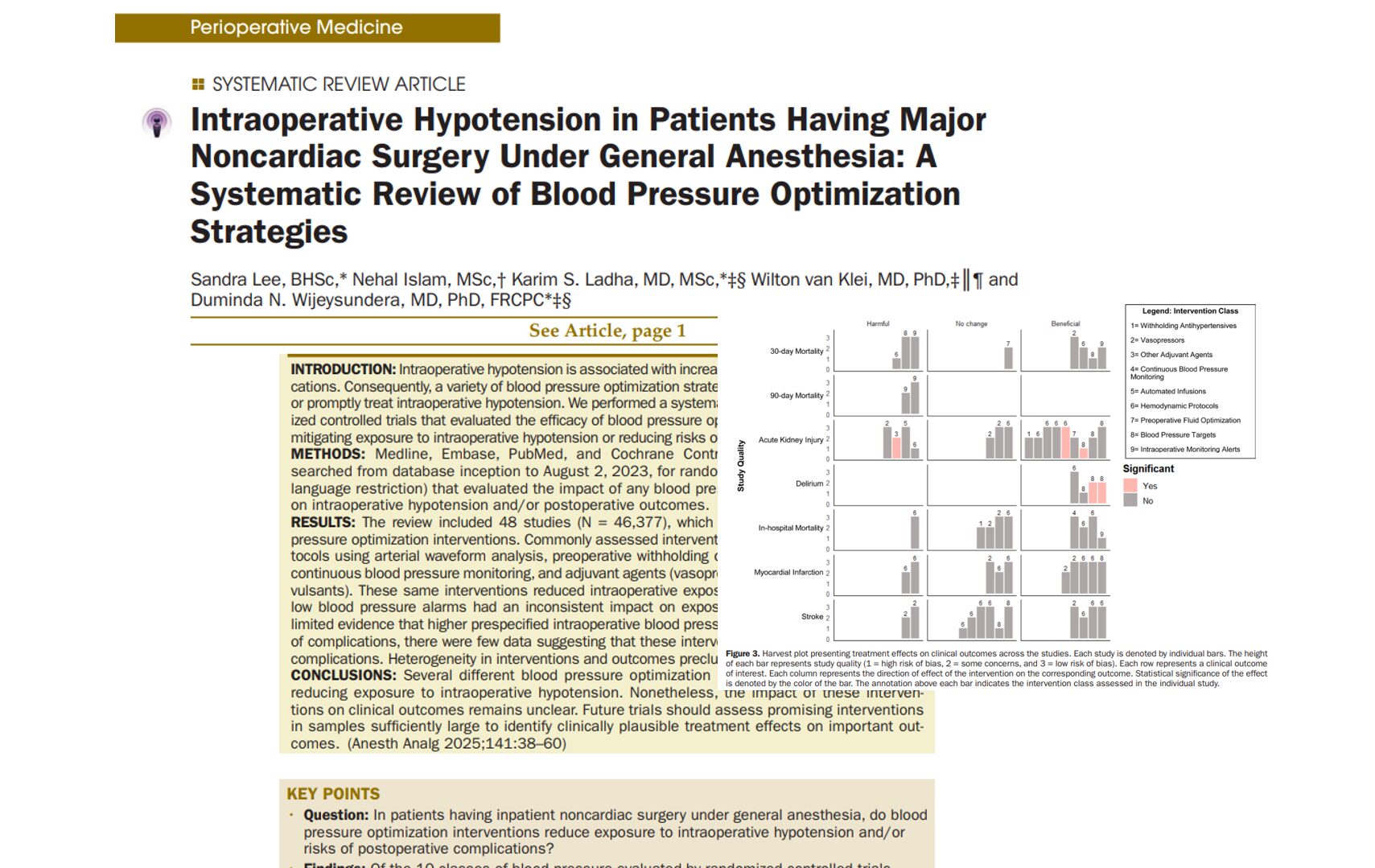
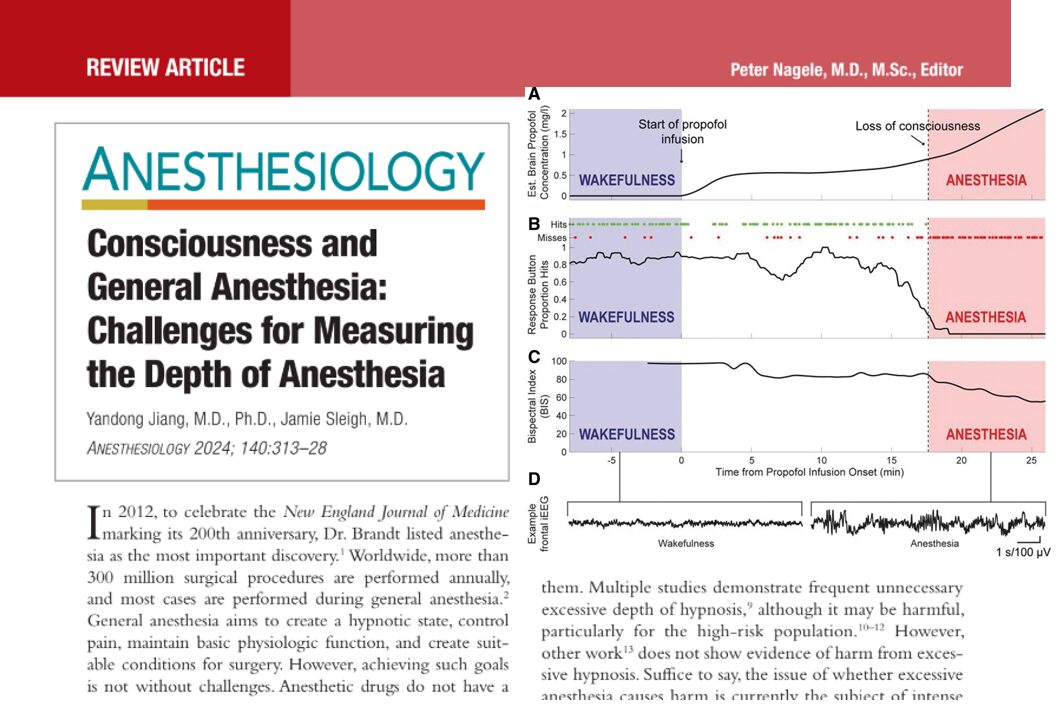
A new phase 3 clinical trial published in the British Journal of Anaesthesia (Zhao et al., 2025) introduces adamgammadex, a novel γ-cyclodextrin-based compound, as a powerful alternative to sugammadex for reversing rocuronium-induced neuromuscular blockade. This breakthrough could reshape perioperative care by offering faster recovery times and an improved safety profile.
What is adamgammadex?
Adamgammadex is a chemically modified cyclodextrin that works similarly to sugammadex—it encapsulates rocuronium molecules to reverse neuromuscular blockade. However, its structural modifications were designed to:
- Increase binding affinity to rocuronium
- Reduce the risk of hypersensitivity reactions
- Improve pharmacokinetics and clearance
Study design at a glance
- Type: Randomized, double-blind, controlled phase 3 trial
- Population: 402 adult patients undergoing elective surgery under general anesthesia
- Intervention: Rocuronium 0.6 mg/kg for neuromuscular block, followed by either:
- Adamgammadex 2 mg/kg (n = 302)
- Sugammadex 2 mg/kg (n = 100)
- Adamgammadex 2 mg/kg (n = 302)
- Primary endpoint: Time to recovery of TOF ratio ≥0.9
- Secondary endpoints: TOF ≥0.8 and ≥1.0, safety outcomes
Results: rapid and reliable recovery
Key efficacy findings
- Time to TOF ratio ≥0.9:
- Adamgammadex: 1.49 ± 0.60 min
- Sugammadex: 1.63 ± 0.78 min
- ➤ Adamgammadex was non-inferior and slightly faster
- Adamgammadex: 1.49 ± 0.60 min
- Time to TOF ≥1.0:
- Adamgammadex: 2.00 ± 0.67 min
- Sugammadex: 2.14 ± 0.89 min
- Adamgammadex: 2.00 ± 0.67 min
Interpretation
- Adamgammadex achieves rapid, consistent recovery from moderate neuromuscular block
- Statistically non-inferior and numerically faster than sugammadex
Safety profile: a major strength
Adverse events:
- Overall adverse event rates:
