologist present who can monitor intraocular pressure and take the appropriate steps to preserve central retinal artery perfusion. Lack of perfusion for even short periods can lead to permanent, devastating loss of vision.
• Surgical decompression may be required, but in most cases, surgery has only to be postponed.
• Venous puncture may occur after both retrobulbar and peribulbar injection. It leads to non-compressive hematoma, the consequences of which are much less severe so that in most cases surgery can be continued.
• For patients on anticoagulants (including even aspirin and similar medications), consider sub-Tenon’s or topical anesthesia to minimize the risk of hemorrhage.
Optic Nerve Trauma
Direct optic nerve trauma by the needle is rare but serious, as it causes blindness. Computed tomography imaging usually shows optic nerve enlargement caused by intraneural hematoma.
Summary
Overall, there is a 1%–3% chance of complications, often necessitating postponement of the planned surgery. Since some complications may be life-threatening if patients are not immediately resuscitated, it is recommended that an anesthesiologist be present to monitor the patient perioperatively.
NYSORA TIPS
Oculocardiac Reflex
• Bradycardia due to a range of stimuli in or around the orbit, such as traction on the extraocular muscles, pressure on the globe, retrobulbar nerve block, ocular trauma or pressure on residual tissues after enucleation.
• Also causes other arrhythmias including ventricular tachycardia and (rarely) asystole.
• The relevant neural pathways are branches of the trigeminal nerve (afferent) and vagus nerve (efferent). While mainly associated with stimulation of the ophthalmic nerve, it can occur with any branch of the trigeminal nerve.
• The incidence is highest in children (up to 90% without pre-treatment with atropine).
• For prophylaxis in children, atropine 0.02 mg/kg or glycopyrrolate 0.01 mg/kg prior to surgery is indicated.
• Intramuscular atropine is not useful due to delayed onset.
• Prophylaxis in adults is not indicated.
• Treatment of bradycardia includes removal of stimulus or asking the surgeon to stop the stimulation, initiation of intravenous anticholinergics (eg atropine 5-10 mcg/kg or glycopyrrolate 2.5-5 mcg/kg), and checking depth of anaesthesia (where GA is used).
TOPICAL ANESTHESIA
Instillation of LA eye drops provides corneal anesthesia, thus allowing for cataract surgery by PhE (Figure 12). This technique is quick and simple to perform and avoids the potential hazards of needle techniques. It has been suggested that the lack of akinesia and intraocular pressure control, associated with the short duration of this technique, may make surgery hazardous;90 however, this observation does not appear to have dented the growing popularity of the technique. Many US surgeons appear to prefer topical anesthesia for routine PhE in more than 90% of their cases.
Nevertheless, use of topical anesthesia should be limited to uncomplicated procedures performed by experienced surgeons in cooperative patients. In parts of the world where PhE is not technically available, and in some specific indications where PhE is not feasible, total akinesia is still required, and the use of topical anesthesia may be questionable.
As to what patients actually prefer, there are conflicting reports. Boezaart has reported that because anesthesia may be incomplete in topical approaches, patients randomly subjected to one of these techniques for one eye and another technique for the other eye preferred the retrobulbar to the topical technique (71% versus 10%).
FIGURE 12.Topical anesthesia.
Patients consistently report less intraoperative discomfort under retrobulbar or sub-Tenon’s anesthesia compared to topical anesthesia. On the other hand, a meta-analysis by Zhao et al. reported that patients overwhelmingly preferred topical anesthesia over needle nerve blocks, citing “fear of needles” as a key reason, despite finding that the same population of patients reported more intraoperative discomfort under topical anesthesia compared to needle nerve blocks. In some experienced hands, no anesthesia at all appears to be as effective as topical anesthesia in selected cases.
An intracameral injection of LA appears to substantially enhance analgesia with a topical approach. This consists of injecting small amounts (0.1 mL) of LA into the anterior chamber at the beginning of or during surgery. Intracameral anesthetic agents must be preservative free. Some concerns have been expressed about the toxicity effects of LA on the corneal endothelium, which is unable to regenerate; however, the safety of intracameral injection now seems well established. Some authors have questioned its analgesic benefit when compared with simple topical anesthesia,and indeed the degree of analgesia does not seem to correlate with intracameral LA concentration. Nevertheless, the combination of topical plus intracameral anesthesia is currently the technique most preferred by US ophthalmic surgeons.
The insertion of sponges soaked in LA into the conjunctival fornices has been proposed. The use of lidocaine jelly instead of eye drops seems to enhance the quality of analgesia of the anterior segment and is becoming very popular for improving patient comfort under topical anesthesia.
Direct Corneal Epithelial Toxicity of Topical Anesthetics
From animal studies, it has been known for some time that repeated applications of virtually all topical anesthetic agents cause some degree of transient corneal epithelial toxicity that is macroscopically apparent, including reversible corneal thickening and opacification. Changes may be evident with even a single topical application, and these may take more than an hour or more to recover. In humans, topical anesthetic abuse is a known cause of severe keratopathy among arc welders and metalworkers, and even in patients who overuse their “comfort drops” after refractive keratectomy.
Transient corneal edema has been noted in patients undergoing cataract surgery under topical ropivacaine or lidocaine. Fernandez et al. reviewed the condition of corneal epithelia by slit-lamp examination the day after cataract surgery under topical levobupivacaine or lidocaine. They found mild epithelial changes in 17–22% of patients and signs of significant epithelial damage (punctate) in 2.4–5.8%, respectively; however, no data were provided on how, when, or whether these changes resolved.
Nevertheless, to date, no cases of severe keratopathy associated with the routine administration of topical anesthesia for eye surgery have been reported. The current relative paucity of hard clinical data may in part explain the diversity of practice across continents, as practitioners may interpret what data there is to affirm their own preferences. Given the growing popularity of the technique (especially in the US), one might assume that, as for extraocular myotoxicity, any clinically significant effect is transient and may be masked by and/or attributed to the effects of surgery during convalescence.
SUBCONJUNCTIVAL ANESTHESIA
Subconjunctival injection of LA, a technique relatively unfamiliar to many anesthesiologists, provides anesthesia of the anterior segment without akinesia. Also known as “perilimbal” anesthesia, it is, in effect, a form of episcleral injection and can also be thought of as a “very anterior” or “very superficial” Sub-tenon’s nerve block. This nerve block is useful for cataract, pterygium, and superficial glaucoma surgery. After pretreatment with one drop of topical anesthetic, a fine-bore (27- to 30-gauge) needle is used to lift the superotemporal or inferotemporal conjunctiva at least 5–8 mm from the limbus (Figure 13). A surgical microscope or loupes can be used to avoid conjunctival vessels and hematoma. Once the needle is under the conjunctiva, 0.5–0.8 mL of local anesthetic solution will cause chemosis, which is dispersed with gentle, constant pressure, either using fingers or a purpose-specific weight or balloon. Hyaluronidase can be added to assist with the spread of solution and dispersal of chemosis. Compared to retrobulbar injection, this technique is less painful and reduces the need for supplemental anesthesia during cataract surgery. Injection at the superotemporal conjunctiva appears to be less painful than injection at the inferotemporal conjunctiva. Subconjunctival injection results in reliable and substantial concentrations of local anesthetic in the aqueous humor.
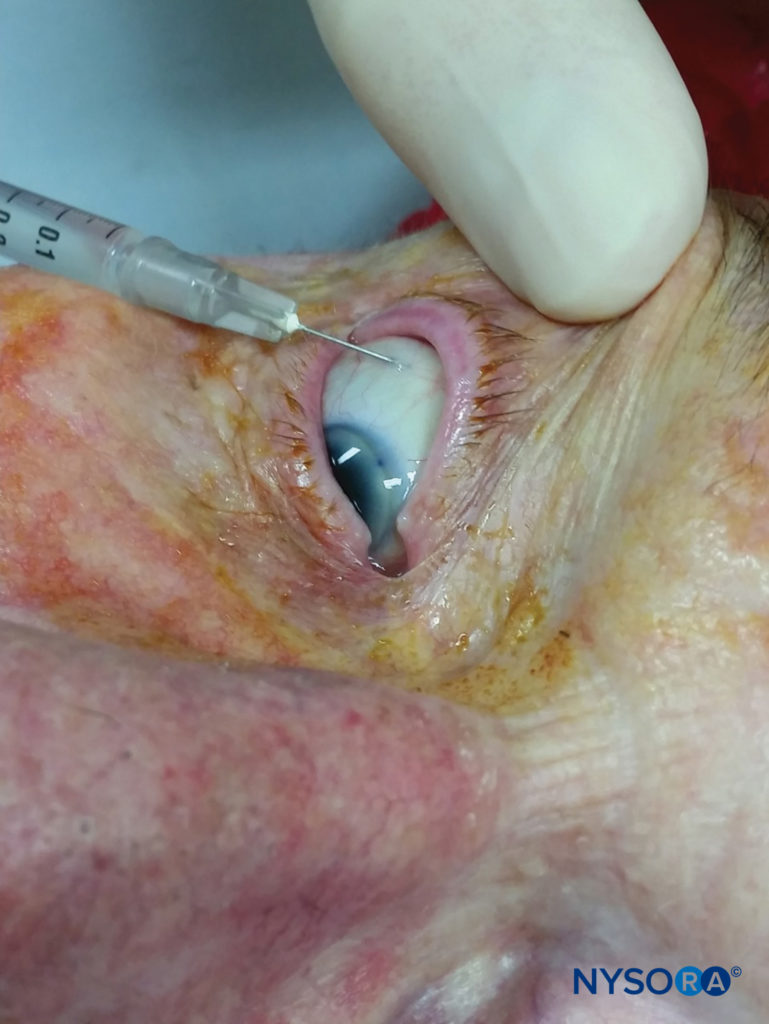
FIGURE 13. Subconjunctival block.
