Anthony M.-H. Ho, Robbert Buck, Malikah Latmore, Matthew Levine, and Manoj K. Karmakar
INTRODUCTION
The intercostal nerves (ICNs) innervate the major parts of the skin and musculature of the chest and abdominal wall. The block of these nerves was first described by Braun in 1907 in the textbook Die Lokalanastesie. In the 1940s, clinicians noticed that intercostal nerve blocks (ICNBs) can reduce pulmonary complications and in opioid requirements after upper abdominal surgery. In 1981, continuous ICNB was introduced to overcome the problems associated with repeated multiple injections. Today, ICNB is used in a variety of acute and chronic pain conditions affecting the thorax and upper abdomen, including breast and chest wall surgery. Introduction of ultrasound guidance to the practice of regional anesthesia further facilitates its practice. The disadvantages of intercostal block, however, include the requirement for technical expertise, risks of pneumothorax, and local anesthetic toxicity with multiple levels of block.
INDICATIONS
ICNB provides excellent analgesia in patients with rib fractures and for postsurgical pain after chest and upper abdominal surgery such as thoracotomy, thoracostomy, mastectomy, gastrostomy, and cholecystectomy. Respiratory parameters typically show impressive improvements on relief of pain. block of the two dermatomes above and the two below the level of surgical incision is required. ICNB does not block visceral abdominal pain, for which a celiac plexus block is required. Neurolytic ICNB is used to manage chronic pain conditions such as postmastectomy pain (T2) and postthoracotomy pain.
CONTRAINDICATIONS
- Disorders of coagulation, although this is not an absolute contraindication
- Local infection, lack of expertise and resuscitating equipment
FUNCTIONAL ANATOMY
As thoracic nerves T1 to T12 emerge from their respective intervertebral foramina, they divide into the following rami (Figure 1):
- The paired gray and white anterior rami communicantes, which pass anteriorly to the sympathetic ganglion and chain.
- The posterior cutaneous ramus, supplying skin and muscle in the paravertebral region.
- The ventral ramus (ICN, the main focus of this chapter).
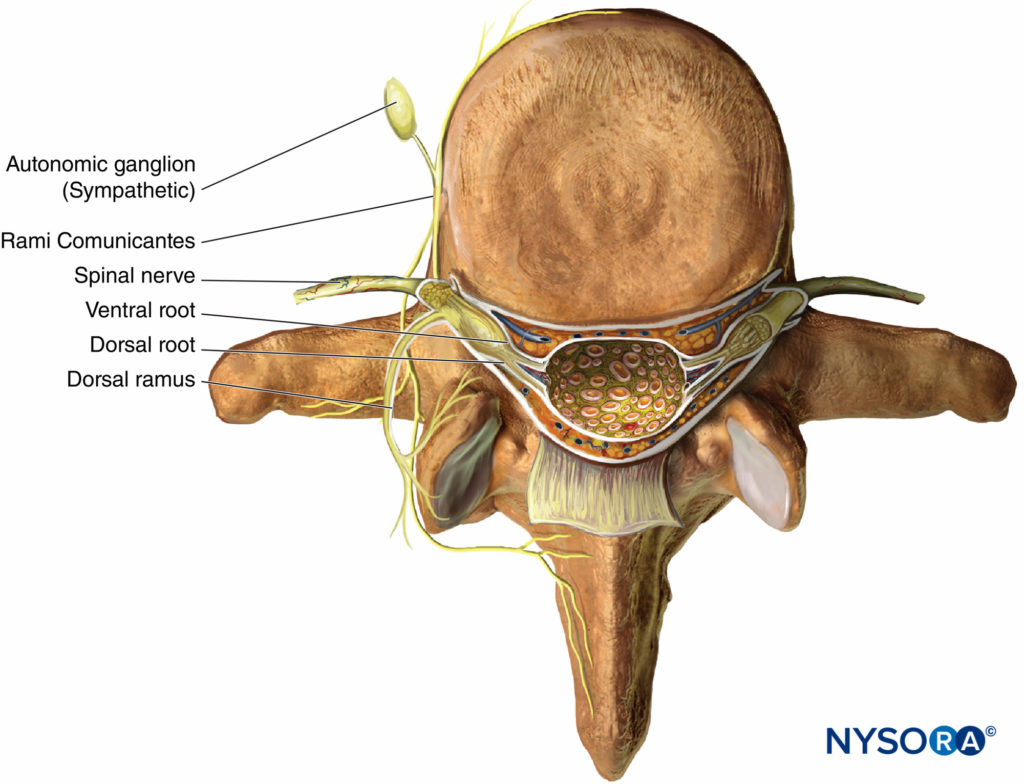
Figure 1. Anatomy of the spinal nerve.
