John-Paul J. Pozek, David Beausang, Kara G. Segna, and Eugene R. Viscusi
INTRODUCTION
Local anesthetics (LAs) are among the most useful drugs in anesthesiology practice and pain management. They are cornerstones in postoperative pain management within a multimodal analgesic pathway to reduce or eliminate opioids and their resulting adverse events. However, currently available LAs display a considerable range of onset and duration as well as tolerability across a wide range of uses, including infiltration, peripheral blocks, and epidural and spinal anesthesia. Their main limitation is duration of action, which in the treatment of postoperative pain may prevent adequate therapy of sufficient duration. For that reason, continuous catheter infusion systems are widely used but introduce challenges, such as catheter placement, catheter migration and maintenance, and the burden of the external pump. Therefore, long-acting LAs with predictable onset, delivery, and duration of action would be a near-ideal solution. Local anesthetics can have considerably different properties depending on the body compartment where they are placed. Controlled-release LAs must be well studied for clinical efficacy and reliability in the various sites and modes of application. At this time, only one controlled-release drug is approved by the Food and Drug Administration (FDA) and is commercially available, although there are several others in development. In this chapter, we summarize the currently available information.
LOCAL ANESTHETIC CARRIERS
Since the 1970s, drug delivery systems for LAs have been the subject of considerable research efforts. Development strategies are typically based on interdisciplinary approaches that combine polymer science, pharmaceutics, bioconjugate chemistry, and molecular biology. The goals of these carriers are to provide a LA depot at the target site to prolong the drug effect and to decrease local and systemic toxicity by reducing the LA concentration and increasing LA permeability and absorption. These factors determine the concentration and the effect of the LA on the nervous tissue, influencing the latency, spread, inten-sity of the block, and the duration of action. Formulation approaches to systemically deliver LA have included the encapsulation in liposomes, complexation in cyclodextrins, association with biopolymers, transdermal nonliposomal carriers, and other carrier systems. Topical delivery systems for LA comprise of a wide spectrum of adjuvants, including viscosity-inducing agents, preservatives, permeation enhancers, and emollients. The physical state of these carriers varies from semisolid (gel, cream, ointment); liquid (emulsion, dispersion); to solid (patch) pharmaceutical forms.
Liposome-Based Local Anesthetic Formulations
Liposomes, widely investigated as drug carriers for improving the delivery of therapeutic agents to specific sites in the body, are nonimmunogenic, biodegradable, nontoxic and work by encapsulating both hydrophilic and hydrophobic materials to deliver drugs. The structural versatility combined with the ability to encapsulate different compounds, such as LAs, is due to microscopic mono- or bi-layer phospholipid vesicles. The polar core of the liposphere allows hydrophilic drug molecules to become encapsulated. Amphiphilic and lipophilic molecules are solubilized within the phospholipid bilayer according to their affinity. Channel proteins can be incorporated into the liposome without a loss of activity within the hydrophobic domain of vesicle membranes, acting as a selective filter. Thus, drugs that are encapsulated with channel proteins are effectively protected from premature degradation by proteolytic enzymes and are able to diffuse through the channel driven by concentration gradients between the interior and exterior “nanocage.” Various types of liposomes can be prepared, depending on the number of lipid layers, size, surface charge, lipid composition, and methods of vesicle formation. In the case of liposomes and micro- or nanoparticle-based systems, the improved pharmacological action is generated by the slow rate of release of the encapsulated drug from these lipid bilayers.
Benefits
Liposomes, composed of naturally occurring substances, offer the advantage of being nontoxic and biodegradable. The ability to entrap drugs in the aqueous or lipid form enables carrying of both hydrophilic and hydrophobic drugs. The advantages of encapsulating LA in liposomes is controlled delivery via slow drug release to prolong anesthetic effect and reduce the risk of cardiovascular and central nervous system toxicity.
NYSORA Tips
- Liposomes are microscopic spheres containing an aqueous core surrounded by a phospholipid bilayer.
Risks/Limitations
Although liposomes are the carrier of choice in many technologies, their use for LAs has not often been adequately explored. This could be because liposomes are considered unstable colloidal systems, either physically due to their size or chemically, as lipids are prone to oxidation.