OCULOPLASTIC NERVE BLOCKS
Many oculoplastic procedures (Figure 14) can be performed under local anesthetic. However, given the sensitive innervation of the face, these nerve blocks are best performed under some form of transient, deep sedation (eg, a small IV dose of propofol), except in the very stoic patient. Note also that, while targeted block of individual nerves is useful, there is often significant overlap of sensory supply. Depending on the site and extent of the proposed operative wound, more than one type of nerve block may be required, and supplemental local infiltration may be necessary. Most oculoplastic surgeons are quite accustomed to this.
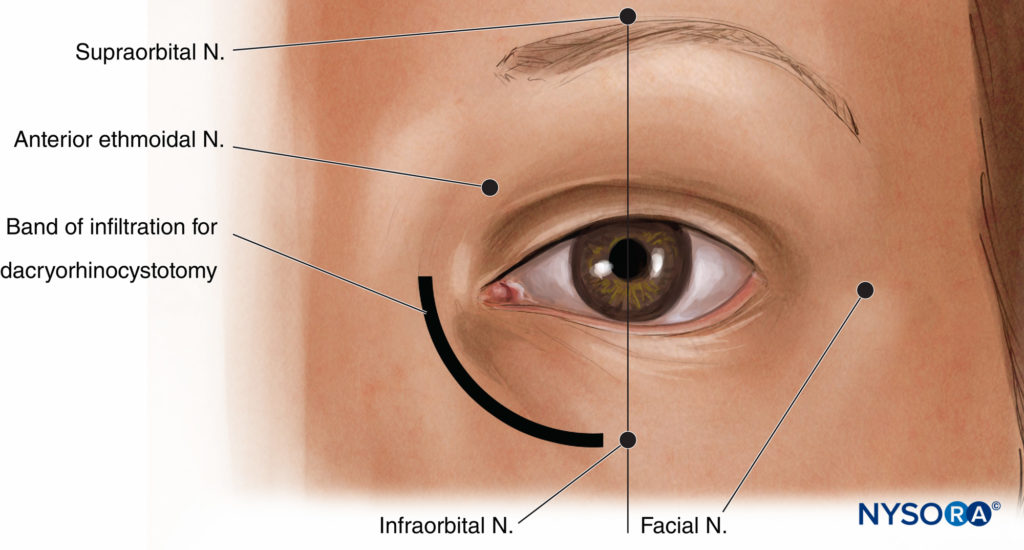
FIGURE 14. Landmarks for oculoplastic nerve blocks.
Upper and Lower Lid nerve blocks
Procedures such as upper and lower blepharoplasty and ectropion repair can be performed under gentle subcutaneous local infiltration of the base of the operative lid. Certain procedures require the surgeon to evert the eyelid under deep sedation and infiltrate distal to the semilunar fold.
Facial Nerve Block
Occasionally, a nerve block of the periocular branches of the facial nerve is required to prevent excessive blinking during eye surgery. Classical approaches include the Van Lint and O’Brien techniques; Atkinson has described a modified technique, variants of which are probably most popular today. A bleb of local anesthetic is raised about 2–3 cm lateral to the lateral rim of the orbit at the level of the lateral canthus, which is somewhere between the locations of the more distal Van Lint and the more proximal O’Brien approaches. Two radii of local infiltration are then injected superiorly and inferiorly from this point forming a “V” that catches the fibers of the facial nerve. A total of 5 mL is typically more than sufficient.
Supratrochlear/Supraorbital Nerve Block
Nerve Blocks of the supraorbital and supratrochlear nerves, branches of the ophthalmic nerve, are useful for procedures that involve the upper eyelid area immediately at or above the eyebrow. The supraorbital notch can usually be felt about 2–3 cm from the midline along the medial part of the superior rim of the orbit, usually in line with the patient’s pupil. Paresthesia can often be elicited by pressing on this notch. A volume of 2 mL of local anesthetic here should provide some anesthesia to the area immediately around the eyebrow, the upper eyelid, and the lower forehead; however, it is usually also necessary to nerve block the supratrochlear nerve by running a medial band of infiltration (an additional 2–3 mL) from the notch to the midline and approximately 1–2 cm laterally to catch lateral supraorbital fibers that can arise through a separate foramen.
Infraorbital Nerve Block
The infraorbital nerve, a major branch of the maxillary nerve, supplies sensation to the lower eyelid and the floor of the orbit. It emerges onto the face through the infraorbital foramen, which is reliably found just below the inferior orbital rim, in line with both the pupil and the supraorbital notch. A volume of 2–3 mL of local anesthetic at this point is usually more than enough. In the traditional approach to this nerve block, the needle is directed slightly upward to align with the orientation of the infraorbital canal; however, note that at least two cases of inadvertent globe penetration have been reported with this technique.
Nasocilary/Infratrochlear/Anterior Ethmoidal Nerve Block
The nasocilary nerve is a branch of the ophthalmic nerve and supplies, via its intratrochlear and anterior ethmoidal branches, sensation to the medial wall of the orbit, the proximal lacrimal sac, the proximal nasolacrimal duct, the mucosa of the nasal cavity, and much of the skin of the nose. This nerve is blocked via an injection of 2–3 mL of local anesthetic by a vertical insertion of a fine-gauge needle parallel to the medial wall of the orbit approximately in line with the bridge of the nose to a depth of about 25 mm. This corresponds with the foramen through which the anterior ethmoidal nerve exits the orbit. It is important that the needle descend freely to the point of injection, as the orbital wall is thin at this level, and perforation of the ethmoidal and even sphenoidal sinuses is quite possible.
Local Anesthesia for Tear Duct Surgery
Local anesthesia for dacryorhinocystostomy and other procedures involving the lacrimal apparatus is possible using a combination of topical anesthesia of the nasal cavity (for the nasal branches of the anterior and posterior ethmoid, sphenopalatine, and nasopalatine nerves), a nasociliary nerve block, an infraorbital nerve block, and a short band of subcutaneous infiltration along the base of the nose from the level of the medial canthus to the level of the infraorbital foramen.
USE OF EYE NERVE BLOCK FOR POSTOPERATIVE ANALGESIA
Regional anesthesia, especially the sub-Tenon’s nerve block, has been proposed as a treatment for postoperative pain.122 However, this is not required for anterior segment surgery, which usually results in minimal or no discomfort postoperatively.
NYSORA TIPS
Postoperative Pain
• Significant pain occurring after cataract surgery is unusual and should raise suspicion for increased intraocular pressure, severe inflammation, or infection; the eye should be examined by an ophthalmologist.
• Postoperative pain is more likely after posterior segment surgery. The use of an indwelling retrobulbar, peribulbar, or sub-Tenon’s catheter has been proposed to improve intraoperative anesthesia, prolong postoperative regional analgesia, and treat intractable eye pain.
CHOICE OF LOCAL ANESTHETIC AND ADJUVANT AGENTS
All available LAs have been used for eye nerve block, either alone or as a combination of two agents. The injected LAs used most often are lidocaine, bupivacaine, ropivacaine, mepivacaine, or a combination of two of these. The choice of LAs should be based on the pharmacologic properties and availability of the drugs, with the primary consideration being the requirement for quick onset (lidocaine, mepivacaine), prolonged effect, or postoperative residual nerve block for analgesia (ropivacaine, bupivacaine) or akinesia (higher concentration). Because the amount of LA injected is usually small (3–11 mL), systemic toxicity is not a major concern.