T1 and T2 send nerve fibers to the upper limbs and the upper thorax, T3 through T6 supply the thorax, T7 through T11 supply the lower thorax and abdomen, and T12 innervates the abdominal wall and the skin of the front part of the gluteal region (Figure 2).
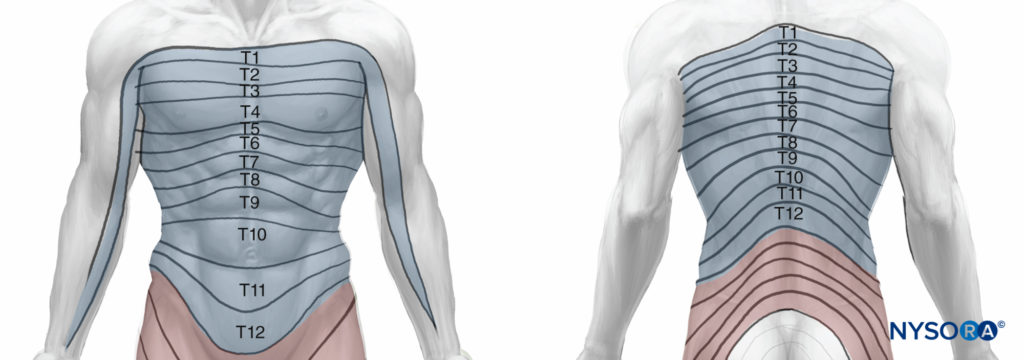
Figure 2. Dermatomal distribution of the intercostal nerves.
Carrying both sensory and motor fibers, the ICN pierces the posterior intercostal membrane about 3 cm (in adults) distal to the intervertebral foramen to enter the subcostal grove where it, for the most part, continues to run parallel to the rib, although branches may often be found anywhere between adjacent ribs. Its course within the thorax is sandwiched between the parietal pleura and innermost intercostal (intercostalis intimus) muscles and the external and internal intercostal muscles (Figures 3 and 4). Just anterior to the midaxillary line, it gives off the lateral cutaneous branch. As the ICN approaches the midline, it turns anteriorly and pierces the overlying muscles and skin to terminate as the anterior cutaneous branch.
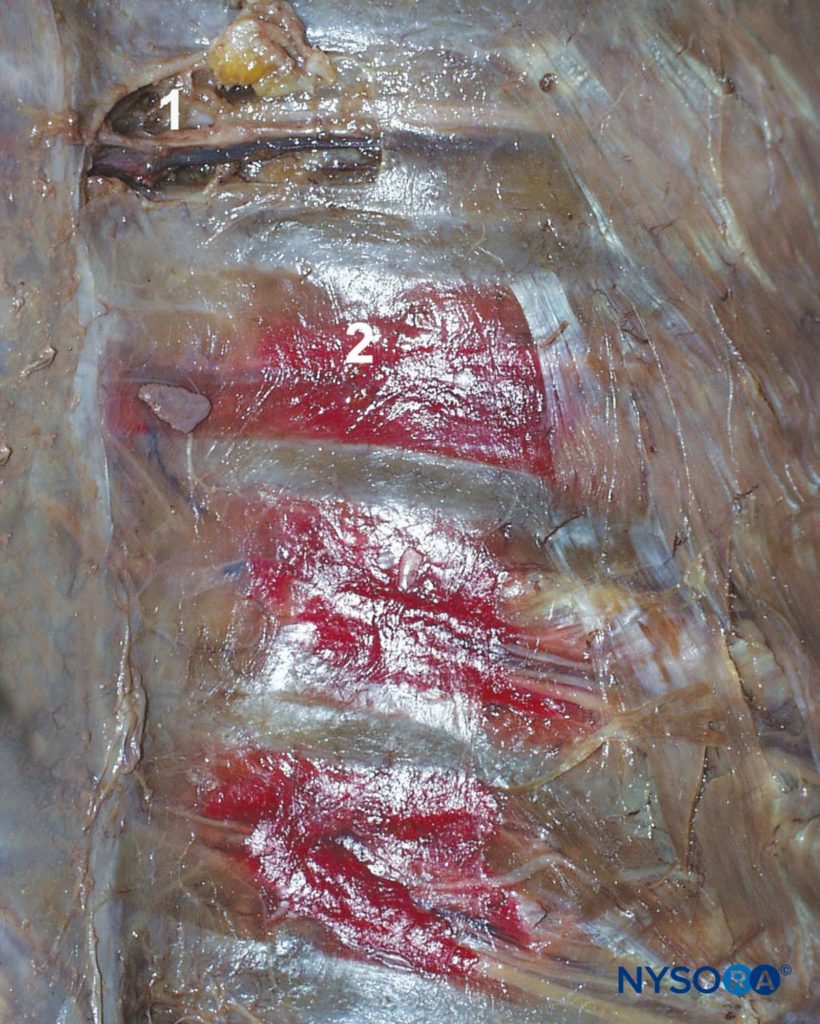
Figure 3 Intercostal nerves (accompanied by intercostal artery and vein) shown in the intercostal sulcus as seen from within the open chest cavity in a cadaver. The red dye illustrates spread of solutions injected into the intercostal sulcus during intercostal block. 1. Intercostal nerve. 2. Distribution of the dye after injection into the intracostal sulcus.
