Brian E. Harrington and Miguel Angel Reina
INTRODUCTION
Postural headaches following interventions that disrupt meningeal integrity are most commonly labeled postdural puncture headaches (PDPHs). This terminology has been officially adopted in the International Classification of Headache Disorders and is used in this section. However, use of the word postdural has been criticized as confusing and probably inaccurate, resulting in the proposal of an alternate term, meningeal puncture headache (MPH), which readers may increasingly encounter. It is also important to acknowledge that references to “dural puncture” throughout the medical literature actually describe puncture of the dura-arachnoid and are more correctly termed and thought of as “meningeal puncture.” Regardless of terminology, the PDPH is well known to the many clinicians whose practice includes procedures that access the subarachnoid space.
Yet, our understanding of this serious complication remains surprisingly incomplete. This section summarizes the current state of knowledge regarding this familiar iatrogenic problem as well as the closely related topics of accidental, or unintentional, dural puncture (ADP or UDP, respectively), and the epidural blood patch (EBP).
HISTORY AND CURRENT RELEVANCE
As one of the earliest recognized complications of regional anesthesia, PDPH has a long and colorful history. Dr. August Bier noted this adverse effect in the first patient to undergo successful spinal anesthesia on August 16, 1898 (Figure 1). Bier observed: “Two hours after the operation his back and left leg became painful and the patient vomited and complained of severe headache. The pain and vomiting soon ceased, but headache was still present the next day” (italics added). The following week, Bier and his assistant, Dr. August Hildebrandt, performed experiments with cocainization of the spinal cord on themselves. In a description of PDPH scarcely improved on in an intervening century, Bier later reported firsthand his experience in the days to follow: “I had a feeling of very strong pressure on my skull and became rather dizzy when I stood up rapidly from my chair. All these symptoms vanished at once when I lay down flat, but returned when I stood up. … I was forced to take to bed and remained there for nine days, because all the manifestations recurred as soon as I got up. … The symptoms finally resolved nine days after the lumbar puncture.” In medical history, few complications have come to be associated as closely to a specific technique as PDPH with spinal anesthesia. Employing the methods of the early 20th century, spinal anesthesia was frequently followed by severe and prolonged headache, casting a long shadow over the development and acceptance of this modality. Investigations into the cause of these troubling symptoms eventually led to the conclusion that they were due to persistent cerebrospinal fluid (CSF) loss through the rent created in the meninges. The most notable successful efforts to minimize the loss of CSF were through the use of smaller-gauge and “noncutting” needles (as convincingly demonstrated in the 1950s by Vandam and Dripps and Hart and Whitacre, respectively). Despite these significant advances in prevention, PDPH remained a frustratingly common occurrence.
The extensive search for effective treatments for PDPH dates to Bier’s time. Yet, efforts through the first half of the 20th century, while often intensive and creative, were questionably worthwhile. In a monograph intended to be a comprehensive review of PDPH from the 1890s through 1960, Dr. Wallace Tourette and colleagues cited dozens of separate and far-ranging treatment recommendations, including such interventions as intravenous ethanol, x-rays to the skull, sympathetic blocks, and manipulation of the spine. Unfortunately, prior to the introduction of the EBP there were no treatment measures that could be described as significant improvements over the simple passage of time. In his 1955 textbook, Complications of Regional Anesthesia, Dr. Daniel C. Moore described in detail a full 3-day treatment protocol for PDPH. He concluded by noting that 3 days was the usual duration of untreated mild-to-moderate headaches, but that, “Nevertheless, the patient feels an attempt to help his problem is being made.” The EBP, a startlingly unique medical procedure, proved to be the major breakthrough in the treatment of PDPH. The concept of using autologous blood to “patch” a hole in the meninges was introduced in late 1960 by Dr. James Gormley, a general surgeon.
Yet, Gormley’s brief report went largely unnoticed for nearly a decade because, to the practitioners of the day, an iatrogenic epidural hematoma raised serious concerns of scarring, infection, and nerve damage. The procedure was only later popularized in anesthesiology circles, and performed as a true epidural injection, largely through the work of Drs. Anthony DiGiovanni and Burdett Dunbar. The EBP procedure was further refined through the 1970s as the volume of blood commonly utilized increased to 20 mL. Today, the EBP is nearly universally employed as the cornerstone for treatment for severe PDPH. Postdural puncture headache remains a prominent clinical concern to the present day. Largely due to modifications in practice that followed the identification of risk factors, rates of PDPH following spinal anesthesia have steadily declined, from an incidence exceeding 50% in Bier’s time, to around 10% in the 1950s, until currently a rate of 1% or less can be reasonably expected. However, as perhaps the highest-risk group, an unfortunate 1.7% of obstetric patients continue to experience PDPH after spinal anesthesia using 27-gauge Whitacre needles. Intending to avoid meningeal puncture, epidural techniques are an attractive alternative to spinal anesthesia.