Septocaine (articaine) is a relatively novel amide-type LA, commonly used in dentistry, with an emergent use in eye nerve blocks. It has a faster onset than bupivacaine/lidocaine preparations in peribulbar and sub-Tenon’s nerve blocks and, because it appears to diffuse through tissues more readily than other LAs, often results in a denser nerve block. There appears to be no clinical advantage of 4% over 2% articaine, at least in dental anesthesia. Despite concerns about its neurotoxic potential in animal studies, there does not seem to any conclusive evidence that it is more toxic than other high-concentration LAs.
Hyaluronidase is an enzyme that has been widely used to facilitate the spread of local anesthetic through connective tissue, thereby improving both the onset and success rate of regional anesthesia for the eye. Another benefit is a reduced incidence of postoperative strabismus connected with its use, possibly by limiting LA myotoxicity owing to its faster spread.
Some authors are less confident about the real benefit of hyaluronidase in improving akinesia. There are also significant concerns about its allergenic potential; however, these may be due to the impurities intrinsic to traditional animal-derived hyaluronidase; newer human recombinant products have been shown to be not only more potent but also to have higher enzyme purity and to be virtually free of hypersensitivity reactions. It would appear that, as the requirement for akinesia relaxes, adjuvants such as hyaluronidase are becoming less popular. Nevertheless, in situations where akinesia is required, hyaluronidase may still have an important role.
α2-adrenergic agonists are well known to ophthalmologists as topical agents that reduce intraocular pressure in glaucoma.
Clonidine has been shown to enhance intraoperative anesthesia and postoperative analgesia when added to the LA in a range of eye nerve blocks. At a dose of 1 mcg/kg, clonidine does not increase the incidence of systemic adverse events such as hypotension or excessive sedation. Moreover, it may help to prevent intraoperative arterial hypertension and lower intraocular pressure. Dexmedetomidine, a more highly selective α2 agonist, has been used increasingly as an effective adjuvant in peripheral nerve blocks, including the sub-Tenon’s nerve block. It has been proposed that the addition of α2 agonists may allow for surgical anesthesia at lower concentrations of LA, thereby limiting
LA-induced myotoxicity. It should be noted that all studies on α2 adjuvants to date have been relatively low-powered, and, while this is a promising area of study, substantially higher sample sizes are required. Epinephrine is sometimes used to increase the duration of eye nerve block. However, the availability of long-acting LAs and the fear of vasospasm with subsequent retinal ischemia have decreased its use. “Wipe-out” (a sudden total sight loss not overtly related to ocular pathology) is a rare but serious risk, particularly in glaucoma patients. LA solutions containing epinephrine have been implicated as a contributing factor.
Alkalinization of local anesthetic solutions has been proposed for decreasing pain during injection and accelerating nerve block onset; however, its efficacy remains unproven. Other adjuvant agents have been proposed but have not gained popularity. Small doses of a muscle relaxant may enhance akinesia, but concern has been expressed about their potential risk for systemic effects.
Opioids do not appear to be more efficient via a regional ophthalmic route than via systemic administration.
Warming the LA may decrease pain on injection and enhance nerve block efficacy, although these benefits do not appear to be clinically relevant.
WHO SHOULD PERFORM EYE NERVE BLOCKS?
Since the 1980s, anesthesiologists have become increasingly involved in eye nerve blocks that previously were performed by surgeons. However, in some countries, anesthesiologists are not available, and surgeons have to manage the nerve block themselves. In other countries, anesthesiologists monitor only the anesthesia care, as the surgeon performs the nerve block. In many developed countries (eg, France and Australia), anesthesiologists are often responsible for administering eye nerve blocks. The same was true in the United Kingdom; however, recent cost constraints have forced managers to reconsider the role of the anesthesiologist in elective ophthalmic procedures that do not require a general anesthetic. There are currently no hard data to support or refute the idea that having the anesthesiologist performing eye nerve blocks, rather than the surgeon, or even having the anesthesiologist in attendance, is safer for this patient population. However, the available literature does suggest that with proper training, anesthesiologists can perform eye nerve blocks with the same degree of safety as in other regional anesthesia techniques. Having one experienced person performing nerve blocks while another operates is more time efficient, and there is a theoretical benefit to having a person skilled in resuscitation present in the unlikely event of a life-threatening complication (which, unsurprisingly, materializes into an actual benefit whenever such a complication occurs).
PERIOPERATIVE MANAGEMENT OF PATIENTS UNDERGOING EYE NERVE BLOCK
Patient Selection and Assessment
Older patients undergoing eye surgery frequently have coexisting diseases such as diabetes mellitus, hypertension, coronary artery disease, or cardiac insufficiency. A preoperative assessment should be routinely done to ensure that coexisting medical conditions are reasonably well controlled. Despite the low morbidity and mortality associated with local ophthalmic anesthesia, patients should be carefully screened for their eligibility for surgery. Patients with severe kyphosis or scoliosis pose obvious practical problems for microscopic surgery. Patients who may not be able to lie flat for the requisite period, due to cardiac or respiratory insufficiency, neurological disease, or dementia, are also challenging. Once draped, patients with profound deafness may be unable to respond to intraoperative commands unless carefully briefed preoperatively.
Briefing Patients
Given the potential problems of excessive sedation (see below), an empathic and interactive preoperative briefing is a useful tool for both reducing anxiety and optimizing cooperation during eye surgery. Patients should be given a clear explanation of what will be done to them, what they are likely to experience, and what they may be asked to do during the operation, in language appropriate to their level of understanding.
Monitoring
Intraoperative monitoring should include basic monitoring (ie, electrocardiogram, pulse oximetry, and automated noninvasive blood pressure measurement). Intravascular access is required for any invasive anesthetic technique.
Sedation
Eye surgery (eg, cataract surgery) carries a low risk of perioperative morbidity and mortality. Eye nerve block is associated with lower perioperative morbidity than is general anesthesia used for ophthalmic surgery, provided that heavy sedation is avoided. Anxiety and residual pain frequently occur during eye surgery under LA. Perfect immobility is required, and the presence of drapes over the head increases patient anxiety and impairs access to the airway. The patient should be positioned as comfortably as possible, with sufficient space to allow free breathing. Intraoperative sedation with judicious doses of sedatives may be used to limit anxiety and pain. However, an excess of sedation may lead to restlessness, sleeping, snoring, or respiratory depression, which, in the absence of any airway access, pose a significant intraoperative challenge. Maintenance of meaningful patient contact is of paramount importance to avoid disasters that can occur with disoriented or combative patients while the surgery is underway.
Another interesting and dangerous phenomenon associated with ophthalmic needle nerve blocks performed under propofol sedation is involuntary sneezing, which may occur in up to one-fifth of patients during injection; this reaction is sight-threatening, as it can lead to accidental globe perforation. It is more likely to occur in patients who are male, have a history of photic sneezing (sneezing with sudden exposure to bright light or sunshine), are under deeper sedation, or who are given concurrent midazolam. It does not appear to occur in patients undergoing nerve blocks with remifentanil sedation or with no sedation at all. Awareness of this phenomenon should better prepare operators to withdraw the nerve block needle immediately if a sneeze is imminent and possibly reconsider their current sedation practice. Dexmedetomidine, a highly selective α-agonist, is gaining some popularity as a sedative agent in a range of clinical settings, including eye surgery.
Do Patients Undergoing Eye Nerve Block Need to Fast?
Fasting practices for patients having procedures under topical, local, or regional nerve block have undergone an evolution in recent years. In the complex machine of public-sector medical practice, the well-intentioned and traditional “NPO from midnight” doctrine often leads to patients fasting well in excess of 12 hours. In addition to an increased likelihood of postoperative nausea and vomiting, inappropriately prolonged fasting can cause discomfort and distress, as well as dehydration and metabolic derangements, all of which can make patients (especially children and the elderly) restless and uncooperative. Moreover, while there is an obvious theoretical case for fasting prior to any surgical procedure, there have been no reported cases of aspiration under local anesthesia during cataract operations. The joint view of the learned colleges in the United Kingdom is that fasting is generally not required for patients undergoing eye surgery without sedation. Moreover, at least one experienced ophthalmic center has performed nerve blocks under “judicious doses” (0.5–2 mg) of IV midazolam in over 5000 unfasted patients without untoward incident. Nevertheless, where it is anticipated that deep sedation or general anesthesia, however brief, will be needed either to perform the nerve block or during the operation, then standard preoperative fasting remains a sensible prerequisite, and, where needed, systemic measures should be explored to minimize inappropriately prolonged fasting. Where fasting is not routinely required, a small proportion of problematic patients will present for surgery unfasted but for whom the need for deep sedation becomes obvious once they are in the operating room. Individual institutions need to audit the prevalence of this phenomenon and to make cost and risk-benefit decisions accordingly. A comprehensive and compassionate preoperative patient briefing may have an important role in minimizing this issue.