However, there are many anatomic variations. The first thoracic nerve (T1) has no anterior cutaneous branch, usually has no lateral cutaneous branch, and most of its fibers leave the intercostal space by crossing the neck of the first rib to join those from C8, while a smaller bundle continues on a genuine intercostal course to supply the muscles of the intercostal space. Some fibers of T2 and T3 give rise to the intercostobrachia nerve, which innervates the axilla and the skin of the medial aspect of the upper arm as far distal as the elbow. In addition, the ventral ramus of T12 is similar to the other ICNs but is called a subcostal nerve because it is not positioned between two ribs.
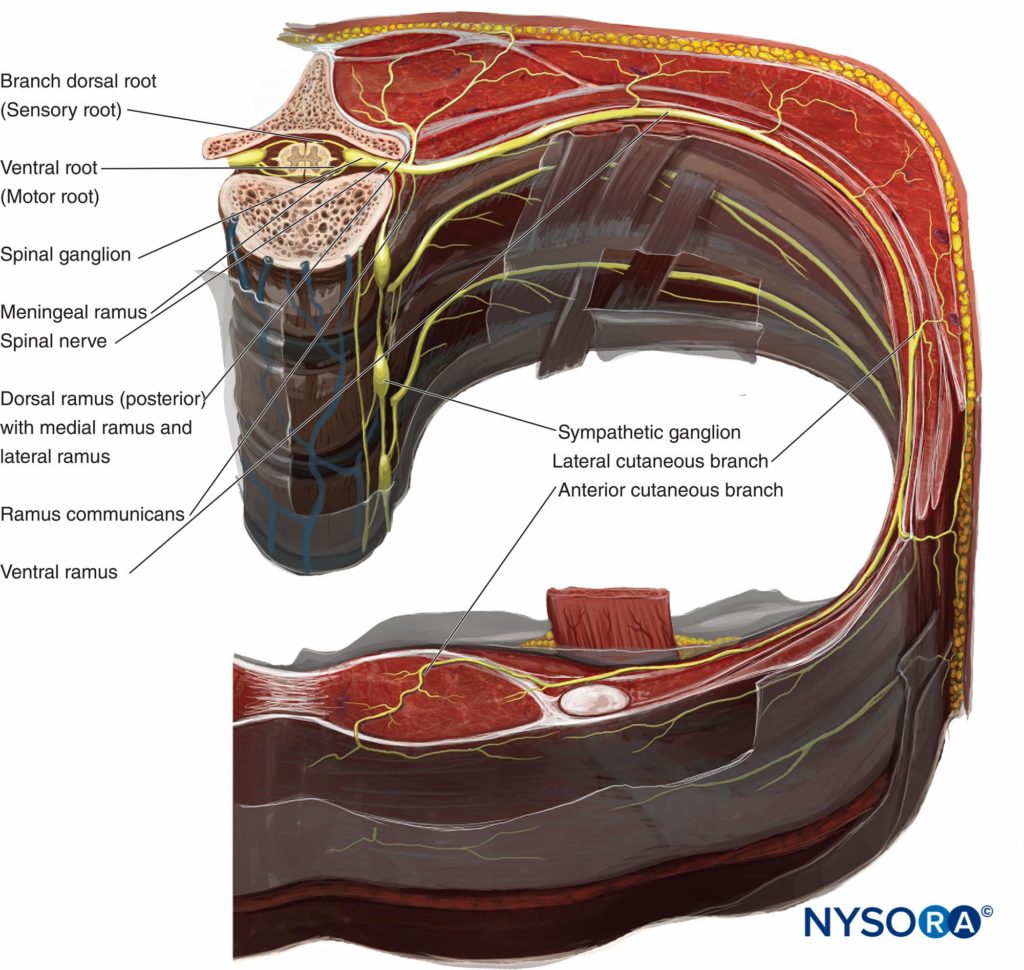
Figure 4. Anatomy of the intercostal nerve.