Yet, occasional ADP, with either the needle or the catheter, is unavoidable (and may be unrecognized at the time in over 25% of patients who eventually develop PDPH). In nonobstetric situations (eg, interlaminar epidural steroid injections), the rate of ADP should be less than 0.5%. However, ADP is of greatest concern in the obstetric anesthesia setting, where the incidence of this adverse event is around 1.5%. Over half of all patients who experience ADP with epidural needles will eventually develop headache symptoms, with many studies in obstetric populations reporting PDPH rates of 75% or greater. Of further concern, ADP in parturients has also been noted to be associated with chronic headache and back pain that is reduced, but not entirely eliminated, by EBP. In addition to anesthesia interventions, PDPH remains a too-common iatrogenic complication following myelography and diagnostic/therapeutic lumbar puncture (LP). In these situations, rates of MPH of around 10% are still commonly cited as practitioners often continue to use large-gauge Quincke needles—considered necessary due to the viscosity of contrast material and to facilitate the timely collection of CSF. Consequently, there is evidence to suggest that the majority of instances of PDPH now have a non-anesthesia-related origin.
NYSORA Tips
• PDPH may carry a risk of medicolegal liability.
• ADP may result in chronic headache and back pain.
• Anesthetic procedures with risk of PDPH require proper informed consent.
The practical significance of PDPH is illustrated by notation in the American Society of Anesthesiologists Closed Claims Project database as one of the most frequent claims for malpractice involving obstetric anesthesia, regional anesthesia, and chronic pain management. Justifiably, headache is the most commonly disclosed risk when obtaining consent for spinal and epidural anesthesia. The potentially serious nature of this complication necessitates inclusion in informed consent involving any procedure that may result in PDPH. As part of this discussion, patients should also be apprised of the normal delayed onset of symptoms and be given clear instructions for the timely provision of advice or management should they experience adverse effects.
PATHOPHYSIOLOGY
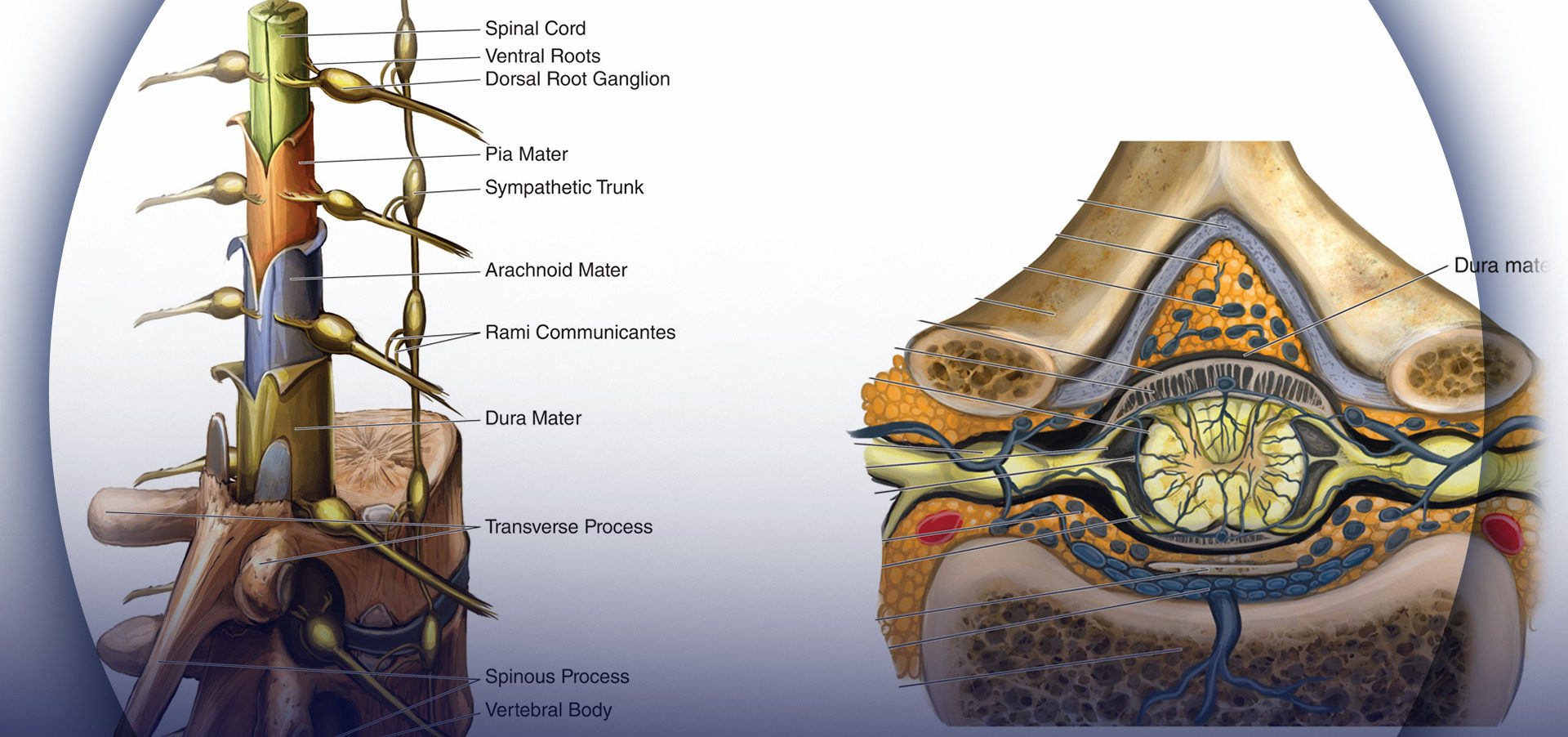
It has long been accepted that PDPH results from a disruption of normal CSF homeostasis. However, despite a great deal of research and observational data, the pathophysiology of PDPH remains incompletely understood. Cerebrospinal fluid is produced primarily in the choroid plexus at a rate of approximately 0.35 mL/min and reabsorbed through the arachnoid villa. The total CSF volume in adults is maintained around 150 mL, of which approximately half is extracranial, and gives rise to normal lumbar opening pressures of 5–15 cm H2O in the horizontal position (40–50 cm H2O in the upright position). It has been shown experimentally that the loss of approximately 10% of total CSF volume predictably results i the development of typical PDPH symptoms, which resolve promptly with reconstitution of this deficit. It is generally agreed that PDPH is due to the loss of CSF through a persistent leak in the meninges. In this regard, it has been postulated that the cellular arachnoid mater (containing frequent tight junctions and occluding junctions) is perhaps more important than the more permeable and acellular dura mater in the generation of symptoms. In fresh cadavers, Reina et al studied lesions of the human dural sac produced by different spinal needles and different bevel orientations. The dura mater has a thickness around 400 μm, and it is formed by randomly distributed fibers, arranged around 80 concentric layers, known as dural laminas, while the arachnoid layer has a thickness around 40 μm1 (Figure 2).