Is Intravenous Access Required in Patients Undergoing Eye Nerve Block?
In recent years, many centers, particularly those performing office-based eye procedures, have abandoned the traditional requirement of securing IV access for all surgical procedures, provided that procedures are performed under “needleless” anesthesia (ie, topical anesthesia or sub-Tenon’s nerve block). According to a joint statement of the Royal College of Anaesthetists and the Royal College of Ophthalmologists in the UK, “Intravenous access … is essential with peribulbar and retrobulbar nerve blocks and when intraoperative sedation is used” and “recommended for long/complex cases, sub-Tenon’s nerve blocks, and patients with poor general health.” In relation to this, the author is in agreement with Guise in making the following observations: (1) Sedation may occasionally be required at very short notice by patients even under topical anesthesia, and prior IV access makes timely sedation easier and less stressful; (2) vasovagal and other dysrhythmic reactions, while uncommon, may also occur, in which case IV access already in situ is beneficial for both patient and resuscitator; and (3) it is a prudent clinical precaution, and no real trouble, if a person experienced in IV cannulation (such as an anesthesiologist) is in attendance.
Eye Nerve Blocks and the Anticoagulated Patient
Traditionally, anesthesiologists would often make a formulaic decision whether to perform an ophthalmic regional nerve block (which was usually a retrobulbar or peribulbar nerve block) based on results of a blood-clotting profile and by nominating an arbitrary cut-off point (eg, an international normalized ratio [INR] of 1.5–2.0 or a platelet count of 50–100). Nowadays, however, there is not only a diversity of anticoagulant therapies (several of which defy easy assessment), but also a range of anesthetic management options. Moreover, the risks of discontinuing anticoagulant therapy in some patients may outweigh the risks of perioperative bleeding on full anticoagulation. Consequently, there is no clear algorithm for managing “patients undergoing eye surgery who are on anticoagulants.” It is best to make a balanced benefit-versus-risk decision in each case based on a number of fundamental questions:
• Which anticogulant(s) is the patient taking? A variety of agents are now available. While there are established management strategies for drugs such as warfarin and heparin, strategies for others are less straightforward.
Dabigatran, for example, is a novel long-acting oral anticoagulant with no direct antidote. The perioperative management of potential bleeding in patients on this drug is currently a matter of much concern, including those undergoing eye surgery. Since there are currently no data on patients on dabigatran undergoing eye nerve block, including sub-Tenon’s nerve block, current recommendations are to withhold the drug for two to five days prior to performing the nerve block and surgery.
• Why is the patient being anticoagulated? Occasionally, patients self-initiate aspirin intake (eg, because they have read about it in a magazine). It is common for patients to be treated with warfarin for acute-onset atrial fibrillation that spontaneously reverts. Such situations make it easier to consider withholding the drug rather than choosing not to perform the nerve block.
• Is the patient in a high-risk group (eg, with a documented history of thromboembolism)? Often, there is not only a clear clinical indication for anticoagulation, but also a clear increased risk if perioperative anticoagulation is not managed correctly, and therefore a greater imperative to use a technique that does not require the patient’s anticoagulant therapy to be modified. When in doubt, consult with the medical team that started the patient on anticoagulant therapy.
• What are the relative bleeding risks of the procedure proposed? Here, the team should consider both the surgical and anesthetic procedures. Oculoplastic and glaucoma surgeries tends to carry a higher bleeding risk, whereas most PhE and vitreoretinal procedures can be done on full anticoagulation (however, discuss individual cases with the surgeon, as the decision is ultimately his or hers).
Generally, topical, subconjunctival, and sub-Tenon’s nerve blocks are considered low risk, and the limited evidence to date supports going ahead with these techniques without altering the patient’s routine anticoagulant therapy. Lowpowered studies suggest that, while minor bleeding complications such as subconjunctival hematoma are more frequent with sub-Tenon’s nerve blocks performed in patients on aspirin, warfarin, or clopidogrel, major complications such as sight-threatening bleeding are not. Retrobulbar and peribulbar nerve blocks are considered high risk.
• What are the surgical requirements for anesthesia? Where akinesia is not required for anterior segment surgery, topical or subconjunctival techniques are more attractive. If posterior segment anesthesia and/or akinesia are needed and there are no other contraindications, sub-Tenon’s nerve block is now seen to be the safest option.
• Is it an elective case or a true emergency? Where there is time to take a more gradual approach that avoids the use of antidotes and prothrombotic agents, this opportunity should be taken.
Working with High-Volume Surgeons
Running a high-turnover cataract list (20 or more patients a day) safely and efficiently is both a science and an art form requiring not only consummate individual technical skill but also good teamwork and communication skills, as well as attention to ergonomic detail. A good surgeon will include all members of his or her theater team in the planning and execution of the list. The anesthesiologist’s role is to support the team both technically and tactfully, monitoring the whole team and signaling when the team should slow down or even stop. Standardization wherever possible—from role allocation and patient trolley positioning to the vocabulary used to describe equipment and procedures—helps to streamline activities and to make deviations from routine more salient. The use of a modified World Health Organization (WHO) surgical safety checklist in a fast-paced eye theater may also help to detect anomalies and reduce errors. Regular feedback and discussion of individual and team performance helps to promote kaizen: the approximation of perfection through small, continuous, incremental improvements over time.
SUMMARY
In developed countries, eye surgery is among the most frequently performed surgical procedures requiring anesthesia.
During the past 20 years, anesthesiologists have assumed a growing role in performing eye nerve blocks. The requirement for a deep anesthetic nerve block with total akinesia has been greatly lessened through the use of PhE for cataract surgery, giving a more prominent role to topical anesthesia.
Needle Nerve blocks carry a low but real risk of serious complications, mainly the result of needle misplacement. Training and practice are required to minimize such problems. The use of the sub-Tenon’s nerve block reduces the risks of needle nerve blocks and is now an established and popular technique, although it does not completely prevent complications. The right anesthetic technique is informed by the anesthesiologist’s skill, experience, and understanding of the surgical procedure and the individual surgeon’s preferences.
Tips from the Compendium of Regional Anesthesia
- Perfect immobility is required. Therefore, the patient should be positioned as comfortably as possible, with sufficient space to allow free breathing.
- Intraoperative sedation with judicious doses of sedatives may be used to limit anxiety and pain. However, an excess of sedation may lead to restlessness, sleeping, snoring, or respiratory depression, which, in the absence of any airway access, pose a significant intraoperative challenge.
- Maintenance of meaningful patient contact is of utmost importance to avoid disasters that can occur with disoriented or combative patients.
- Fasting is generally not required for patients undergoing eye surgery without sedation. Nevertheless, standard preoperative fasting remains a prerequisite when it is anticipated that deep sedation or general anesthesia, however brief, will be needed (either to perform the block or during surgery).
- Oculoplastic and glaucoma surgeries tend to carry a higher bleeding risk, whereas most phacoemulsification and vitreoretinal procedures can be done on full anticoagulation (however, discuss individual cases with the surgeon).
- Topical, subconjunctival, and sub-Tenon’s blocks have a low bleeding risk, whereas retrobulbar and peribulbar blocks are considered high-risk.
APPENDIX: THE OCULOCARDIAC REFLEX
The oculocardiac reflex, first described in 1908; is a decrease in heart rate over 20% below baseline values often seen during traction on the extrinsic muscles of the eye, particularly during strabismus surgery. However, it can also occur during the administration of eye nerve blocks, particularly retrobulbar nerve block.
Other triggers include ocular trauma, intraorbital hematoma, acute glaucoma, a sudden increase in intraocular pressure, stretching of the eyelid muscles, and conjunctiva. In unprepared subjects, bradycardia can be quite dramatic, and occasionally leading to asystole in the patient (and tachycardia in the anesthesiologist), which lasts as long as the triggering stimulation is applied. While sinus slowing of the heart is classical, any arrhythmia including ventricular fibrillation may occur.
The reflex is exaggerated in children (in up to 90% without pretreatment) and in the presence of hypoventilation, hypoxemia, and acidosis.