Lateral Cutaneous Branch
The lateral cutaneous branches of T2 through T11 pierce the internal and external intercostal muscles obliquely before divid-ing into the anterior and posterior branches (see Figure 4). These branches supply the muscles and skin of the lateral torso. The anterior branches supply of T7–T11 innervate the skin as far forward as the lateral edge of the rectus abdominis. The posterior branches of T7–T11 supply the skin overlying the latissimus dorsi. The lateral cutaneous branch of T12 does not divide. Most of the ventral ramus of T12 joins that of L1 to form the iliohypogastric, ilioinguinal, and genitofemoral nerves; the rest pierces the transverse abdominal muscle (TAM) to travel between TAM and the internal oblique muscle.
Anterior Cutaneous Branch
The anterior cutaneous branches of T2 through T6 pierce the external intercostals and pectoralis major muscles to enter the superficial fascia near the lateral border of the sternum to supply the skin of the anterior part of the thorax near the midline and slightly beyond (see Figure 4). Smaller branches (T1 through T6) exist to supply the intercostal muscles and parietal pleura, and these branches may cross to adjoining intercostal spaces. The anterior cutaneous branches of T7 through T12 pierce the posterior rectus sheath to supply motor nerves to the rectus muscle and sensory fibers to the skin of the anterior abdominal wall. Some final branches of T7 through T12 continue anteriorly and, together with L1, innervate the parietal peritoneum of the abdominal wall. Their anterior course continues and becomes superficial near the linea alba to provide cutaneous innervation to the midline of the abdomen and a couple of centimeters beyond. For additional information, see Functional Regional Anesthesia Anatomy.
MECHANISM OF BLOCK AND DISTRIBUTION OF ANESTHESIA
ICNB blocks the ipsilateral sensory and motor fibers of the ICNs. Local anesthetic solution injected into the subcostal groove spreads both distally and proximally; some of the injectate may enter paravertebral space as well. (see Figure 3).
TECHNIQUE
An intravenous line should be established, and resuscitation drugs should be readily available. Sedation and analgesia are always used judiciously. ICNB may be performed in an anesthetized patient, although spinal anesthesia has been reported in patients when ICNB was performed under general anesthesia, and there is a concern that the risk of pneumothorax may be increased in a patient under positive pressure ventilation. After the block, the patient should be monitored for potential complications, particularly delayed pneumothorax, local anesthetic toxicity, hematoma, and occurrence of spinal anesthesia (rare).
The ICN can be blocked anywhere proximal to the midaxillary line, where the lateral cutaneous branch takes off. In children, the block is commonly carried out at the posterior axillary line or, alternatively, just lateral to the paraspinal muscles, at the angle of the rib. In adults, the most common site for ICNB is at the angle of the rib (6–8 cm from the spinous processes; Figure 5). At the angle of the rib, the rib is relatively superficial and easy to palpate, and the subcostal groove is the widest. The nerve is inferior to the posterior intercostal artery, which is inferior to the intercostal vein (Figure 6) (mnemonic: VAN [vein/artery/nerve]). VAN are surrounded by adipose tissue and are sandwiched between the internal inter-costal and the interior intercostal (intercostalis intimus) muscles. The nerve often runs as three or four separate bundles, without an enclosing endoneural sheath, making it easily accessible to block. Blocking intercostal nerves medial to the angle of the rib is not recommended because the nerves lies deep to the posterior intercostal membrane with very little tissue between it and the parietal pleura, and the overlying sacrospinalis muscle makes rib palpation difficult. On the other hand, block distal to the anterior axillary line is more difficult because the nerve has left the subcostal groove and reentered the intercostal space and lies in the substance of the internal intercostal muscle.