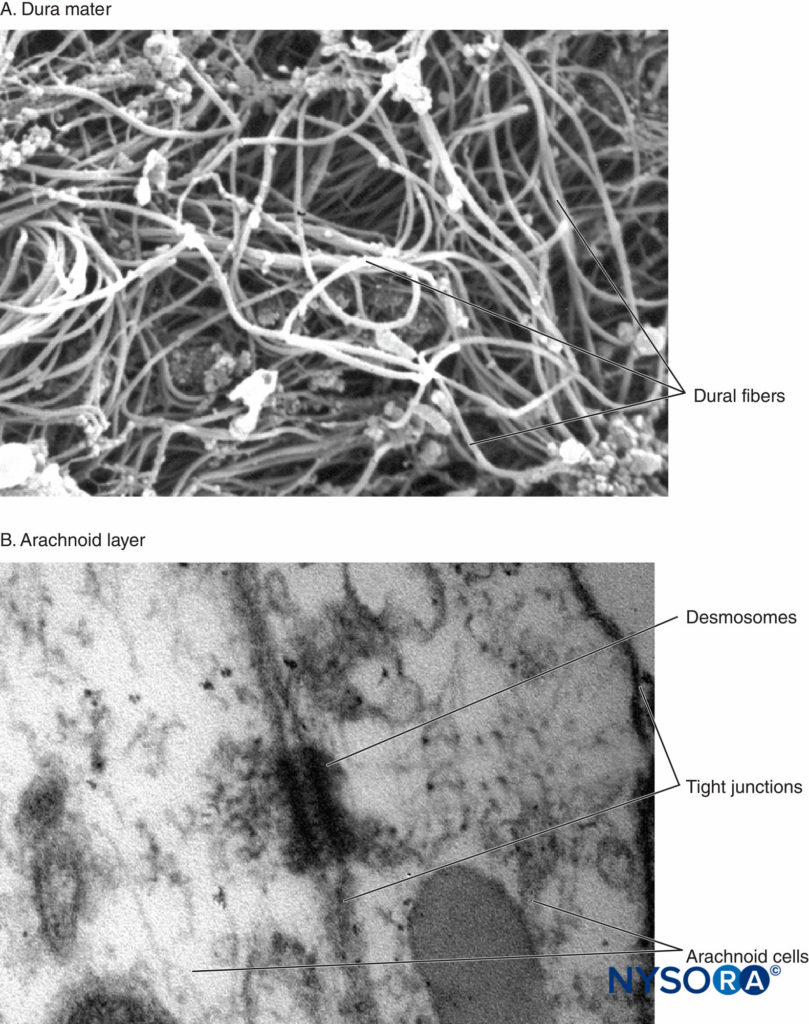
FIGURE 2. A: Human spinal dura mater. Collagen fibers in a random direction. Scanning electron microscopy. Magnification x6500. Reproduced with permission from Dittmann M, Reina MA, López García A: New results in the visualization of the spinal dura mater with scanning electron microscopy. Anaesthesist. 1998 May;47(5):409-413. B: Human spinal arachnoid layer. Arachnoid cells. Transmission electron microscopy. Magnification x150000. Reproduced with permission from Reina MA1, Prats-Galino A, Sola RG, et al: Structure of the arachnoid layer of the human spinal meninges: a barrier that regulates dural sac permeability. Rev Esp Anestesiol Reanim. 2010 Oct;57(8):486–492.
Recently, these authors reported on the possible importance of the arachnoid layer in the closure of dural and arachnoid lesions. The arachnoid membrane may exhibit tissue closure in relation to the dura because its main function is to act as a barrier; therefore, it may lack the elastic properties of the dural layer. The arachnoid layer limits the escape of fluid, so the amount of CSF lost through the punctured orifice is likely related to the speed of closure of the arachnoid lesion (Figures 3 to 6).