The reflex arc has been well described (Figure 15), the afferent limb consisting of the long and short branches of the ciliary nerves, the ophthalmic nerve, the geniculate ganglion, and the main sensory nucleus of the trigeminal nerve. Short internuncial fibers then convey the reflex to the efferent limb, consisting of the motor nucleus of the vagus, the vagus nerve, and the cardiac depressor nerve. The oculocardiac reflex is a specific variant of the trigeminocardiac reflex, a wider constellation of brainstem-mediated reactions—bradycardia, sweating, breath holding, yawning, sneezing, and so on—triggered by stimulation of any of the sensory branches of the trigeminal nerve (primarily the ophthalmic nerve).
The best treatment in children is prophylaxis: IV atropine 10–20 mcg/kg or glycopyrrolate 5–10 mcg/kg on induction. Intramuscular agents are less useful due to their delayed onset. Prophylaxis in adults is usually not indicated; however, it is prudent to have an IV anticholinergic predrawn and available.
Acute treatment consists of removing the stimulus: Ask the surgeon to stop whatever they are doing. If the pulse does not improve, give an anticholinergic (eg, IV atropine 5–10 mcg/kg or glycopyrrolate 2.5–5 mcg/kg). Check the depth of anesthesia in cases of GA.
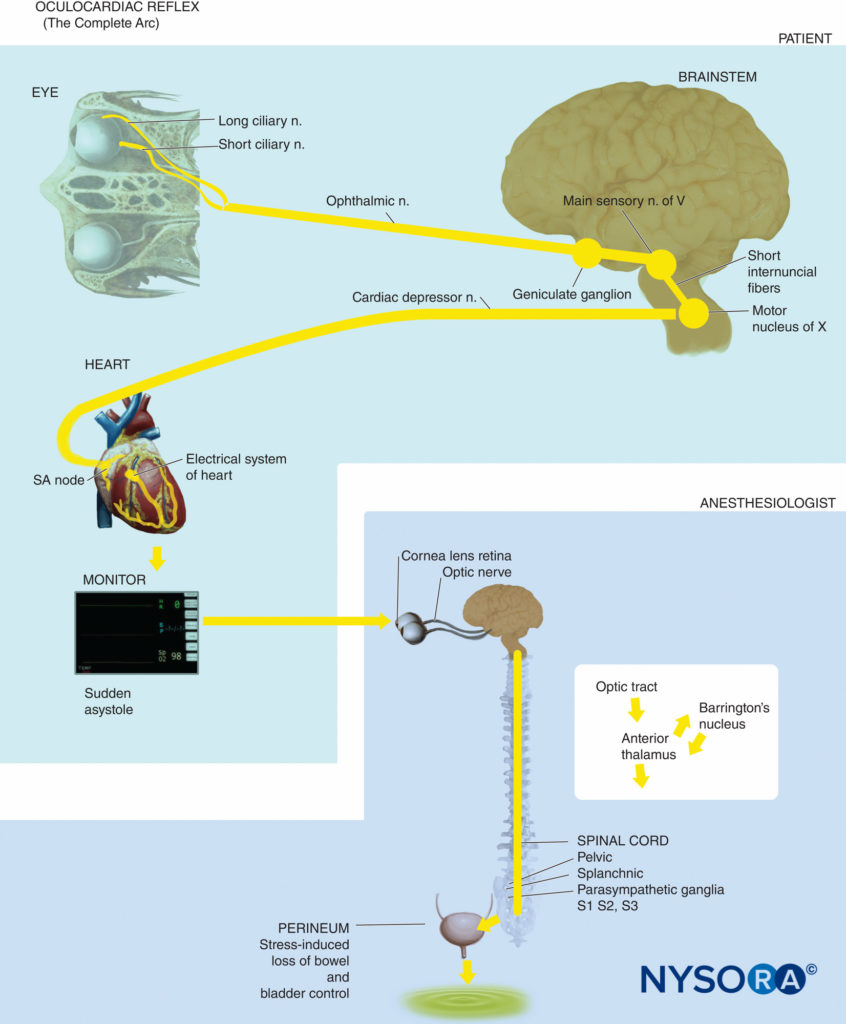
FIGURE 15. Oculocardiac reflex arc.
Acknowledgments
The author would like to thank Jacques Ripart, Kenneth Mehbridge, and Robert Della Rocca for their first-edition version of this chapter and without whom this revision would not have been possible. Thanks with deep gratitude also to Keith Allman, Tom Eke, Phil Guise, and Chandra Kumar, all of whom devoted substantial time and effort to offer invaluable suggestions and additional references.
*Sources: Aschner B. Ueber einen bisher noch nicht beschrieben Reflex vom Auge auf kreislauf and Atmung. Verschwinden des Radialpulsen bei Druck aut des auge. Wiener Klinische Wochenschrift 1908;21:1529–1530; Dagnini G. Intemo ad un riflesso provocato in alcuni emiplegici collo stimolo della come e colla pressione sul bulbo oculare. Bull Sci Med 1908;8:380–381.
Continue reading Nerve Blocks of the Face
REFERENCES
- Leaming DV: Practice styles and preferences of ASCRS members—2003 survey. J Cataract Refract Surg 2004;30:892–900.
- Gild WM, Posner KL, Caplan RA, et al: Eye injuries associated with anesthesia. A closed claims analysis. Anesthesiology 1992;76:204–208.
- Quigley HA: Mortality associated with ophthalmic surgery: A 20-year experience at the Wilmer Institute. Am J Ophthalmol 1974;77:517–524.
- Breslin PP: Mortality in ophthalmic surgery. Int Ophthalmol Clin 1973; 13:215–226.
- McKibbin M: The pre-operative assessment and investigation of ophthalmic patients. Eye 1996;10:138–140.
- Bass EB, Steinberg EP, Luthra R: Do ophthalmologists, anesthesiologists, and internists agree about preoperative testing in healthy patients undergoing cataract surgery? Arch Ophthalmol 1995;113:1248–1256.
- Maltzman BA, Cinotti AA, Calderone JP Jr: Preadmission evaluation and elective cataract surgery. J Med Soc N J 1981;78:519–520.
- Gilvarry A, Eustace P: The medical profile of cataract patients. Trans Ophthalmol Soc UK 1982;102:502–504.
- Fisher SJ, Cunningham RD: The medical profile of cataract patients. Clin Geriatr Med 1985;1:339–344.
- Hardesty DC: The ophthalmic surgical patient. In Wolfsthal S (ed): Medical Perioperative Management. Norwalk, CT: Appleton & Lange,
1989, pp 417–426. - Hodgkins P, Luff A, Morrell A: Current practice of cataract extraction and anaesthesia. Br J Ophthalmol 1992;76:323–326.
- Hamilton R, Gimble H, Strunin L: Regional anaesthesia for 12,000 cataract extraction and intraocular lens implantation procedures. J Can Anaesth 1988;35:615–623.
- Eke T, Thompson J: The national survey of local anaesthesia for ocular surgery. II. Survey methodology and current practice. Eye 1999;13: 196–204.
- Eichel R, Goldberg I: Anaesthesia techniques for cataract surgery: a survey of delegates to the Congress of the International Council of Ophthalmology, 2002. Clin Exp Ophth 2005;33:469–472
- Thampy R, Hariprasad M, Saha B: Local anaesthesia for ophthalmic surgery: a multicentre survey of current practice amongst anaesthetists in the North West. Anaesthesia 2007;62:101–103.
- Vohra SB, Murray PI: Sub-tenon’s block. a national United Kingdom survey. Ophth Surg Lasers Imag 2008;39:379–385.
- Leaming DV, Duffey R: Highlights of the 2008 ASCRS Member Practice Style Survey. http://ascrs2009.abstractsnet.com/handouts/000101_Highlights_of_the_2008_ASCRS_member_practice_style.ppt. Accessed June 29, 2013.
- Leaming DV: Surveys of American Society of Cataract and Refractive Surgery members 1999–2012. www.analeyz.com. Accessed June 29, 2013.
- Williams PL (ed): Gray’s Anatomy, 38th ed. London: Churchill Livingstone, 1995; passim but esp. pp 1225–1241, 1321–1366.
- Ropo A, Nikki P, Ruusuvaara P, et al: Comparison of retrobulbar and periocular injections of lignocaine by computerized tomography. Br J Ophthalmol 1991;75:417–420.
- Koornneef L: The architecture of the musculo-fibrous apparatus in the human orbit. Acta Morphol Neerl Scand 1977;15:35–64.
- Koornneef L: Details of the orbital connective tissue system in the adult. Acta Morphol Neerl Scand 1977;15:1–34.
- Ripart J, Lefrant J, de La Coussaye J, et al: Peribulbar versus retrobulbar anesthesia for ophthalmic surgery. An anatomical comparison of extraconal and intraconal injections. Anesthesiology 2001;94:56–62.