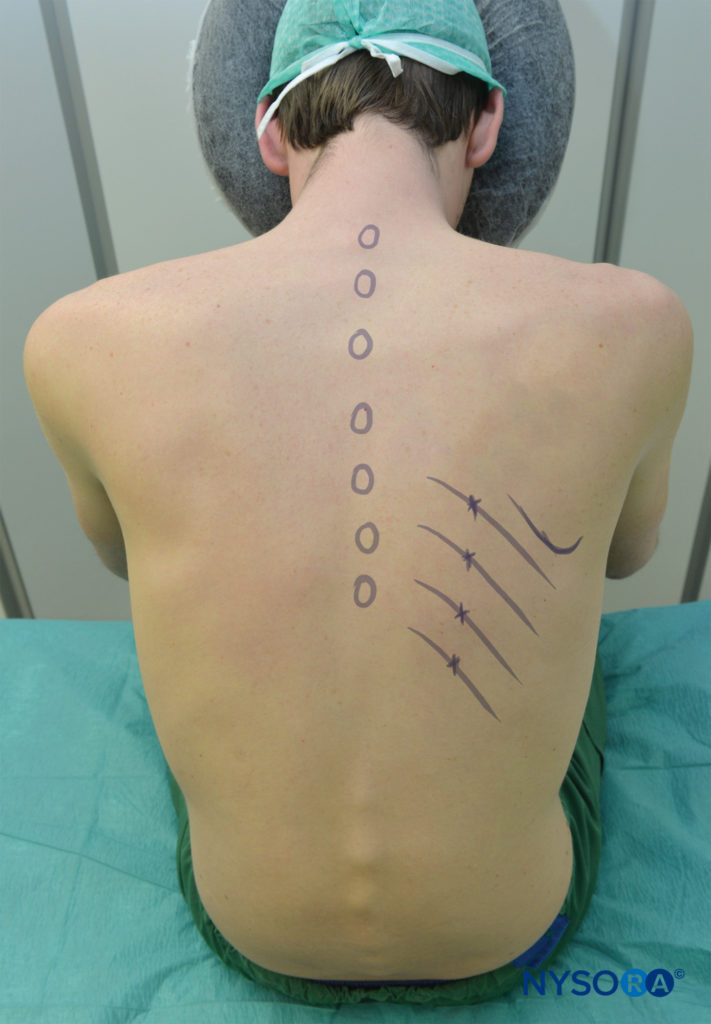
Figure 5. The sitting patient should lean slightly forward and be supported. The arms should pull the scapulae laterally to facilitate access to the posterior rib angles above T7. The inferior edges of the ribs to be blocked are marked just lateral to the lateral border of the sacrospinalis (paraspinous) muscle group, corresponding to the angles of the ribs. Points of needle entry are marked at 6–8 cm from the midline in most adults.
ICNB can be performed with the patient in the prone, sitting, or lateral position (block side up). In prone position, a pillow should be placed under the patient’s upper abdomen, and the arms are allowed to hang off the sides. The sitting patient should lean slightly forward holding a pillow and be supported. The arms should be forward. The position of the arm in either position is to pull the scapulae laterally and facilitate access to the posterior rib angles above T7 (see Figure 5). Under aseptic conditions, the block sites are identified.
NYSORA Tips
- Ribs can be counted starting from the twelfth rib, or from the seventh rib (inferior tip of the scapula).
- The inferior edges of the ribs to be blocked are marked just lateral to the lateral border of the sacrospinalis (paraspinous) muscle group (usually 6–8 cm from the mid-line at the lower ribs and 4–7 cm from the midline at the upper ribs), corresponding to the angles of the ribs.