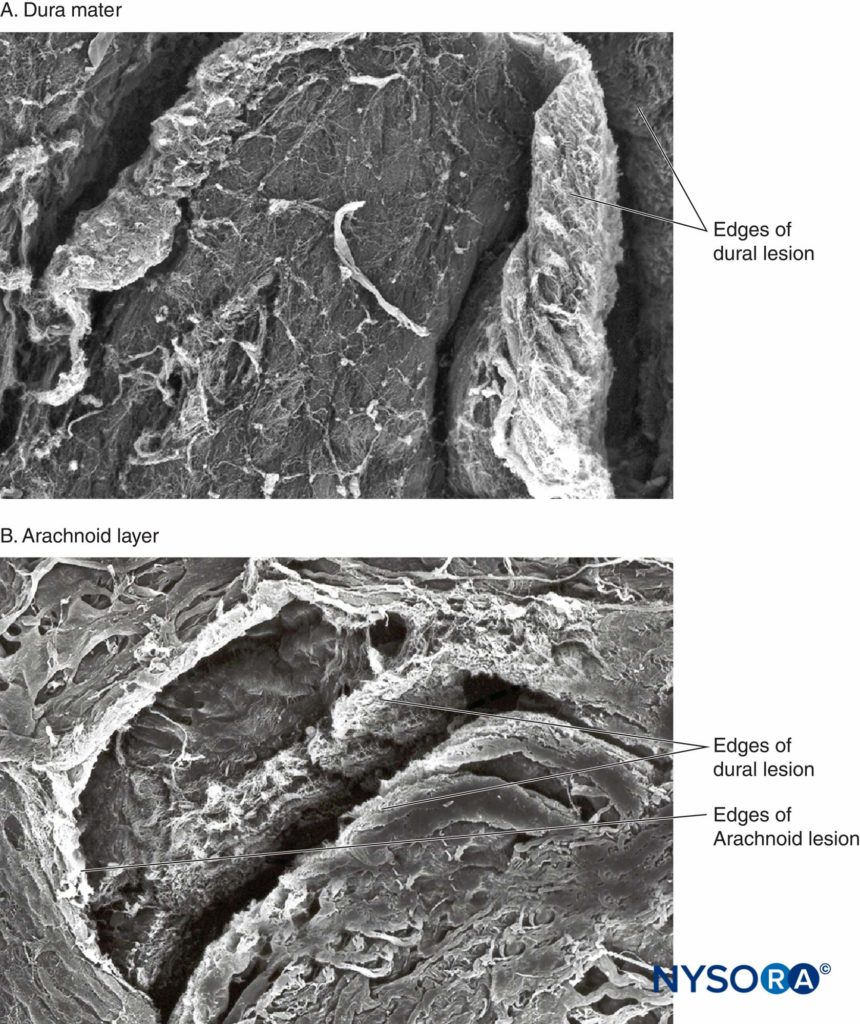
FIGURE 3. Human dura mater. Dura-arachnoid lesion produced by 25-gauge Quincke needle. Scanning electron microscopy. Magnification ×200. A: Dural surface. B: Arachnoid surface.
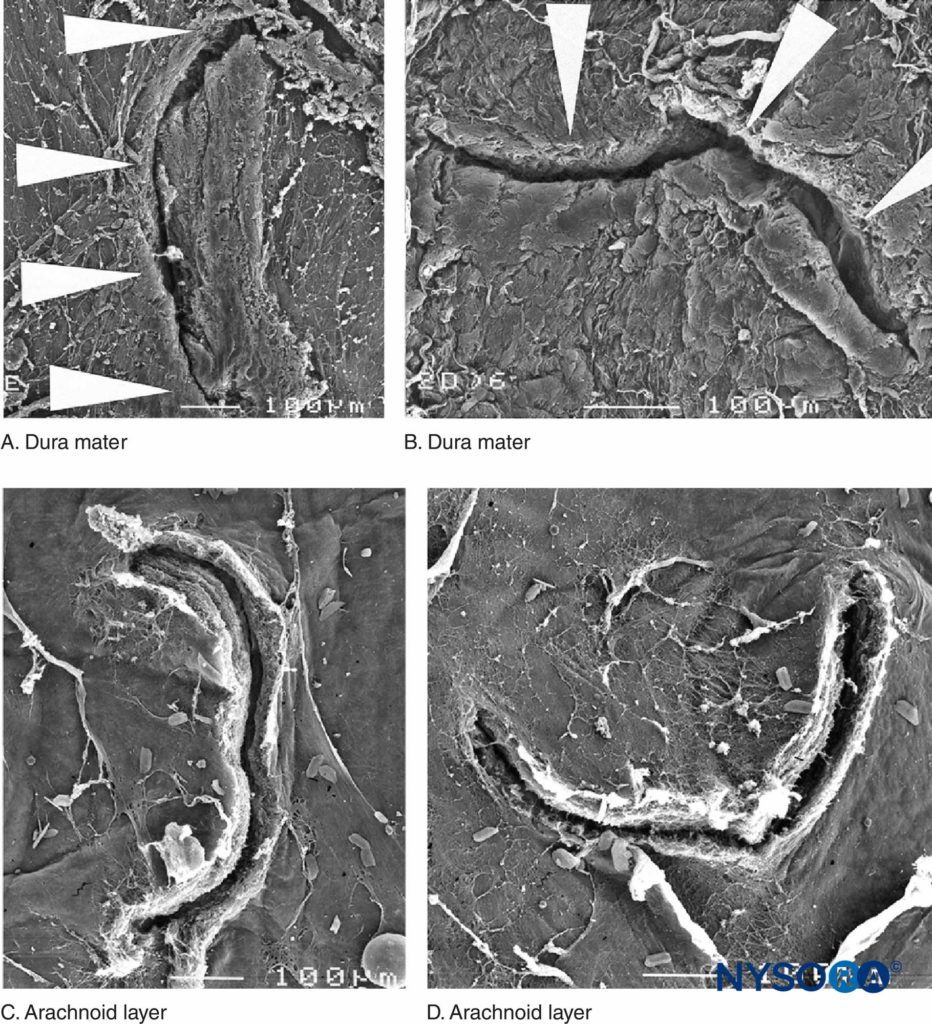
FIGURE 4. Human dura-arachnoid lesion produced by 22-gauge Quincke needle. Scanning electron microscopy. Magnification ×100. A and B: Dural surface. C and D: Arachnoid surface. (Reproduced with permission from Reina MA, López A, Badorrey V, et al: Dura-arachnoid lesions produced by 22 gauge Quincke spinal needles during a lumbar puncture. J Neurol Neurosurg Psychiatry. 2004 Jun;75(6):893–897.)