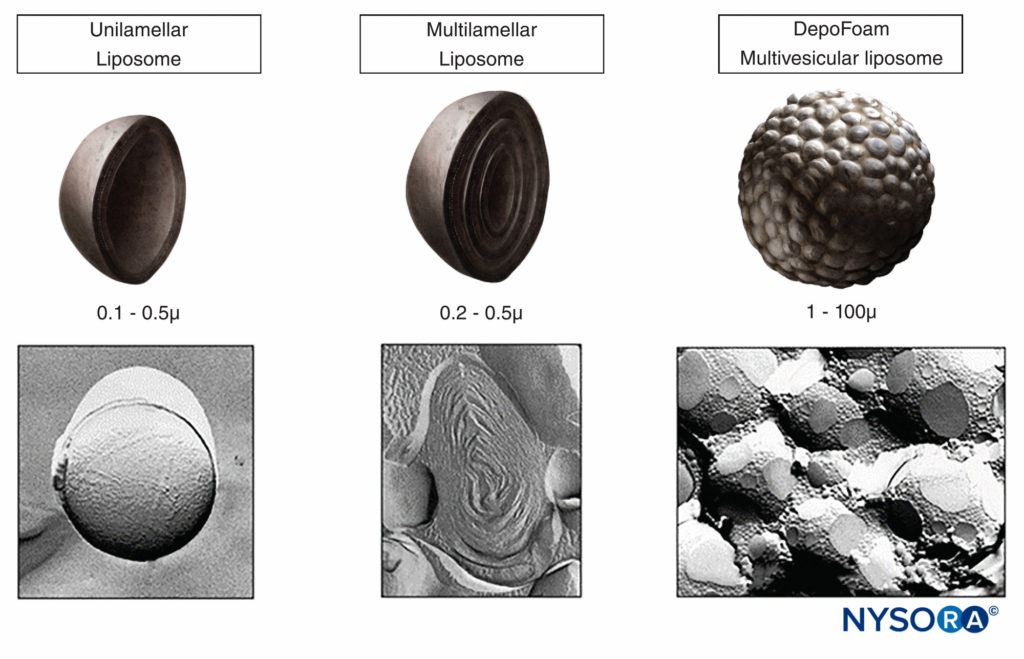
DepoFoam®
DepoFoam® consists of microscopic, spherical, lipid-based particles (Figure 1A). The particles are composed of numerous polyhedral, nonconcentric, aqueous chambers containing the drug in solution. Each chamber in this multivesicular liposome is separated from adjacent chambers by lipid membranes (Figure 1B). DepoFoam particles are distinguished structurally from unilamellar vesicles, multilamellar vesicles, and neosomes (Figure 2) by these closely packed, nonconcentric vesicles. The particles are tens of micrometers in diameter and have a large trapped volume. This allows delivery of relatively large quantities of medications in the encapsulated form with only a small volume of the formulation. Importantly, the liposomal platform that encapsulates the drug does so without altering the molecular structure. Therefore, a number of methods based on a manipulation of the lipid and aqueous composition may be used to control the rate of sustained release over a desired period from 1 to 30 days via erosion or reorganization of the lipid membranes. DepoFoam has been used in to-date, two FDA-approved commercial products, including DepoCyt(e)® (cytarabine liposome injection), as well as EXPAREL® (bupivacaine liposome injectable suspension). DepoFoam can be released into the bloodstream via the interstitial space subcutaneously or intra-muscularly or it can be delivered locally to a body compartment or joint via intrathecal, intraperitoneal, subcutaneous, epidural, or intraocular methods.
NYSORA Tips
- DepoFoam technology consists of lipid-based particles with polyhedral, nonconcentric, aqueous chambers that contain the medication. This technology can be used with a number of different medications.
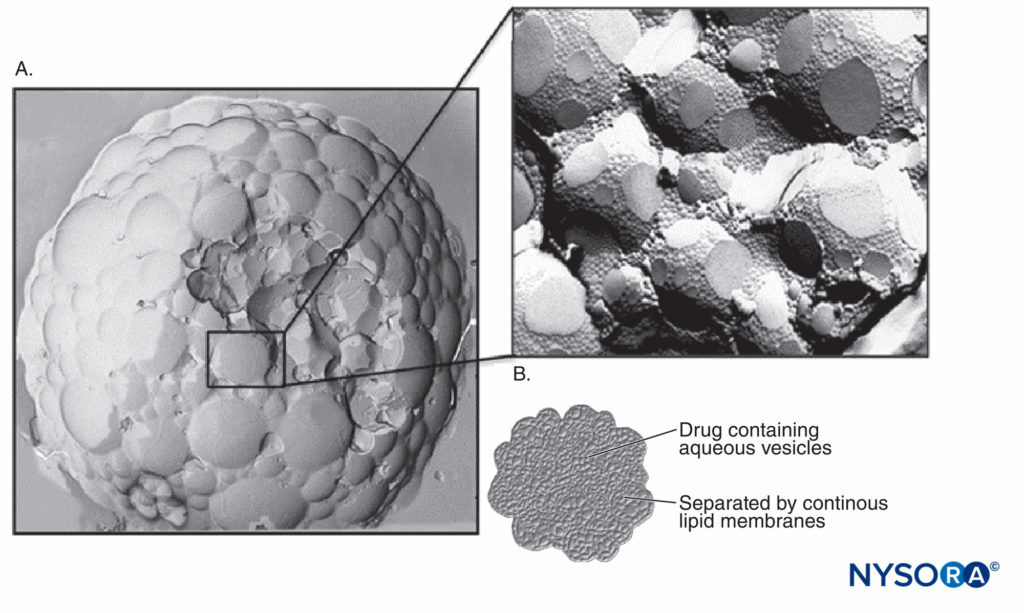
FIGURE 1. A: Scanning electron micrographic image of DepoFoam with bupivacaine. B: Diagram representing the polyhedral, nonconcentric aqueous chambers filled with medication. (Used with permission from Pacira Pharmaceuticals, Inc.)