- Adamgammadex: 15.2%
- Sugammadex: 17.0%
- Adamgammadex: 15.2%
- Injection site pain was the most common event
- No hypersensitivity reactions were observed with adamgammadex
- Bradycardia, nausea, and vomiting occurred at similar low frequencies in both groups
Why this matters:
- Sugammadex, while effective, has been associated with anaphylaxis and hypersensitivity, prompting the need for safer alternatives
- Adamgammadex may offer a lower risk of immune-mediated side effects
Comparing adamgammadex and sugammadex

Clinical implications
If approved, adamgammadex could be a game-changer in anesthesia practice by:
- Offering faster recovery
- Reducing perioperative complications
- Avoiding hypersensitivity reactions associated with sugammadex
- Serving as an important alternative in patients with known allergy risks
Final thoughts
This trial positions adamgammadex as a highly effective and potentially safer option for rapid neuromuscular block reversal. As the anesthesia community seeks to enhance safety and efficiency, adamgammadex may soon become a cornerstone of modern perioperative care.
For more detailed information, refer to the full article in BJA.
Zhao Y et al. Efficacy and safety of adamgammadex for reversing rocuronium-induced deep neuromuscular block: a multicentre, randomised, double-blind, positive-controlled phase III trial. Br J Anaesth. 2025;135:331-339.
Get this and more in the AA App
This content is also available in the NYSORA Anesthesia Assistant App — your smart, expert-reviewed tool for:
- Point-of-care decisions: Instant, evidence-based guidance
- AI support (MAIA): Tools like DoseCalc and Case Manager
- Weekly updates: Protocols, tips, and exam-ready knowledge
Built on science, reviewed by experts — smarter (and safer) than a web search.
Download the AA App now to put trusted anesthesia guidance in your pocket.