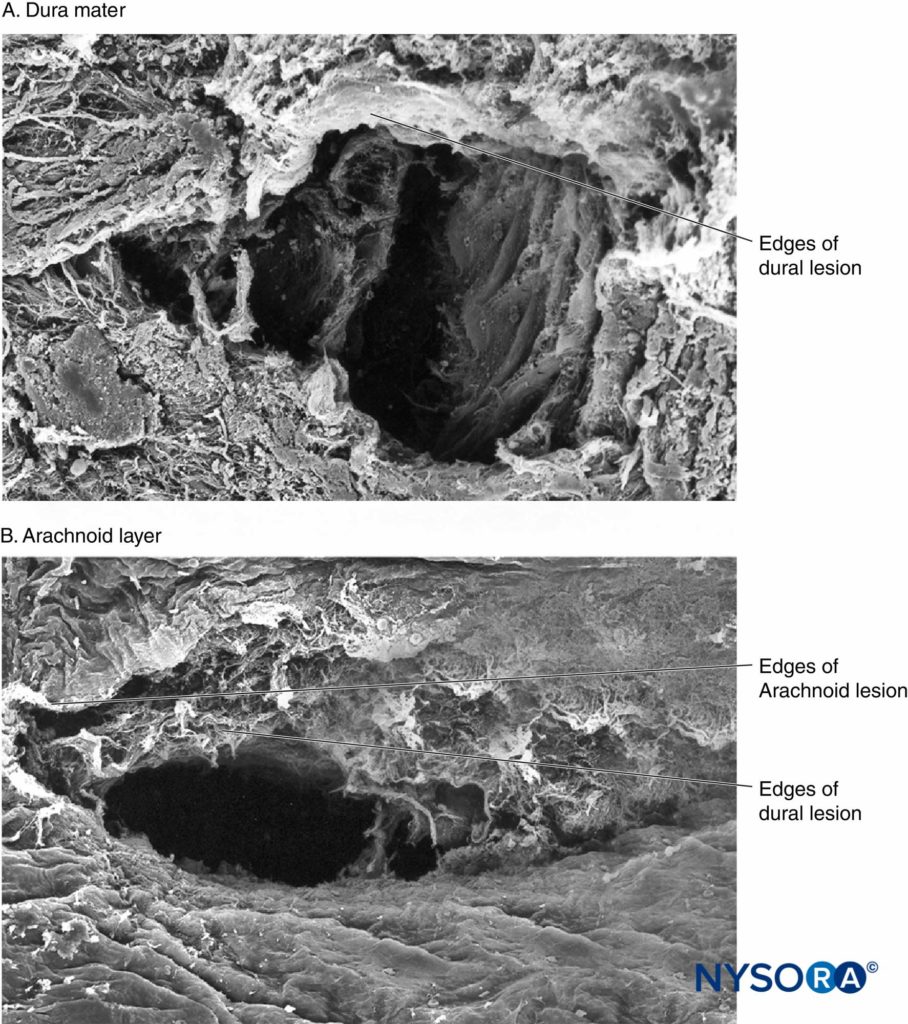
FIGURE 5. Human dura-arachnoid lesion produced by 25-gauge Whitacre needle. Scanning electron microscopy. Magnification ×200. A: Dural surface. B: Arachnoid surface. (Reproduced with permission from Reina MA, López-García A, de Andrés-Ibáñez JA, et al: Electron microscopy of the lesions produced in the human dura mater by Quincke beveled and Whitacre needles. Rev Esp Anestesiol Reanim. 1997 Feb; 44(2):56–61)
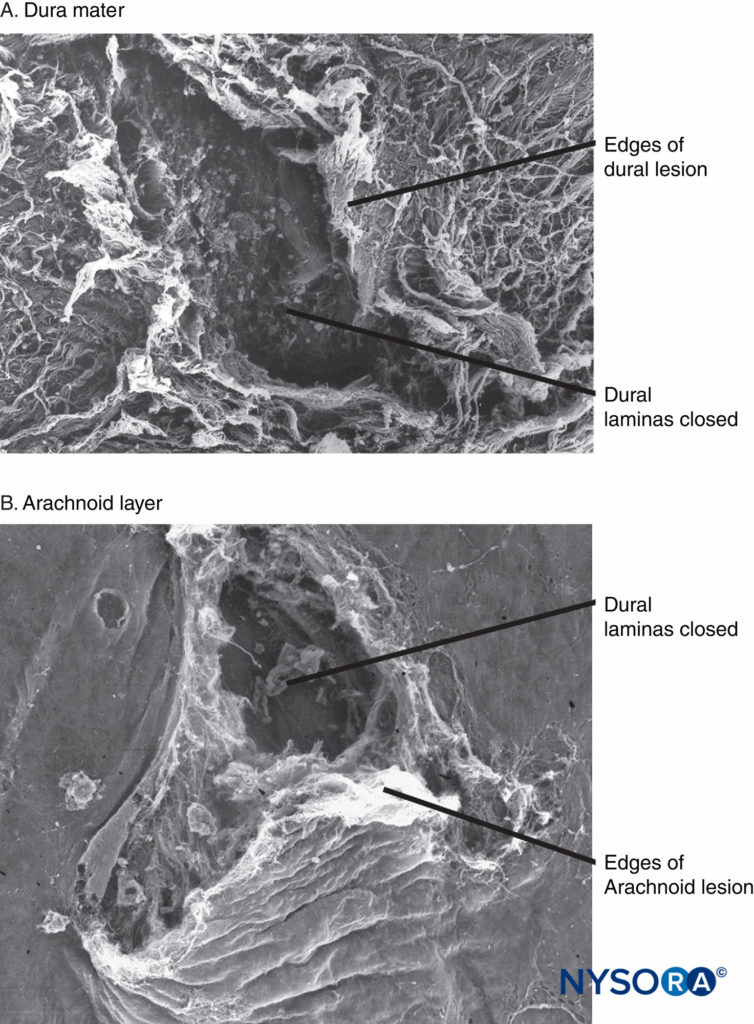
FIGURE 6. Human spinal dura mater. Dura-arachnoid lesion produced by 25-gauge Whitacre needle. Scanning electron microscopy. Magnification ×200. A: Dural surface. B: Arachnoid surface. (Reproduced with permission from Reina MA, de Leon-Casasola OA, Lopez A, et al: An in vitro study of dural lesions produced by 25-gauge Quincke and Whitacre needles evaluated by scanning electron microscopy. Reg Anesth Pain Med. 2000 Jul-Aug;25(4):393–402.)
Thus, the anatomically supported term meningeal puncture headache (MPH) has been proposed as an alternative to the rather ambiguous postdural puncture headache (PDPH). The apparent role of the arachnoid mater in this disorder further calls into question the significance of many published studies that involve isolated dura mater in vitro. The actual means by which CSF hypotension generates headache is somewhat controversial and currently ascribed to a bimodal mechanism involving both loss of intracranial support and cerebral vasodilation (predominantly venous). Diminished buoyant support is thought to allow the brain to sag in the upright position, resulting in traction and pressure on pain-sensitive structures within the cranium (dura, cranial nerves, bridging veins, and venous sinuses). Adenosine-mediated vasodilation may occur secondary to diminished intracranial CSF (in accordance with the Monro-Kellie hypothesis, which states that intracranial volume must remain constant) and reflexively secondary to traction on intracranial vessels. Multiple neural pathways are involved in generating the symptoms of PDPH. These include the ophthalmic branch of the trigeminal nerve (cranial nerve [CN] V1) in frontal head pain, cranial nerves IX and X in occipital pain, and cervical nerves C1–C3 in