- Edward DP, Kaufmann LM: Anatomy, development and pathology of the visual system. Pedatr Clin North Am 2003;50:1–23.
- Bloomberg L: Administration of periocular anesthesia. J Cataract Refract Surg 1986;12:677–679.
- Davis D, Mandel M: Posterior peribulbar anesthesia: an alternative to retrobulbar anesthesia. J Cataract Refract Surg 1986;12:182–184.
- Spaeth GL, Danesh-Meyer H, Goldberg I, Kampik, A (eds): Ophthalmic Surgery: Principles and Practice, 4th ed. Amsterdam, Netherlands: Elsevier, 2012.
- Ortiz M, Blanco D, Serra J, Vidal F: Peribulbar anaesthesia: the role of local anaesthetic volumes and Thiomucase in motor block and intraocular pressure. Eur J Anaesthesiol 1995;12:603–607.
- Wong DH: Regional anaesthesia for intraocular surgery. Can J Anaesth 1993;40:635–657.
- Rowley SA, Hale JE, Finlay RD: Sub-Tenon’s local anaesthesia: the effect of hyaluronidase. Br J Ophthmalmol 2000;84:435–436.
- Aziz ES, Rageh M: Deep topical fornix nerve block versus peribulbar block in one-step adjustable suture horizontal strabismus surgery. Br J Anaesth 2002;88:129–132.
- Scott IU, McCabe CM, Flynn HW: Local anesthesia with intravenous sedation for surgical repair of selected open globe injuries. Am J Ophthalmol 2002;134:707–711.
- Amadasun FE, Isele TO: Vitreous humour extrusion after suxamethonium induction of anaesthesia in a polytraumatized patient: a case report. Case Rep Med 2010;2010:913763.
- Ripart J, Prat-Pradal D, Charavel P, et al: Medial canthus single injection episcleral (sub-Tenon) anesthesia anatomic imaging. Clin Anat 1998;11:390–395.
- Ripart J, Metge L, Prat-Pradal D, et al: Medial canthus single injection episcleral (sub-Tenon) anesthesia computed tomography imaging. Anesth Analg 1998;87:43–45.
- Ripart J, Lefrant JY, Vivien B, et al: Ophthalmic regional anesthesia: medial canthus episcleral (sub-tenon) anesthesia is more efficient than peribulbar anesthesia: a double-blind randomized study. Anesthesiology
2000;92:1278–1285. - Li H, Abouleish A, Grady J, et al: Sub-Tenon’s injection for local anesthesia in posterior segment surgery. Ophthalmology 2000;107:41–47.
- Mein C, Flynn HW: Augmentation of local anesthesia during retinal detachment surgery [letter]. Arch Ophthalmol 1989;107:1084.
- Stevens JD: A new local anaesthesia technique for cataract extraction by one quadrant sub-Tenon’s infiltration. Br J Ophthalmol 1992;76: 670–674.
- MacNeela BJ, Kumar CM: Sub-Tenon’s block using ultrashort cannula. J Cataract Refract Surg 2004;30:858–862.
- Kumar CM, Mac Neela BJ: Ultrasonic localization of anaesthetic fluids using sub-Tenon’s cannulae of three different lengths. Eye 2003;17:1–5.
- Guise P: Sub-Tenon’s anesthesia: a prospective study of 6000 blocks. Anesthesiology 2003;98:964–968.
- Heatley CJ, Marshall J, Toma M: “A Thrip to eye casualty” an unusual complication of sub-Tenon’s anaesthesia. Eye 2006;20:738–739.
- Costen MT, Bolton K, Boase DL: Lash foreign body complicating sub-Tenon’s anaesthesia. Eye 2004;18:192–193.
- Allman KG, Theron AD, Bayles DB: A new technique of incisionless minimally invasive sub-Tenon’s anesthesia. Anaesthesia 2008;63: 782–783.
- Nouvellon E, L’Hermite J, Chaumeron A, et al: Medial canthus single injection sub-Tenon’s anesthesia: a 2000 case experience. Anesthesiology 2004;100:370–374.
- Guise P: Sub-Tenon’s anesthesia: an update. Local Reg Anesth 2012;5: 35–46.
- Ciulla TA, Starr MB, Masket S: Bacterial endophthalmitis prophylaxis for cataract surgery: an evidence-based update. Ophthalmology 2002;109:13–24.
- Atkinson W: Retrobulbar injection of anesthetic within the muscular cone (cone injection). Arch Ophthalmol 1936;16:495–503.
- Liu C, Youl B, Moseley I: Magnetic resonance imaging of the optic nerve in the extremes of gaze. Implications for the positioning of the globe for retrobulbar anaesthesia. Br J Ophthalmol 1992;76:728–733.
- Unsöld R, Stanley J, Degroot J: The CT-topography of retrobulbar anesthesia. Anatomic-clinical correlation of implications and suggestion of a modified technique. Albrecht Von Graefes Arch Klin Exp Ophthalmol 1981;217:125–136.
- Hamilton R, Loken R: Modified retrobulbar block. Can J Anaesth 1993;40:1219–1220.
- Galindo A, Keilson L, Mondshine R, et al: Retro-peribulbar anesthesia: special technique and needle design. Ophthalmol Clin North Am 1990;3:71–81.
- Chang WM, Stetten GD, Lobes LA Jr, Shelton DM, Tamburo RJ: Guidance of retrobulbar injection with real-time tomographic reflection. J Ultrasound Med 2002;21:1131–1135.
- Luyet C, Eichenberger U, Moriggl B, Remonda L, Greif R: Real-time visualization of ultrasound-guided retrobulbar block: an imaging study. Br J Anaesth 2008;101:855–859.
- Guyer S, Shaw ES: Ocular ultrasound guided anaesthesia. In Singh AD (ed): Ophthalmic Ultrasonography. Amsterdam, Netherlands: Elsevier, 2012.
- Kumar C, personal communication, June 23, 2013.
- Bloomberg L: Anterior periocular anesthesia: five years experience.
J Cataract Refract Surg 1991;17:508–511. - Davis D, Mandel M: Efficacy and complication rate of 16224 consecutive peribulbar blocks. A prospective multicenter study. J Cataract Refract Surg 1994;20:327–337.
- Hustead R, Hamilton R, Loken R: Periocular local anesthesia: medial orbital as an alternative to superior nasal injection. J Cataract Refract Surg 1994;20:197–201.
- Wang BC, Bogart B, Hillman DE, et al: Subarachnoid injection—a potential complication of retrobulbar block. Anesthesiology 1989;71: 845–857.
- Kumar CM, Lawler PG: Pulmonary oedema after peribulbar block. Br J Anaesth 1999;82:777–779.
- Demirok A, Simsek S, Cinal A, et al: Peribulbar anesthesia: one versus two injections. Ophthalmic Surg Lasers 1997;28:998–1001.
- Ball JL, Woon WH, Smith S: Globe perforation by the second peribulbar injection. Eye 2002;16:663–665.
- Sarvela J, Nikki P: Comparison of two needle lengths in regional ophthalmic anesthesia with etidocaine and hyaluronidase. Ophthalmic Surg 1992;23:742–745.
- Karampatakis V, Natsis K, Gisgis P, Stangos N: The risk of optic nerve injury in retrobulbar anesthesia: a comparative study on 35 and 40 mm retrobulbar needles in 12 cadavers. Eur J Ophthalmol 1998;8:184–187.
- Katsev D, Drews RC, Rose BT: Anatomic study of retrobulbar needle path length. Ophthalmology 1989;96:1221–1224.
- Waller S, Taboada J, O’Connor P: Retrobulbar anesthesia risk: do sharp needles really perforate the eye more easily than blunt needles? Ophthalmology 1993;100:506–510.
- Demediuk O, Dhaliwal R, Papworth D, et al: A comparison of peribulbar and retrobulbar anesthesia for vitreoretinal surgical procedures. Arch Ophthalmol 1995;113:908–913.
- Aldrete J, Romo-Salas F, Arora S, et al: Reverse arterial blood flow as a pathway for central nervous system toxic responses following injection of local anesthetics. Anesth Analg 1978;57:428–433.
- Singer SB, Preston R, Hodge WG: Respiratory arrest following peribulbar anesthesia for cataract surgery: case report and review of literature. Can J Ophthalmol 1997;32:450–454.
- Loken R, Mervyn Kirker GE, Hamilton RC: Respiratory arrest following peribulbar anesthesia for cataract surgery: case report and review of the literature. Can J Ophthalmol 1998;33:225–226.