Benefits
DepoFoam is a ready-to-use product and can be administered with small-gauge needles and pen systems. With a flexible delivery system, it is designed to offer an immediate-release dose, followed by sustained delivery. DepoFoam is less than 3% lipid that is naturally occurring or a synthetic analogue of common lipids, including phospholipids, cholesterol, and triglycerides; therefore, it is biodegradable and biocompatible. Clinical trials have demonstrated limited to no adverse effects of DepoFoam. There is already considerable clinical experience with the delivery system as the formulation has been in use in products approved by the FDA and European Medicine Agency. Furthermore, at similar doses, this formulation can reduce systemic exposure and toxicity by reducing peak serum levels of a drug.
Polymeric Micro- and Nanoparticle Formulations
Polymeric micro- or nanoparticles represent drug delivery systems made of natural or artificial polymer spheres or capsules, which must be biocompatible and biodegradable for drug delivery purposes. Nanoparticles act as potential carriers for several classes of drugs, such as anticancer agents, antihypertensive agents, immunomodulators, and hormones, and for macromolecules such as nucleic acids, proteins, peptides, and antibodies. Nanoparticles can be designed for the site-specific delivery of drugs. The targeting and release capability of nanoparticles is influenced by particle size, surface charge, surface modification, and hydrophobicity. The performance of nanoparticles in vivo is influenced by morphological characteristics, surface chemistry, and molecular weight. Polymer (micro- or nanoparticle) technologies are claimed to be applicable to all commercially available LA compounds. A variety of natural and synthetic polymers have been explored for the preparation of nanoparticles, of which poly(lactic acid) (PLA) and poly(glycolic acid) (PGA) and their copolymer poly(lactic-co-glycolic acid) (PLGA) have been extensively investigated for their biocompatibility and biodegradability.
PLGA, one of the most successfully developed biodegradable polymers, attracted considerable attention due to the FDA and European Medicine Agency giving approval for parenteral administration. Other properties include well-described formulations and methods of production adapted to various types of drugs (eg, hydrophilic or hydrophobic small molecules or macromolecules) and protection of the drug from degradation. Use of PLGA allows for the possibility of sustained release, the possibility to modify surface properties to provide better interaction with biological materials, and even a possibility toward targeting nanoparticles to specific organs or cells. Of note, after systemic administration, PLGA-based drug delivery systems are preferentially taken up by the reticuloendothelial system (RES) and present a high and selective uptake in inflamed areas. One of the reasons for the success of the carrier is that hydrolysis leads to the metabolite monomers lactic acid and glycolic acid, which are endogenous and easily metabolized by the body via the Krebs cycle. The PLGA delivery system is associated with a nearly negligible potential for toxicity.
Benefits
Biodegradable nanoparticles have been used frequently as drug delivery vehicles due to their improved bioavailability, better encapsulation, and controlled release. The literature describes that micro- or nanoencapsulation of LA greatly prolongs the duration of block and reduces systemic toxicity.
Risks/Limitations
Despite the existing research on biodegradable microparticles containing macromolecular drugs, the effects of critical parameters influencing drug encapsulation are not sufficiently investigated for nanoscale carriers. However, many novel techniques for preparation of drug-loaded nanoparticles are being developed and refined. The crux of the problem is the stability of nanoparticles after preparation, which is being addressed by freeze-drying using different classes of lyoprotectants. Another issue is that precise determination of the drug content is not easy because nanoparticles are colloidal systems. Encapsulation efficiency of drugs varies from 6% to 90% for dexamethasone and paclitaxel, respectively, while mean encapsulation efficiency is around 60% to 70% for various drugs, such as estradiol or xanthones. Another major pitfall of PLGA-based nanoparticles is that although PLGA-based nanoparticles often can present with high encapsulation efficiencies, the drug loading is generally poor (around 1%, which means that nanoparticles contain 1 mg active ingredient per 100 mg polymers of nanoparticles). Yet another important pitfall is the consideration of high burst release of drug from nanoparticles. This phenomenon is described for most PLGA-based nanoparticles. Consequently, the drug might not be able to reach the target tissue or cells, leading to a loss of efficacy. Drug release mechanisms depend on the polymer used and on the loading efficiency. Generally, the rapid initial release is attributed to drug adsorbed to the nanoparticles’ surface. Work is still being conducted to address these issues.
LIPOSOMAL BUPIVACAINE
In October 2011, the FDA approved the use of single-injection liposomal bupivacaine for surgical site infiltration. To date, this is the only FDA-approved controlled-release LA. Liposomal bupivacaine produces reliable plasma levels of bupivacaine up to 72 hours following infiltration. By comparison, traditional bupivacaine HCl has a duration of action of roughly 7 hours following tissue infiltration. Liposomal bupivacaine encapsulates bupivacaine HCl within the carrier, DepoFoam. Prior to this development, extending the duration of action of an LA relied on indwelling catheters and infusion pumps. Infusion technology with an indwelling catheter carries a risk of infection, drug-filling errors, labeling errors, and variable infusion rates, particularly with elastometric pumps. Replacement of elastomeric bags and targeted catheters with LA encapsulated in a liposome is a novel approach for providing analgesia. To date, liposomal bupivacaine has been studied in patients undergoing soft tissue surgery (hemorrhoidectomy, inguinal hernia repair, augmentation mammoplasty) or orthopedic surgery (bunionectomy and total knee arthroplasty). Currently, it is approved for tissue infiltration.