neck and shoulder pain. Nausea is attributed to vagal stimulation (CN X). Auditory and vestibular symptoms are secondary to the direct communication between the CSF and the perilymph via the cochlear aqueduct, which results in decreased perilymphatic pressures in the inner ear and an imbalance between the endolymph and perilymph. Significant visual disturbances are thought to represent a transient palsy of the nerves supplying the extraocular muscles of the eye (CN III, IV, and VI). Here, the lateral rectus muscle is most often involved, which is attributed to the long, vulnerable intracranial course of the abducens nerve (CN VI). Other, much less-frequent cranial nerve palsies of the trigeminal (CN V), facial (CN VII), and auditory (CN VIII) nerves have also been reported.
CLINICAL PRESENTATION AND CHARACTERISTICS
Although many clinical variations have been described, most cases of PDPH are characterized by their typical onset, presentation, and associated symptoms.
NYSORA Tips
Most cases of PDPH will be typical (see text for details) in
• Onset—often delayed, but within 48 hours
• Presentation—symmetric, bilateral headache
• Associated symptoms—more likely with severe headache
Onset
Onset of symptoms is generally delayed, with headache usually beginning 12–48 hours and rarely more than 5 days following meningeal puncture. In their landmark observational study, Vandam and Dripps reported onset of headache symptoms within 3 days of spinal anesthesia in 84.8% of patients for whom such data were available. More recently, Lybecker and colleagues performed a detailed analysis of 75 consecutive patients with PDPH following spinal anesthesia (primarily using 25-gauge cutting-point needles). While none of their patients noted the onset of symptoms during the first hour following meningeal puncture, 65% experienced symptoms within 24 hours and 92% within 48 hours. An onset of symptoms within 1 hour of neuraxial procedures is suspicious for pneumocephalus, especially in the setting of an epidural loss of-resistance technique using air. Occasional reports of unusually delayed onset of PDPH highlight the importance of seeking a history of central neuraxial instrumentation whenever positional headaches are evaluated.