- Nicoll J, Acharya P, Ahlen K, et al Central nervous system complication after 6000 retrobulbar blocks. Anesth Analg 1987;66:1298–1302.
- Duker J, Belmont J, Benson W, et al: Inadvertent globe perforation during retrobulbar and peribulbar anesthesia. Ophthalmology 1991; 98:519–526.
- Edge R, Navon S. Scleral perforation during retrobulbar and peribulbar anesthesia. Risk factor and outcome in 50,000 consecutive injections. J Cataract Refract Surg 1999;25:1237–1244.
- Vohra S, Good P: Altered globe dimensions of axial myopia as risk factors for penetrating ocular injury during peribulbar anaesthesia. Br J Anaesth 2000;85:242–245.
- Carlson B, Rainin E: Rat extraocular muscle regeneration. Repair of local anesthetic-induced damage. Arch Ophthalmol 1985;103:1373–1377.
- Porter JD, Edner DP, et al Extraocular myotocxicity of the retrobulbar anesthetic bupivacaine hydrochloride. Inv Ophth Vis Sci 1988;29: 163–174.
- Gomez-Arnau JI, Yanguela J, Gonzalez A, et al: Anaesthesia-related diplopia after cataract surgery. Br J Anaesth 2003;90:189–193.
- Han SK, Kim JH, Hwang JM: Persistent diplopia after retrobulbar anesthesia. J Cataract Refract Surg 2004;30:1248–1253.
- Taylor G, Devys JM, et al: Early exploration of diplopia with magnetic resonance imaging after peribulbar anaesthesia. Br J Anaesth 2004;92: 899–901.
- Zhang C, Phamonvaechavan P, et al: Concentration-dependent bupivacaine myotoxicity in rabbit extraocular muscle. J AAPOS 2010;14:323–327.
- Yagiela JA, Benoit PW, et al. Comparison of myotoxic effects of lidocaine with epinephrine in rats and humans. Anesth Analg 1981;60:471–480.
- Scott AB, Alexander DE, Miller JM: Bupivacaine injection of eye muscles to treat strabismus. Br J Ophthalmol 2007;91:146–148.
- Zink W, Graf BM: Local anesthetic myotoxicity. Reg Anesth Pain Med 2004;29:333–340.
- Nouette-Gaulain K, Dadure C, et al. Age-dependent bupivacaine-induced muscle toxicity during continuous peripheral nerve block in rats. Anesthesiology 2009;111:1120–1127.
- Edge K, Nicoll M: Retrobulbar hemorrhage after 12,500 retrobulbar blocks. Anesth Analg 1993;76:1019–1022.
- Hersch M, Baer G, Diecker JP, et al. Optic nerve enlargement and central retinal-artery occlusion secondary to retrobulbar anesthesia. Ann Ophthalmol 1989;21:195–197.
- Rubin AP: Complications of local anaesthesia for ophthalmic surgery. Br J Anaesth 1995;75:93–96.
- Rebolleda G, Munoz-Negrete FJ, Gutierrez-Ortiz C: Topical plus intracameral lidocaine versus retrobulbar anesthesia in phacotrabeculectomy: prospective randomized study. J Cataract Refract Surg 2001;27:1214–1220.
- Waddell KM, Reeves BC, Johnson GJ: A comparison of anterior and posterior chamber lenses after cataract extraction in rural Africa: a within patient randomized trial. Br J Ophthalmol 2004;88:734–739.
- Bourne R, Minassian D, et al: Effect of cataract surgery on the corneal endothelium: modern phacoemulsification compared with extracapsular surgery. Ophthalmology 2004;11:679–685.
- Boezaart A, Berry R, Nell M: Topical anesthesia versus retrobulbar block for cataract surgery: the patient’s perspective. J Clin Anesth 2000;12: 58–60.
- Sekundo W, Dick HB, Schmidt JC: Lidocaine-assisted xylocaine jelly anesthesia versus one quadrant sub-Tenon infiltration for self-sealing sclero-corneal incision routine phacoemulsification. Eur J Ophthalmol 2004;14:111–116.
- Davison M, Padroni S, Bunce C, Rüschen H: Sub-Tenon’s anaesthesia versus topical anaesthesia for cataract surgery. Cochrane Database Syst Rev 2007;3:CD006291.
- Zhao LQ, Zhu H, et al: Topical anesthesia versus regional anesthesia for cataract surgery: a meta-analysis of randomized controlled trials. Ophthalmology 2012;119:659–667.
- Pandey S, Werner L, et al: No-anesthesia clear corneal phacoemulsification versus topical and topical plus intracameral anesthesia. Randomized clinical trial. J Cataract Refract Surg 2001;27:1643–1650.
- Karp CL, Cox TA, et al: Intracameral anesthesia. A report by the American Academy of Ophthalmology. Ophthalmology 2001;108: 1704–1710.
- Heuerman T, Hartman C, Anders N: Long term endothelial cell loss after phacoemulsification: peribulbar anesthesia versus intracameral lidocaine 1%. Prospective randomized study. J Cataract Refract Surg 2002;28:638–643.
- Pang MP, Fujimoto DK, Wilkens LR: Pain, photophobia, and retinal and optic nerve function after phacoemulsification with intracameral lidocaine. Ophthalmology 2001;108:2018–2025.
- Roberts T, Boytell K: A comparison of cataract surgery under topical anaesthesia with and without intracameral lignocaine. Clin Experiment Ophthalmol 2002;30:19–22.
- Boulton J, Lopatazidis A, Luck J, et al: A randomized controlled trial of intracameral lidocaine during phacoemulsification under topical anesthesia. Ophthalmology 2000;107:68–71.
- Bardocci A, Lofoco G, Perdicaro S, et al: Lidocaine 2% gel versus lidocaine 4% unpreserved drops for topical anesthesia in cataract surgery. A randomized controlled trial. Ophthalmology 2003;110:144–149.
- Barequet IS, Soriano ES, Green WR, et al: Provision of anesthesia with single application of lidocaine gel. J Cataract Refract Surg 1999;25: 626–631.
- Judge AJ, Najafi K, et al: Corneal endothelial toxicity of topical anesthesia. Ophthalmology 1997;104:1373–1379.
- Guzey M, Satici A, Dogan Z, Karadede S: The effects of bupivacaine and lidocaine on the corneal endothelium when applied into the anterior chamber at the concentrations supplied commercially. Ophthalmologica
2002;216:113–117. - Carney LG, O’Leary DJ, Millodot M: Effect of topical anesthesia on corneal epithelial fragility. Int Ophthalmol 1984;7:71–73.
- Yagci A, Bozkurt B, Egrilmez S, Palamar M, Ozturk BT, Pekel H: Topical anesthetic abuse keratopathy: a commonly overlooked health care problem. Cornea 2011;30:571–575.
- Rao SK, Wong VW, Cheng AC, Lam PT, Lam DS: Topical anesthesiainduced keratopathy after laser-assisted subepithelial keratectomy. J Cataract Refract Surg 2007;33:1482–1484.
- Lee JK, Stark WJ: Anesthetic keratopathy after photorefractive keratectomy. J Cataract Refract Surg 2008;34:1803–1805.
- Martini E, Cavallini GM, Campi L, Lugli N, Neri G, Molinari P: Lidocaine versus ropivacaine for topical anesthesia in cataract surgery(1). J Cataract Refract Surg 2002;28:1018–1022.
- Fernandez SA, Dios E, Diz JC: Comparative study of topical anaesthesia with lidocaine 2% vs levobupivacaine 0.75% in cataract surgery. Br J Anaesth 2009;102:216–220.
- Wood CC, Menon G, Ayliffe W: Subconjunctival block for cataract extraction and keratoplasty. Br J Anaesth 1999;83:969.
- Kongsap P: Superior subconjunctival anesthesia versus retrobulbar anesthesia for manual small-incision cataract surgery in a residency training program: a randomized controlled trial. Clin Ophthalmol 2012;
6:1981–1986. - Yuen JS, Prineas S, Pham T, Liu H: Effectiveness of superior versus inferior subconjunctival anaesthesia for cataract surgery. Anaesth Intensive Care 2007;35:945–948.
- Prineas SN: Unpublished data. Available by contacting the author directly.
- Van Lint M: Paralysie palpébrale transitoire provoquée dans l’opération de la cataracte. Ann Ocul 1914;151:420–424.
- O’Brien, CS: Akinesis during cataract extraction. Arch Ophthal 1929;1: 447–449.
- Atkinson WS: Akinesia of the orbicularis. Am J Ophthalmol 1953;36: 1255–1258.