NYSORA Tips
- Liposomal bupivacaine is a controlled-release LA that is FDA approved for wound (surgical site) infiltration.
Formulation
DepoFoam serves as the lipid-based carrier of bupivacaine HCl. When compared with other carriers, such as DepoDur® and DepoCyt, the major difference is the incorporation of dierucoylphosphatidylcholine into the DepoFoam. It is comprised of nonemetogenic, naturally occurring or synthetic analogues of common lipids, making it generally well tolerated, although a tissue infiltration with DepoFoam bupivacaine in rabbits and dogs resulted in granulomatous inflammation, considered a natural reaction against the liposomes.
Pharmacology
Liposomal bupivacaine is currently packaged in a 20-mL vial at a 1.3% concentration. Single-dose administration is recommended, not exceeding 266 mg (one vial). Approximately 3% of the LA in liposomal bupivacaine is present in the free form. Because of this, the drug exhibits two peaks in plasma concentration Tmax following tissue infiltration (Table 1). This was observed by Langford et al in their study of patients receiving infiltration of liposomal bupivacaine for inguinal hernia repair. The first Tmax occurs within the first hour, followed by a second Tmax within 12 hours. Systemic absorption depends on the total dose of drug administered, administration route, and vascularity of the administration site. Liposomal bupivacaine has a 24-hour duration of action. As with traditional bupivacaine, liposomal bupivacaine is metabolized by the liver following its release from the drug delivery system. Caution is recommended when using liposomal bupivacaine for patients with severe hepatic dysfunction. In phase 1, trial patients with moderate hepatic impairment had a 1.5-fold increase in the maximum plasma concentration Cmax compared with healthy controls following single 300-mg infiltration of liposomal bupivacaine. However, this is likely not of great clinical significance with single administration of liposomal bupivacaine. Significant accumulation of bupivacaine or its metabolites is not expected despite impaired liver function. Approximately 6% of bupivacaine is excreted unchanged in urine.
TABLE 1. Pharmacokinetics of controlled-release local anesthetics.
| Drug | Carrier | Tmax (h) | Cmax (ng/mL) |
|---|---|---|---|
| Liposomal bupivacaine | DepoFoam | 1–12a | 365b |
| SABER-bupivacaine | SAIB | 24–48c | 625–989c |
| Bupivacaine-collagen implant | Bioidegradeable collagen matrix | 0.5–20d | 200d |
SAIB = sucrose acetate isobutyrate.
Dosing and Administration
Dilution is recommended with sterile saline up to a maximum total volume of 300 mL. Hypobaric solutions, such as sterile water, may disrupt the liposomal carrier, potentially leading to loss of sustained efficacy and high system drug levels. Diluting liposomal bupivacaine with other drugs, such as lidocaine or bupivacaine HCl, may cause disruption of the carrier, accelerated release of bound bupivacaine and toxicity. Additional LA, of any kind, is not recommended for 24 hours following liposomal bupivacaine administration. The liposomal carrier will maintain its integrity with injection through as small as 30-gauge needles.
NYSORA Tips
- Diluting liposomal bupivacaine with other LA may cause disruption of the lipid carrier, possibly unbinding bupivacaine.
Clinical Evidence
In a phase 3 trial, an infiltration of 266 mg liposomal bupivacaine was compared with placebo in patients receiving hemorrhoidectomy. This randomized, double-blind study of 189 patients found that patients receiving liposomal bupivacaine had significantly less pain and fewer patients required opiate rescue. A significant difference was also observed with regard to 72-hour opioid consumption, which was 45% lower compared with placebo. Following this study, Onel and colleagues compared liposomal bupivacaine to bupivacaine HCl in a similar cohort of patients. This double-blind, randomized, controlled study examined 100 patients for hemorrhoidectomy. Patients had significantly less pain (47%) and required significantly less opioid (66%) over the first 72 hours with liposomal bupivacaine.
NYSORA Tips
- Patients receiving wound infiltration with liposomal bupivacaine had significantly less pain and opioid usage than those who received bupivacaine HCl for hemorrhoidectomy and bunionectomy.