Presentation
The cardinal feature of PDPH is its postural nature, with headache symptoms worsening in the upright position and relieved, or at least improved, with recumbency. The International Headache Society (IHS) diagnostic criteria further describe this positional quality as worsening within 15 minutes of sitting or standing and improving within 15 minutes after lying. Headache is always bilateral, with a distribution that is frontal (25%), occipital (27%), or both (45%). Headaches are typically described as “dull/aching,” “throbbing,” or “pressure type.” The severity of headache symptoms, a feature with important ramifications for treatment, varies considerably among patients. Although there is no universally accepted severity scale, one practical approach is to have patients simply rate their headache intensity using a 10-point analog scale, with 1–3 classified as “mild,” 4–6 “moderate,” and 7–10 “severe.” Lybecker et al further categorized patients according to restriction in physical activity, degree of confinement to bed, and presence of associated symptoms. Using this classification system, they prospectively determined that 11% of their PDPH cases after spinal anesthesia were mild, 23% moderate, and 67% severe.
Associated Symptoms
The IHS criteria for PDPH require that headache be accompanied by at least one of the following symptoms: neck stiffness, tinnitus, hypoacusia, photophobia, and nausea. However, these criteria may need to be revisited as many patients (29% in one recent study) have been noted to suffer from PDPH in the absence of any symptoms apart from the headache itself. It can be said that the more severe the headache, the more likely it is to be accompanied by associated symptoms.
NYSORA Tips
The IHS criteria for PDPH are as follows:
• Headache accompanied by at least one of these symptoms:
• neck stiffness
• tinnitus
• hypoacusia
• photophobia
• nausea
The most common associated symptom is nausea, which may be reported by a majority of patients (especially if questioned specifically) and can lead to vomiting. Pain and stiffness in the neck and shoulders are also common and are seen in nearly half of all patients experiencing PDPH. Uncommonly, patients may experience auditory or visual symptoms, and the risk for either appears to be directly related to needle size. In Vandam and Dripps’s large observational study of PDPH, auditory and visual symptoms were each seen in 0.4% of patients. Auditory symptoms include hearing loss, tinnitus, and even hyperacusis, and can be unilateral. It is interesting to note that subclinical hearing loss, especially in the lower frequencies, has been found to be common following spinal anesthesia, even in the absence of PDPH. Closely associated with an auditory function, vestibular disturbances (dizziness or vertigo) may also occur. Visual problems include blurred vision, difficulties with accommodation, mild photophobia, and diplopia. In contrast to headache complaints, which are consistently bilateral, nearly 80% of episodes of diplopia secondary to meningeal puncture involve unilateral cranial nerve palsies.
RISK FACTORS
Risk factors for PDPH can be broadly categorized into patient characteristics and procedural details.
Patient Characteristics
The patient characteristic having the greatest impact on risk of PDPH is age. Uncommonly reported in children less than 10 years of age, PDPH has a peak incidence in the teens and early 20s. The incidence then declines over time, becoming much less frequent in patients over 50 years of age. Gender is also a significant risk factor, with nonpregnant females having approximately twice the risk for PDPH when compared with age-matched male subjects. While the etiology behind this gender difference has not been convincingly elucidated, a number of physiological, anatomical, social, perceptual, and behavioral explanations have been proposed.
NYSORA Tips
Major patient-related risk factors for PDPH include:
• Age: It is uncommon in patients less than 10 years of age; peak incidence is in the teens and early 20s.
• Gender: Nonpregnant females have twice the risk compared to age-matched men.
Pregnancy has traditionally been regarded as a risk factor for PDPH, but this consideration largely reflects a young female cohort as well as the high incidence of ADP in the gravid population. Although controversial, pushing during the second stage of labor, thought to promote the loss of CSF through a hole in the meninges, has been reported to influence the risk of PDPH following ADP. Angle and colleagues noted that the cumulative duration of bearing down correlated with the risk of developing PDPH in patients who had experienced ADP.41 They also found that patients who avoided pushing altogether (proceeded to cesarean delivery prior to reaching second-stage labor) had a much lower incidence of PDPH (10%) than those who pushed (74%). Furthermore, they noted a marked difference in the requirement for EBP to treat PDPH between those who pushed and those who did not (81% vs. 0%). Body mass index (BMI) appears to be a mixed-risk factor. Morbid obesity presents obvious technical difficulties for central neuraxial procedures, increasing the likelihood of multiple needle passes and ADP. Yet, low BMI has been reported to be an independent risk factor for PDPH, and high BMI (ie, obesity) may actually decrease risk, possibly secondary to a beneficial effect of increased intra-abdominal pressure. Recently, a retrospective analysis reported cigarette smoking to be associated with a lower risk of PDPH. It can be hoped that this observation will promote further insights into the mechanism of PDPH symptoms and pharmacologic treatment options. Postdural puncture headaches appear to have an interesting association with other headaches. Patients who report having had a headache within the week prior to LP have been observed to have a higher incidence of PDPH. On further analysis, only those with chronic bilateral tension-type headaches were found to be at increased risk. A history of unilateral headache or migraine has not been linked to an increased risk of PDPH. Menstrual cycle, a factor in migraine headaches did not influence the rate of PDPH in one small pilot study. Patients with a history of previous PDPH, particularly women, appear to have an increased risk for new PDPH after spinal anesthesia. With epidural procedures, patients with a history of ADP have been shown to be at slightly increased risk for another ADP (and subsequent PDPH).