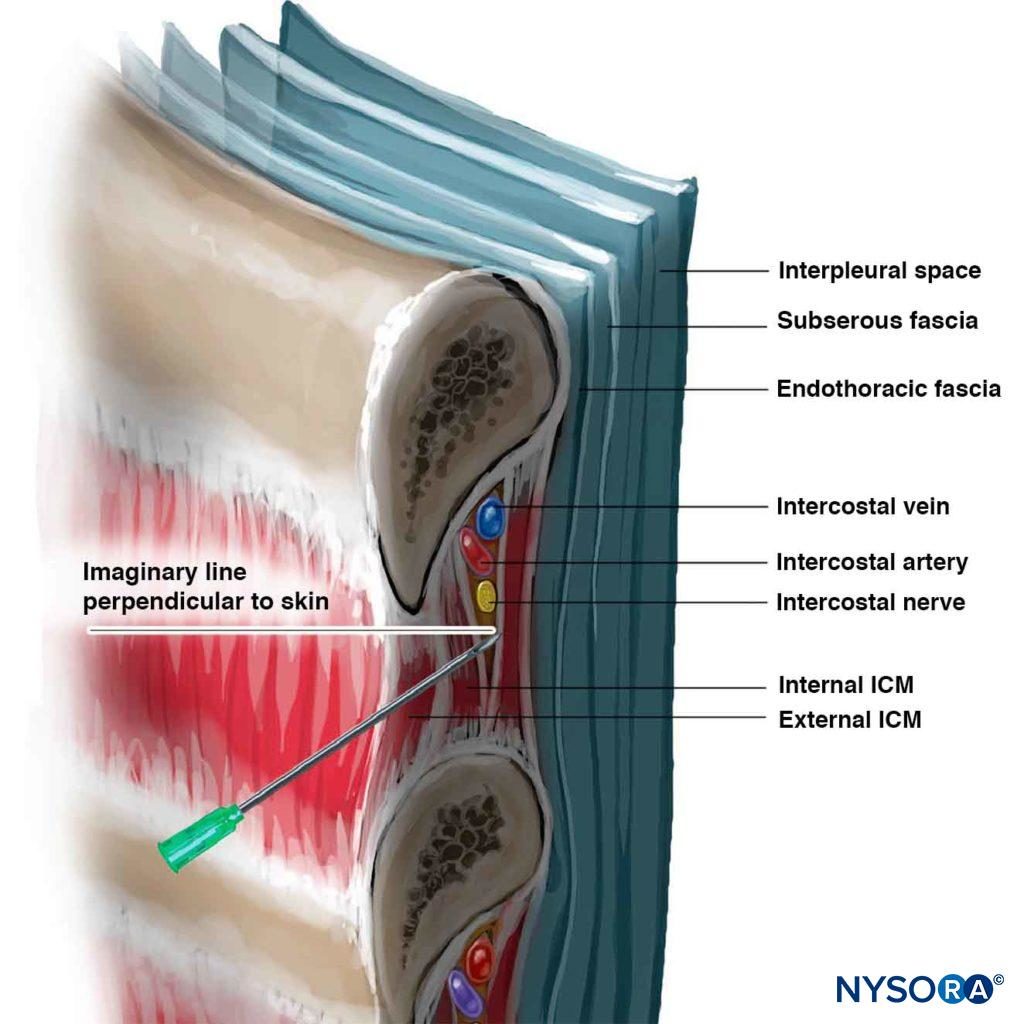
Figure 6. Needle angle required to enter intercostal sulcus. Note the relationship of the intercostal vessels to the nerve.
Inferior borders of the ribs to be blocked are palpated and marked (see Figure 5). The needle entry sites are infiltrated with lidocaine 1%–2%. A site of entry is well placed when a needle introduced through it at 20 degrees cephalad (sagittal plane; see Figure 6) scrapes underneath the inferior border of the rib and reaches the subcostal groove. The skin is first drawn cephalad with the palpating hand by about 1 cm, and a 4- to 5-cm, 22- to 24-gauge (for single-shot injection) needle is introduced through the chosen entry site at a 20-degree cephalad angle with the bevel facing cephalad. The needle is advanced until it contacts the rib at a depth of less than 1 cm in most patients. A small amount of local anesthetic may be injected to anesthetize the periosteum. With the palpating hand holding the needle firmly and resting securely on the patient’s back, the injecting hand gently “walks” the needle caudally while the skin is allowed to move back over the rib (Figure 7).