- Saeedi OJ, Wang H, Blomquist PH: Penetrating globe injury during infraorbital nerve block. Arch Otolaryngol Head Neck Surg 2011;137:396–397.
- Chan BJ, Koushan K, Liszauer A, Martin J: Iatrogenic globe penetration in a case of infraorbital nerve block. Can J Ophthalmol 2011;46: 290–291.
- Duker J, Nielsen J, Vander JF, Rosenstein RB, Benson WE: Retrobulbar bupivacaine irrigation for postoperative pain after scleral buckling surgery. Ophthalmology 1991;98:514–518.
- Jonas JB, Jäger M, Hemmerling T: Continuous retrobulbar anesthesia for scleral buckling surgery using an ultra-fine spinal anesthesia catheter. Can J Anesth 2002;49:487–489.
- Allman KG, Barker LL, Werrett GC, Gouws P, Sturrock GD, Wilson IH:
Comparison of articaine and bupivacaine/lidocaine for peribulbar anaesthesia by inferotemporal injection. Br J Anaesth 2002;88:
676–678. - Gouws P, Galloway P, Jacob J, English W, Allman KG: Comparison of articaine and bupivacaine/lidocaine for sub-Tenon’s anaesthesia in cataract extraction. Br J Anaesth 2004;92:228–230.
- Hintze A, Paessler L: Comparative investigations on the efficacy of articaine 4% (epinephrine 1:200,000) and articaine 2% (epinephrine 1:200,000) in local infiltration anaesthesia in dentistry—a randomised
double-blind study. Clin Oral Investig 2006;10:145–150. - Hillerup S, Bakke M, Larsen JO, Thomsen CE, Gerds TA: Concentrationdependent neurotoxicity of articaine: an electrophysiological and stereological study of the rat sciatic nerve. Anesth Analg 2011;112:
133–138. - Snoeck M: Articaine: a review of its use for local and regional anesthesia. Loc Reg Anesth 2012;5:23–33.
- Guise P, Laurent S: Sub-Tenon’s block. The effect of hyaluronidase on speed of onset and block quality. Anaesth Intensive Care 1999:27:
179–181. - Brown SM, Coats DK, Collins ML, Underdahl JP: Second cluster of strabismus cases after periocular anesthesia without hyaluronidase. J Cataract Refract Surg 2001;27:1872–1875.
- Strouthidis NG, Sobha S, Lanigan L, Hammond CJ: Vertical diplopia following peribulbar anesthesia: the role of hyaluronidase. J Pediatr Ophthalmol Starbismus 2004;41:25–30.
- Alwitryy A, Chaudhary S, Gopee K, Butler TK, Holden R: Effect of hyaluronidase on ocular motility in sub-Tenon’s anesthesia: randomized controlled trial. J Cataract Refract Surg 2002;28:1420–1423.
- Dempsey GA, Barrett PJ, Kirby IJ: Hyaluronidase and peribulbar block. Br J Anaesth 1997;78:671–674.
- Quhill F, Bowling B, Packard RB: Hyaluronidase allergy after peribulbar anesthesia with orbital inflammation. J Cataract Refract Surg 2004;30:916.
- Eberhart AH, Weiler CR, Erie JC: Angioedema related to the use of hyaluronidase in cataract surgery. Am J Ophthalmol 2004;138:142.
- Escolano F, Parés N, Gonzalez I, Castillo J, Valero A, Bartolomé B: Allergic reaction to hyaluronidase in cataract surgery. Eur J Anaesth 2005;22:729–730.
- Silverstein SM, Greenbaum S, Stern R: Hyaluronidase in ophthalmology. J App Res 2012;12:1–13.
- Mjahed K, el Harrar N, Hamdani M, Amraoui M, Benaguida M: Lidocaine-clonidine retrobulbar block for cataract surgery in the elderly. Reg Anesth 1996;21:569–575.
- Baroni MF, Lauretti GR, Lauretti-Fo A, Pereira NL: Clonidine as co-adjuvant in eye surgery: comparison of peribulbar vs oral administration. J Clin Anesth 2002;14:140–145.
- Madan R, Bharti N, Shende D, Khokhar SK, Kaul HL: A dose response study of clonidine with local anesthetic mixture for peribulbar block: a comparison of three doses. Anesth Analg 2001;93:1593–1597.
- Khan B, Bajwa SJ, Vohra R, et al: Comparative evaluation of ropivacaine and lignocaine with ropivacaine, lignocaine and clonidine combination during peribulbar anaesthesia for phacoemulsification cataract surgery. Ind J Anaes 2012;56:21–26.
- Cabral SA, Carraretto AR, Brocco MC, Abreu Baptista JF, Gomez RS: Effect of clonidine added to lidocaine for sub-Tenon’s (episcleral) anesthesia in cataract surgery. J Anesth 2014;28:70–75.
- Bharti N, Madan R, Kaul HL, et al: Effect of addition of clonidine to local aneasthetic mixture for peribulbar block. Anaesth Intensive Care 2002;30:438–441.
- Eskandr AM, Elbakry AE, Elmorsy OA: Dexmedetomidine is an effective adjuvant to subtenon block in phacoemulsification cataract surgery. Egypt J Anaes 2014;30:261–266.
- Moster MR, Azura-Blanco A: Wipe-out after glaucoma filtration surgery. J Curr Glauc Prac 2007;1:45–47.
- Kücükyavuz Z, Arici MK: Effects of atracurium added to local anesthetics on akinesia in peribulbar block. Reg Anesth Pain Med 2002; 27:487–490.
- Hemmerling TM, Budde WM, Koppert W, Jonas JB: Retrobulbar versus systemic application of morphine during titratable regional anesthesia via retrobulbar catheter in intraocular surgery. Anesth Analg 2000;
91:585–588. - Krause M, Weindler J, Ruprecht KW: Does warming of anesthetic solutions improve analgesia and akinesia in retrobulbar anesthesia? Ophthalmology 1997;104:429–432.
- Hansen TE: Practice styles and preferences of Danish cataract surgeons—1995 survey. Acta Ophthalmol Scand 1996;74:56–59.
- Eke T, Thompson JR: Eye: the national survey of local anesthesia for ocular surgery II. Safety profile of local anesthesia techniques. Eye 1999;13:196–204.
- Glantz L, Drenger B, Gozal Y: Perioperative myocardial ischemia in cataract surgery patients: General vs local anesthesia. Anesth Analg 2000;91:1415–1419.
- Katz J, Feldman MA, Bass EB, et al. Adverse intraoperative medical events and their association with anesthesia management strategies in cataract surgery. Ophthalmology 2001;108:1721–1726.
- Abramson DC. Sudden unexpected sneezing during the insertion of peribulbar block under propofol Sedation. Can J Anaesth1995;42(8): 740–3.
- Ahn ES, Mills DM, Meyer DR, Stasior GO: Sneezing reflex associated with intravenous sedation and periocular anesthetic injection. Am J Ophthal 2008;146:31–35.
- Boezaart AP, Berry RA, Nell MA, van Dyk AL: A comparison of propofol and remifentanil for sedation and limitation of movement during periretrobulbar block. J Clin Anesth 2001;13:422–426.
- Morley AM, Jazayeri F, Ali S, Malhotra R: Factors prompting sneezing in intravenously sedated patients receiving local anesthetic injections to the eyelids. Ophthalmology 2010;117:1032–1036.
- Schaack E, Diallo B et al. [Inadvertent globe perforation during peribulbar anaesthesia and sedation with propofol]. Ann Fr Anesth Reanim 2006;25(1):43-5.
- Steeds C, Mather SJ. Fasting regimens for regional ophthalmic anaesthesia: a survey of members of the British Ophthalmic Anaesthesia Society. Anaesthesia 2001;56:638
- Crenshaw JT, Winslow EH: Preoperative fasting: old habits die hard. Am J Nurs 2002;102:36–44.
- The Royal College of Anaesthetists. Guidelines for the Provision of Anaesthetic Services. Chapter 13: Guidance on the Provision of Ophthalmic Anaesthesia Services. London, 2015 weblink http://www.rcoa.ac.uk/system/files/GPAS-2015-13-OPHTHAL.pdf accessed 31 August 2016
- Royal College of Anaesthetists, Royal College of Ophthalmologists: Local anaesthesia for ophthalmic surgery. Joint guidelines from the Royal College of Anaesthetists and the Royal College of Ophthalmologists. London, 2012. http://www.rcoa.ac.uk/system/files/LA- Ophthalmicsurgery-2012.pdf accessed 31 August 2016
- Guise P, personal communication, July 16, 2013.