In a double-blind, randomized trial of 193 patients receiving bunionectomy with first-metatarsal osteotomy, liposomal bupivacaine showed significantly reduced pain at 24 and 36 hours when compared with placebo. Although there was no statistically significant difference in pain scores, a liposomal bupivacaine dose-finding study of patients having unilateral inguinal hernia repair demonstrated benefits for secondary endpoints. The liposomal bupivacaine group trended toward lower opioid requirements in patients at all doses (155, 200, 266, 310 mg) compared with 100 mg bupivacaine HCl. In a randomized trial of women having bilateral breast augmentation surgery, subjects were randomized to injection of either 133 or 266 mg liposomal bupivacaine in one breast and 75 mg bupivacaine HCl in the contralateral breast. In both groups, the subjects complained of more pain in the breast receiving bupivacaine HCl. The difference in opioid consump-tion between the two groups only reached significance after 48 hours which is commensurate with the delayed release of bupivacaine from liposome carriers. Use of liposomal bupivacaine in patients after implant-based breast reconstruction demonstrated significantly decreased visual analog scale (VAS) pain scores at 4–24 hours postoperatively when compared to bupivacaine HCl and placebo. There was no difference in opiod and antiemetic usage between the three treatment groups. Multiple studies investigating analgesic efficacy of liposomal bupivacaine in wound infiltration after total knee arthroplasty have been performed. A study by Bagsby et al compared periarticular injection with 2.6% liposomal bupivacaine versus 0.5% ropiva-caine. Patients reported similar mean pain scores at 24 hours, but for the remainder of the hospitalization, pain scores were significantly increased in the liposomal bupivacaine group. Half of the ropivacaine group reported their pain as mild, compared with only 17% of patients receiving liposomal bupivacaine. A recent, large, randomized, controlled trial compared peri-articular injection of liposomal bupivacaine versus bupivacaine HCl. All patients concurrently received multimodal analgesia. The two groups had no significant difference in terms of least, worst, and average daily pain at all time points. Furthermore, there was no difference in consumption of opioids. A recent randomized prospective study compared local infiltration of liposomal bupivacaine with a single-injection femoral nerve block of ropivacaine and tetracaine. The nerve block group had significantly less pain in the first 24 hours postoperatively, but total opioid consumption was unchanged between the two groups. Interestingly, the nerve block group had less opioid during the first day postoperatively, while the liposomal bupivacaine group consumed less on the second day. The two treatments demonstrated no effect on total ambulation, but a greater percentage of patients ambulating and greater total distance was seen in the liposomal bupivacaine group.
Safety
It is recommended that the dose of liposomal bupivacaine should not exceed the single 266-mg vial. Repeat LA administration is not recommended within 72 hours following infiltration. To ensure the liposomal carrier’s integrity, liposomal bupivacaine should be diluted only with normal saline and administered through a needle that is 25 gauge or larger. To avoid possible toxic levels of lidocaine and bupivacaine, liposomal bupivacaine infiltration should follow lidocaine infiltration by at least 20 minutes. Overall, however, in over 1 million patient exposures, liposomal bupivacaine demonstrated a remarkable safety systemic toxicity profile. Safety of liposomal bupivacaine in peripheral nerve blocks (PNBs) is discussed further in the chapter.
NYSORA Tips
- Injection of liposomal bupivacaine should occur at least 20 minutes after infiltration of lidocaine to avoid potential toxicity.
Experimental ApplicationsPeripheral Nerve Blocks
The use of liposomal bupivacaine in PNBs has generated sig-nificant interest as a possible FDA-approved method to prolong nerve block without indwelling catheters. At the time of publication, liposomal bupivacaine has not been approved by the FDA for this indication. Data from preclinical toxicology studies demonstrated no signs of neurotoxicity in animal models. Similarly, a phase 1 study in healthy volunteers demonstrated no nerve injury with single-injection PNB. Efficacy of higher-dose liposomal bupivacaine was seen in femoral nerve blocks for patients receiving tricompartment knee arthroplasty. Patients receiving 133 and 266 mg had significantly decreased resting pain at 24 hours when compared with patients receiving 67 mg of liposomal bupivacaine or saline. A study by Ilfeld et al with variable doses of liposomal bupivacaine (0–80 mg) demonstrated prolonged motor and sensory block with higher doses of the medication. All patients had motor and sensory block more than 24 hours in the 40-mg treatment group and more than 90% in the 80-mg treatment group. A recent review of literature examined the safety of liposomal bupivacaine over six studies with healthy volunteers and patients undergoing various surgical procedures. The most common side effects of perineural liposomal bupivacaine injection were nausea, pyrexia, constipation, vomiting, and pruritus. There was no difference in adverse effects between liposomal bupivacaine and placebo. Treatment-related adverse events had lower incidence in the liposomal bupivacaine versus bupivacaine HCL groups, with the most common adverse event being hypesthesia. Potential deterrents for widespread use of liposomal bupivacaine in PNBs are a possible inability to achieve surgical anesthesia, inferior analgesia compared to bupivacaine HCl over the first 12 postoperative hours, and inability to titrate the LA to effect. Prolonged sensory and motor block may affect early rehabilitation and increase fall risks. If approved for use in PNBs, single-injection liposomal bupivacaine may present a long-acting alternative to continuous PNB. There is potential for increased procedure efficiency and more widespread use of PNBs without the placement and fixation of a perineurial catheter, and the patient would avoid possible adverse events related to catheter placement.
Epidural Anesthesia
Liposomal bupivacaine is currently not approved for epidural administration, although its pharmacologic profile following a single epidural injection has been studied. Viscusi, Candiotti, and colleagues performed a phase 1 randomized, double-blind, active-control, dose-escalating pilot study evaluating a single dose of liposomal bupivacaine at 89, 155, or 266 mg compared with bupivacaine HCl 50 mg in healthy volunteers. Their study concluded that epidural liposomal bupivacaine at 266 mg resulted in a longer duration of sensory block than liposomal bupivacaine 89 or 155 mg or bupivacaine HCl 50 mg. Interestingly, incidence of some degree of motor block was less with liposomal bupivacaine 266 mg versus bupivacaine HCl 50 mg. The liposomal bupivacaine group had fewer patients who were unable to ambulate after 4 hours and a quicker resolution of complete motor block. The high sensorimotor block ratio suggests significant utility for liposomal bupivacaine in epidural anesthesia, but further study is needed to document safety and efficacy.