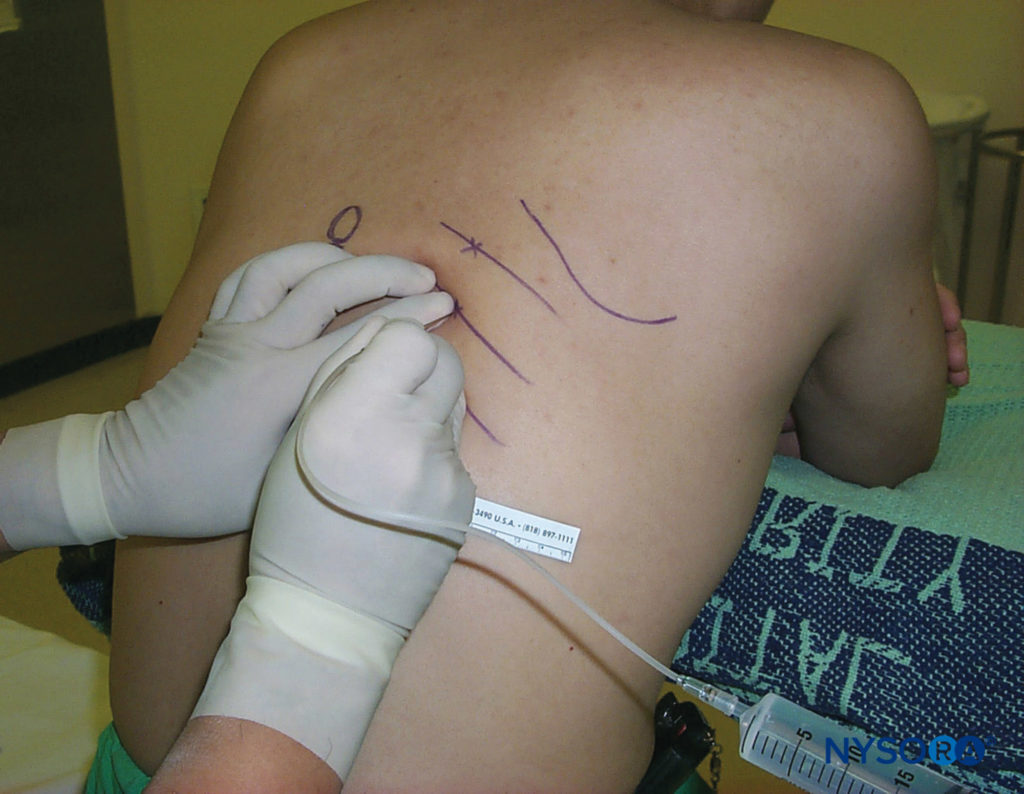
Figure 7. With the palpating hand holding the needle firmly and resting securely on the patient’s back to control the needle advancement, the injecting hand gently “walks” the needle caudally while the skin is allowed to move back over the rib.
The needle is now advanced farther a few mm, while maintaining the 20-degree tilt angle cephalad (even a slight caudad-pointing angle by the needle greatly reduces the chance of success). A subtle “give” or “pop” of the fascia of the internal intercostal muscle may be felt, especially if a short-beveled needle is used. As the average distance from the posterior aspect of the rib to the pleura averages 8 mm, advancement of the needle much beyond a few mm increases the risk of pneumothorax. Paresthesia, although not actively sought, occasionally occurs as an additional confirmation of the correct needle placement. Radiologic guidance is advised for neurolytic blocks. At this point, on negative aspiration for blood, 3–5 mL of local anesthetic is injected. For a single ICNB, it is desirable to block at least one ICN cephalad and one caudad because some degree of overlapping innervation from adjacent ICNs is common. To ensure that the tip of the needle remains in the optimal location, unaffected by hand and chest movements, some clinicians prefer to connect extension tubing between the needle and the syringe and have an assistant perform the aspiration and injection.
block of T1 through T7 is technically more challenging because of the scapulae and the rhomboid muscles. For this reason, we prefer to perform a thoracic paravertebral block or an epidural block when high thoracic block is required.
EQUIPMENT
- Needle: Single shot: 20- to 22-gauge 4- to 5-cm needle (adults)
- Catheter placement: 18- to 20-gauge Tuohy needle (adults)
- Syringe and needle for local infiltration
- Syringe with extension tubing
- Sterilizing and resuscitation equipment and drugs, drapes, marking pen, pillow, portable fluoroscope (for neurolytic blocks)
Learn more about Equipment for Peripheral Nerve Blocks.
