The search for safer anesthesia protocols has intensified in recent years, driven by the opioid crisis and the growing awareness of postoperative complications linked to opioid use. The SOFA (Study of Opioid-Free Anesthesia) trial, published in Anesthesiology in 2024, adds important evidence to this evolving field by comparing opioid-free anesthesia (OFA) to standard opioid-based techniques in major surgeries.
What is opioid-free anesthesia?
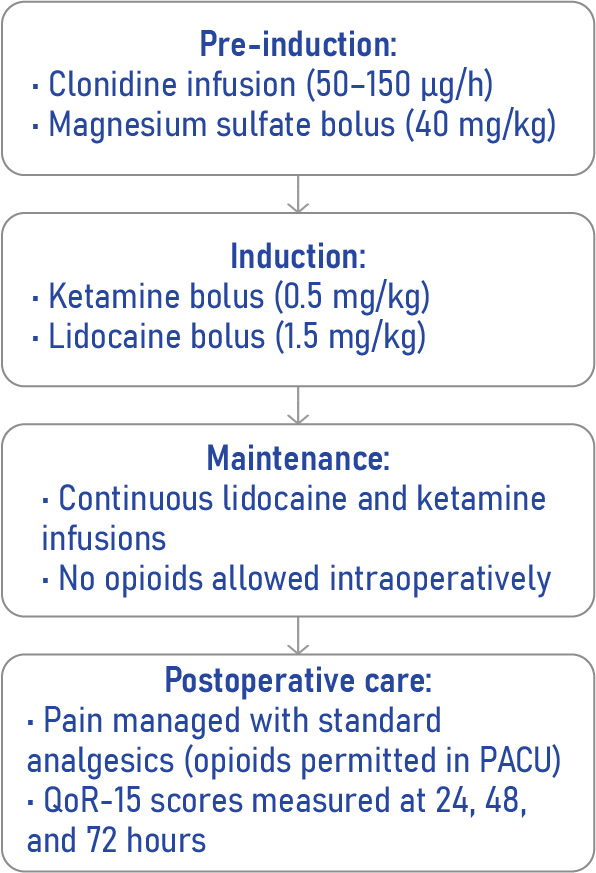
Opioid-free anesthesia is a multimodal anesthesia approach that omits opioids during surgery, aiming to reduce the risk of short- and long-term opioid-related complications. Instead, it uses combinations of:
- Ketamine: an NMDA receptor antagonist
- Lidocaine: a local anesthetic with systemic analgesic effects
- Clonidine: an α2-adrenergic agonist
- Magnesium sulfate: enhances analgesia and reduces anesthetic requirements
Why does this matter?
Traditional general anesthesia often includes opioids like fentanyl or sufentanil for intraoperative pain control. However, these drugs are associated with:
- Respiratory depression
- Postoperative nausea and vomiting (PONV)
- Hyperalgesia
- Delayed gastrointestinal recovery
- Risk of prolonged opioid use post-discharge
The SOFA trial investigated whether OFA could enhance the quality of recovery (QoR) in the early postoperative period.
Key findings from the SOFA trial
Study design at a glance
- Trial type: Single-center, randomized, controlled, patient- and assessor-blinded
- Duration: July 2021 – February 2022
- Setting: University Hospital Center of Angers, France
- Sample size: 135 adult patients undergoing major elective surgery (excluding bone procedures)
- Groups:
- OFA group: Received at least two of ketamine, lidocaine, clonidine, or magnesium sulfate
- Standard group: Received standard opioid-based anesthesia (sufentanil/remifentanil)
- OFA group: Received at least two of ketamine, lidocaine, clonidine, or magnesium sulfate
Primary outcome: Quality of recovery (QoR-15)
- At 24 hours: OFA group scored 114.9 vs. 108.7 in the standard group
- At 48 hours: OFA group scored 123.0 vs. 114.3
- At 72 hours: OFA group scored 129.2 vs. 121.9
Although statistically significant, these improvements did not reach the minimal clinically important difference (6–8 points), indicating modest clinical impact.
How opioid-free anesthesia was implemented
Pros and cons of OFA
Pros:
- Reduced incidence of PONV
- Slight improvement in early QoR
- Avoids opioid-related complications
Cons:
- Requires more drug preparation
- Increased intraoperative hypertension risk
- No reduction in postoperative opioid use
- Not broadly studied in high-risk populations
Final thoughts
The SOFA trial offers promising, if modest, support for OFA in improving early postoperative recovery. While the benefits in QoR scores were statistically significant, they fell short of strong clinical impact. Nonetheless, OFA appears safe and may play a role in reducing opioid-related side effects for select patient groups.
As the medical community continues seeking solutions to the opioid epidemic, OFA represents an important direction, one that calls for larger, multicenter trials and inclusion of more diverse surgical populations.
Reference: Léger M et al. Opioid-free Anesthesia Protocol on the Early Quality of Recovery after Major Surgery (SOFA Trial): A Randomized Clinical Trial. Anesthesiology. 2024;140:679-689.
Read more about this topic in the Anesthesia Updates section of the Anesthesia Assistant App. Prefer a physical copy? Get the latest literature and guidelines in book format. For an interactive digital experience, check out the Anesthesia Updates Module on NYSORA360!