EXPERIMENTAL MEDICATIONS
SABER-Bupivacaine
SABER (sucrose acetate isobutyrate extended release) technology (Durect Corporation) has been developed as a bioerodable injectable depot system with the potential of delivering a drug over a period of days to 3 months.
Formulation
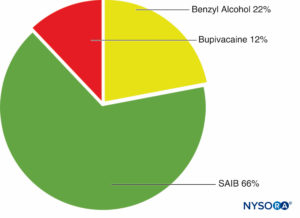
The SABER delivery system consists of sucrose acetate isobutyrate (SAIB), additives, and a solvent. SAIB is a hydrophobic, esterified sucrose derivative that exists as a viscous liquid (Figure 3). The SABER system can be mixed with a drug and injected subcutaneously or intramuscularly with up to a 25-gauge needle.
SABER-bupivacaine (Posidur™), developed by the Durect Corporation, awaits FDA approval.
NYSORA Tips
- SABER-bupivacaine consists of a SAIB delivery system that is mixed with LA. After infiltration, the delivery system dissolves within tissues.
Pharmacology
Solvent type and amount, drug loading, and other additives are possible variables to customize the duration of drug delivery. SABER formulations can carry a drug payload as high as 30%. On injection, the drug forms a depot in subcutaneous tissue, and its release begins immediately. The delivery system dissolves in situ, eliminating the need for removal. In a study comparing differing doses of SABER-bupivacaine (12% bupivacaine), the dose concentration response exhibited linear pharmacokinetics. A large review of 11 clinical trials with both healthy subjects and those undergoing varied surgical pro-cedures demonstrated a varied Tmax at 24–48 hours (Table 1). This seems to differ with surgical procedure, as Tmax with administration after shoulder surgery was shorter when compared with abdominal surgery. This is possibly due to rapid absorption of the drug when confined to a smaller surgical area.
Clinical Evidence
In a 2012 double-blinded, randomized, controlled trial of 124 patients receiving open hernia repair, SABER-bupivacaine out-performed placebo after surgical site administration. A dose of 5 mL of SABER-bupivacaine (12% bupivacaine) had a significantly lower area under the curve (AUC) for mean pain inten-sity from 1 to 72 hours, compared to placebo (2.47 vs. 3.61; p = .0036). The 5-mL group achieved significantly reduced pain with movement and opioid consumption and increased time to first opioid when compared to placebo. Notably, the 2.5-mL formulation of SABER-bupivacaine did not reach the same levels of significance. A 2014 multicenter, randomized, double-blind study of 98 patients undergoing abdominal surgery showed clinically and statistically significant decreased pain for 3 days in patients given SABER-bupivacaine.
Safety
Due to incomplete evidence of clinical safety, the FDA did not approve SABER-bupivacaine’s new drug application in 2013. In 2012, Hadj et al reported no adverse events resulting from SABER-bupivacaine. Wound healing was unchanged among the groups. Gan et al failed to identify any evidence of bupivacaine toxicity through evaluation of vital signs, physical exam, laboratory results, and Holter monitoring.
Bupivacaine-Collagen Implant
A collagen-based implant with LA that is currently waiting for phase 3 testing is a bupivacaine-collagen implant (XaraColl®). This medication is being developed by Innocoll Pharmaceuticals for implantation in sites of surgical trauma to provide postsurgical analgesia.
Formulation
XaraColl is composed of a biodegradable and fully resorbable collagen matrix that is impregnated with bupivacaine (Figure 4). The matrix is implanted during surgery and is purported to begin releasing LA immediately.
NYSORA Tips
- A bupivacaine-collagen implant is composed of a collagen matrix that is impregnated with LA. While the collagen matrix is resorbed, LA is released.

FIGURE 4. Delivery system of bupivacaine-collagen implant. (Used with permission from Innocoll Inc. Website. Accessed November 2015.)
Pharmacology
Collagen implants have been studied with varying concentrations of bupivacaine. With slow resorption of the collagen matrix, controlled release of LA occurs. Systemic bupivacaine levels were demonstrated to be well below the toxicity threshold with a mean Cmax of 0.22 μg/mL (Table 1). Similar to liposomal bupivacaine, this medication demonstrated a biphasic peak of increased concentration. In a study by Cusack, Tmax ranged from 30 minutes to 20 hours, depending on which peak predominated.
Clinical Evidence
Two independent studies in men after unilateral inguinal hernia repair indicated a significant treatment effect for bupivacaine-collagen implants when compared to placebo. In one study, pain scores were significantly decreased in patients treated with implants versus placebo at both 24 and 48 hours with no sig-nificant change in opioid usage. In the second study, pain scores did not differ, but opioid usage decreased significantly in patients with bupivacaine-collagen implants. Pooled analysis of these studies suggested that this treatment effect extended over 72 hours postoperatively.