CHOICE OF LOCAL ANESTHETIC
The choice of local anesthetic for single-shot ICNB includes bupivacaine 0.25%–0.5%, lidocaine 1%–2% with epinephrine 1/200,000–1/400,000, and ropivacaine 0.5%. Three to 5 mL of local anesthetic is injected at each level during a multiple-injection ICNB. The duration of action is usually 12 ± 6 h. Addition of epinephrine to bupivacaine or ropivacaine does not significantly prolong the duration of the block but may slow the systemic absorption and increase the maximum allowable dose with a single shot by 30%. Maximum bupivacaine dose is 2 (for plain solution) to 3 (with epinephrine) mg/kg/injection (total at one time)7 and 7–10 mg/kg/day. Maximum lidocaine dose is up to 5–7 (with epinephrine) mg/kg/injection7 and 20 mg/kg/day. Volunteers reportedly may tolerate 30% more ropivacaine than bupivacaine before neurologic symptoms develop. The maximum single injection dose for ropivacaine is 2.5 mg/kg and 4 mg/kg with epinephrine, whereas the maximum daily dose is 9–12 mg/kg/24 h. The maximum single injection of epinephrine as an additive is 4 mcg/kg. Vascular sites favor more rapid local anesthetic absorption, and the blood levels of local anesthetics after ICNB are higher than for most other regional anesthetic procedure. As such, it is advisable to leave a safety margin between the doses given and the maximum recommended dosages, especially in young children; the elderly; debilitated patients; and those with underlying cardiac, hepatic, or renal impairment. For continuous infusion, patients can usually tolerate a gradual build-up of the plasma local anesthetic level better than acute rises. One recommended regimen is a loading dose of 0.3 mL/kg followed by an infusion of 0.1 mL/kg/h of either bupivacaine 0.25% or lidocaine 1%.
NYSORA Tips
- The best needle insertion site for ICNB is the angle of the rib, about 7 cm lateral to the midline in adults.
- The ideal angle of entry into the subcostal groove is about 20 degrees cephalad.
- Epidural analgesia may be better-suited alternative to bilateral ICNBs because of the risk of bilateral pneumothorax and the potential for local anesthetic toxicity due to the large dose of local anesthetic required.
- ICNB above T7 may be difficult because of the scapulae; an alternative technique such as paravertebral or epidural block should be considered.
COMPLICATIONS
The foremost concern is a pneumothorax, which may occur in about 1%. Tension pneumothorax and the subsequent need for tube thoracostomy, however, is rare. If an asymptomatic pneumothorax is detected, the best management is observation, reassurance, and, if necessary, supplemental oxygen. The peritoneum and abdominal viscera are at risk of penetration when lower ICNs are blocked. Absorption of local anesthetic from the intercostal space is rapid; arterial plasma concentration peaks in 5-10 minutes, and venous plasma concentration peaks several minutes later.
SUMMARY
ICNB is a useful regional anesthesia technique; which is very effective in the controlling pain involving the thorax and upper abdomen. Although there is the risk of pneumothorax and local anesthetic toxicity, these can be reduced with proper technique and consideration given to the maximum allowable drug dose. The proper use of ICNB includes balancing its advantages and disadvantages against those of alternative techniques such as epidural and paravertebral block. With expertise and proper indications, intercostal nerve block may provide a uniquely suitable anesthetic option in patients in whom general or other regional anesthesia choices may be limited.
REFERENCES
- Strømskag KE, Kleiven S: Continuous intercostals and interpleural nerve blocks. Tech Reg Anesth Pain Manage 1998;2:79–89.
- Karmakar MK, Ho AMH: Acute pain management of patients with multiple fractured ribs. J Trauma 2003;54:612–615.
- Karmakar MK, Critchley LAH, Ho AMH, et al: Continuous thoracic paravertebral infusion of bupivacaine for pain management in patients with multiple fractured ribs. Chest 2003;123:424–431.
- Kopacz DJ, Thompson GE: Intercostal blocks for thoracic and abdominal surgery. Tech Reg Anesth Pain Manage 1998;2:25–29.
- Nunn JF, Slavin G: Posterior intercostal nerve block for pain relief after cholecystectomy. Anatomical basis and efficacy. Br J Anaesth 1980;52:253–60.
- Barron DJ, Tolan MJ, Lea RE: A randomized controlled trial of continuous extra-pleural analgesia post-thoracotomy: efficacy and choice of local anaesthetic. Eur J Anaesthesiol 1999;16:236–245.
- Lagan G, McLure HA: Review of local anaesthetic agents. Curr Anaesth Crit Care 2004;15:247–254.
- Scott DB, Lee A, Fagan D, et al: Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth Analg 1989;69:563–569.
- Vandepitte C, Gautier P, Bellen P, Murata H, Salviz EA, Hadzic A. Use of ultrasound-guided intercostal nerve block as a sole anaesthetic technique in a high-risk patient with Duchenne muscular dystrophy. Acta Anaesthesiol Belg. 2013;64(2):91-94.
Anthony M.-H. Ho, Robbert Buck, Malikah Latmore, Matthew Levine, and Manoj K. Karmakar