Safety
Most common adverse events after implantation of bupivacaine-collagen implants were constipation, nausea, and headache. One study demonstrated elevated liver enzymes and abnormal phosphorous levels after implantation, although none of these were clinically significant and resolved spontaneously. Visual disturbances possibly indicating bupivacaine toxicity were found in one patient, but serum sampling showed a low systemic concentration of bupivacaine. Phase 3 trials show a statistically significant decrease in pain scores 48 hours postoperatively in inguinal hernia repair when compared to placebo.
SUMMARY
The clinical practice need for longer duration of analgesia and avoidance of the time-inefficient and procedurally more complex indwelling catheters has spurred interest in controlled-release LAs. Every technology to date has inherent compromises. Evidence to date suggests a clear utility for single-injection extended-released LAs but a continued role for delivery of LA by catheter and indwelling pump. Currently, the only medication in this class with FDA approval is liposomal bupivacaine, which is approved for wound infiltration. The search for new indications has inspired research in multiple modalities. Of particular interest to regional anesthesia and acute pain medicine are its potential use in PNBs and epidural anesthesia. FDA approval for use in these areas has the potential to positively affect the practice of regional anesthesia and quality of postoperative pain management. Controlled-release LAs are likely to become an important inherent part of a multimodal analgesia regimen. Controlled-release LAs, along with other analgesics, may further reduce the reliance on opioids as the primary post-operative analgesia consistent with all current published acute pain guidelines.
REFERENCES
- Samad A, et al: Liposomal drug delivery systems: An update review. Curr Drug Deliv 2007;4(4):297–305.
- Minkowitz HS, Singla NK, Evashenk MA, et al: Pharmacokinetics of sublingual sufentanil tablets and efficacy and safety in the management of postoperative pain. Reg Anesth Pain Med 2013;38:131–139.
- Volltexte, et al: Cyclodextrins as drug carrier molecule: A review. Sci Pharm 2008;76:567–598.
- Kulkarni PR, et al: Liposomes: A novel drug delivery system. Int J Curr Pharm Res 2011;3(2):10–18.
- Formulary: Liposomal bupivacaine: A long acting local anesthetic for postsurgical analgesia.
- Lambert WJ: DepoFoam multivesicular liposomes for the sustained release of macromolecules. In Rathbone MJ, Hadgraft J, Roberts MS, Lane ME (eds): Modified Release Drug Delivery Technology, 2nd ed. Informa Healthcare, 2008:207–214.
- Angst MS, Drover DR: Pharmacology of drugs formulated with Depo-foam: A sustained drug delivery system for parenteral administration using multivesicular liposome technologoy. Clin Pharmacokinet 2006;45(12):1153–1176.
- Howell SB: Clinical applications of a novel sustained-release injectable drug delivery system: Depofoam technology. Cancer J 2001;7 (3): 219–227.
- Bala I, et al: PLGA nanoparticles in drug delivery: the state of the art. Crit Rev Ther Drug Carrier Syst 2004;21(5):387–422.
- Danhier F, et al: PLGA-based nanoparticles: An overview of biomedical applications. J Control Release 2012;161(2):505–522.
- Pathak P, Nagarsenker M: Formulation and evaluation of lidocaine lipid nanosystems for dermal delivery. AAPS PharmSciTech 2009;10(3): 985–992.
- Mundargi RC, et al: Nano/micro technologies for delivering macromolecular therapeutics using poly (D,L-lactide-co-glycolide) and its derivatives. J Control Release 2008;125(3):193–209.
- Marcaine (Bupivacaine HCl) [US prescribing information]. Hospira Inc., 2009.
- ISMP: ISMP Calls for Safety Improvements in Use of Elastomeric Pain Relief Pumps. Institute for Safe Medication Practices, 2009.
- Richard BM, et al: Safety evaluation of EXPAREL (DepoFoam Bupivacaine) administered by repeated subcutaneous injection in rabbits and dogs: Species comparison. J Drug Deliv 2011;2011:467429.
- Richard BM, Ott, LR, et al: The safety and tolerability evaluation of DepoFoam bupivacaine administered by incision wound infiltration in rabbits and dogs. Expert Opin Investig Drugs 2011;20(10):1327–1341.
- Langford RM, et al: A single administration of depobupivacaine intraoperatively results in prolonged detectable plasma bupivacaine and analgesia in patients undergoing inguinal hernia repair. Presented at 62nd Postgraduate Assembly in Anesthesiology, December 12–16, 2008, New York, Poster 9088.
- Exparel (Bupivacaine Liposome Extended-Release Injectable Suspension) [prescribing information]. Pacira Pharmaceuticals Inc., 2011.
- Clinical Trial no SKY0402-C-110. An open-label, phase I study to assess the pharmacokinetics and safety of SKY0402 in subjects with impaired hepatic function. Pacira Pharmaceuticals Inc. (date on file).
- 20. Hadzic A, Abikhaled JA, Harmon WJ: Impact of volume expansion on the efficacy and pharmacokinetics of liposome bupivacaine. Local Reg Anesth 2015;8:105–111.
- Gorfine SR, et al: Bupivacaine extended-release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: A multicenter, randomized, double-blind, placebo-controlled trial. Dis Colon Rectum 2011;54(12)1552–1559.
- Onel E, et al: Exparel, a liposomal bupivacaine local analgesic, extends pain relief and decreases opioid use. Presented at Annual Meeting of the American Society of Anesthesiologists, October 16–20, 2010, San Diego, CA.
- Golf M, et al: A phase 3, randomized, placebo-controlled trial of DepoFoam® bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Adv Ther 2011;28(9):776–788.
- Clinical Trial no. SKY0402-C-210. A randomized, double-blind, active-control study to evaluate the safety and efficacy of a single local administration of SKY0402 for prolonged postoperative analgesia in subjects undergoing augmentation mammoplasty. Pacira Pharmaceuticals Inc. (date on file)
- Butz DR, Shenaq DS, Rundell VL, et al: Postoperative pain and length of stay lowered by use of exparel in immediate, implant-based breast reconstruction. Plast Reconstr Surg Glob Open 2015;3(5):e391.
- Bagsby DT, Ireland PH, Meneghini RM: Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty 2014;29(8):1687–1690.
- Alijanipour et al: Peri-articular injection of liposomal bupivacaine offers no benefit over standard bupivacaine injection in total knee arthroplasty: a prospective, randomized, controlled trial. Presented at 2016 Annual Meeting of the American Academy of Orthopedic Surgeons, March 1, 2016. Orlando, FL.
- Surdam JW, et al: The use of Exparel (liposomal bupivacaine) to manage postoperative pain in unilateral total knee arthroplasty patients. J Arthroplasty 2015;30:325–329.
- Viscusi ER: The safety of liposome bupivacaine 2 years post-launch: A look back and a look forward. Expert Opin Drug Saf 2015;14(12): 1801–1803.
- Ilfeld BM, Viscusi ER, Hadzic A, et al: Safety and side effect profile of liposome bupivacaine (Exparel) in peripheral nerve blocks. Reg Anesth Pain Med 2015;40(5):572–582.
- McAlvin JB, et al: Multivesicular liposomal bupivacaine at the sciatic nerve. Biomaterials 2014;35:4557–4564.
- Damjanovska M, Cvetko E, Hadzic A, et al: Neurotoxicity of perineural vs intraneural-extrafascicular injection of liposomal bupivacaine in the porcine model of sciatic nerve block. Anaesthesia 2015;70(12): 1418–1426.
- Ilfeld BM, et al: Liposomal bupivacaine as a single-injection peripheral nerve block: A dose-response study. Anesth Analg 2013;117:1248–1256.
- Ilfeld BM, et al: Safety and side effect profile of liposome bupivacaine (Exparel) in peripheral nerve blocks. Reg Anesth Pain Med 2015;40: 572–582.
- Ilfeld BM, et al: A 4-day peripheral nerve block? Liposome bupivacaine: An introduction and update. ASA Newsletter 2014;78(8).
- Viscusi ER, Candiotti KA, Onel E, Morren M, Ludbrook GL: The pharmacokinetics and pharmacodynamics of liposome bupivacaine administered via a single epidural injection to healthy volunteers. Reg Anesth Pain Med 2012;37(6):616–622.
- Hadj A, et al: Safety and efficacy of extended-release bupivacaine local anesthetic in open hernia: A randomized controlled trial. ANZ J Surg 2012;82:251-257.
- Sekar M, et al: Drug delivery of biologics: A controlled release strategy. Presented at IBC’s 17th Annual TIDES Conference, May 3–6, 2015, San Diego, CA.
- Shah J, et al: The PK profile of SABER-bupivacaine in humans across surgical models demonstrates sustained 72-hour drug delivery. Presented at 2014 Annual Meeting of the American Society of Anesthesiologists, October 15, 2014, New Orleans, LA.
- Gan T, et al: SABER-bupivacaine reduced pain intensity for 72 hours following abdominal surgery relative to bupivacaine HCl. Presented at 2014 Annual Meeting of the American Society of Anesthesiologists, October 15, 2014, New Orleans, LA.
- Cusack SL, et al: Clinical evaluation of XaraColl, a bupivacaine-collagen implant, for postoperative analgesia in two multicenter, randomized, double-blind, placebo-controlled pilot studies. J Pain Res 2012;5: 217–225.
- Cusack SL, et al: The pharmacokinetics and safety of an intraoperative bupivacaine-collagen implant (XaraColl®) for postoperative analgesia in women following total abdominal hysterectomy. J Pain Res 2013;6: 151–159.
- Hu D, et al: Pharmacokinetic profile of liposome bupivacaine injection following a single administration at the surgical site. Clin Drug Investig 2013;33:109–115.
- Clinical Trial NCT02523599. A Phase 3, Randomized, Double-blind, Placebo-controlled Study to Investigate the Efficacy and Safety of the Xaracoll® Bupivacaine Implant (300mg Bupivacaine Hydrochloride) After Open Laparotomy Hernioplasty. Innocoll. (date on file)