Procedural Details
Needle size and tip design are the most important procedural factors related to PDPH. Needle size is directly related to the risk of PDPH. Meningeal puncture with larger needles is associated with a higher incidence of PDPH, more severe headaches, more associated symptoms, a longer duration of symptoms, and a greater need for definitive treatment measures. Needle tip design is also a major influence, with “noncutting” needles clearly associated with a reduced incidence of PDPH when compared with “cutting” (usually Quincke) needles of the same gauge (Figure 7). In general, noncutting needles have an opening set back from a tapered (“pencil-point”) tip and include the Whitacre, Sprotte, European, Pencan, and Gertie Marx needles. Adding to this somewhat-confusing terminology, noncutting needles are sometimes still incorrectly referred to as “atraumatic” needles, this despite being shown with electron microscopy to produce a more traumatic rent in the dura than cutting needles (perhaps resulting in a better inflammatory healing response). The influence of needle size on risk of PDPH appears to be greatest for cutting needles (in other words, the reduction seen in the incidence of PDPH between 22- and 26-gauge sizes is greater for cutting than noncutting needles). Insertion of cutting needles with the bevel parallel to the long axis of the spine significantly reduces the incidence of PDPH. This observation was for many years attributed to spreading rather than cutting of longitudinally oriented dural fibers. However, scanning electron microscopy revealed the dura to be made of many layers of concentrically directed fibers, and the importance of needle bevel insertion is now thought to be due to longitudinal tension on the meninges, particularly in the upright position, and its influence on CSF leakage through holes having differing orientations.
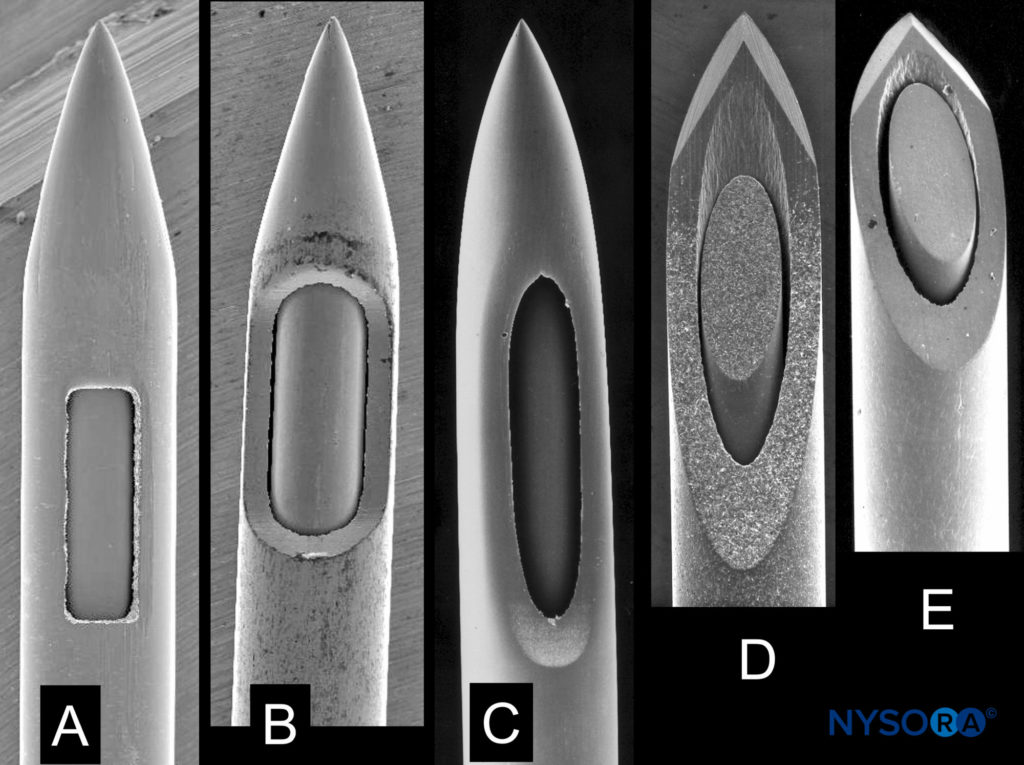
FIGURE 7. Spinal needles of different manufacturers with same external diameter. A: Whitacre type. B: Spinal type. C: Sprotte type. D, E: Quincke type. Scanning electron microscopy. Magnification ×40. (Reproduced with permission from Reina MA: Atlas of Functional Anatomy for Regional Anesthesia and Pain Medicine. Heidelberg: Springer; 2015.)
NYSORA Tips
• The most important equipment-related details for risk of PDPH are needle gauge (larger > smaller) and needle tip design (cutting > noncutting).
Not surprisingly, the experience/comfort/skill of the operator appear to be significant factors in the development of PDPH. A larger number of meningeal punctures, frequently associated with inexperience, have been shown to increase the rate of PDPH. De Almeida et al noted higher rates of PDPH when LP was performed by inexperienced providers. Higher rates of ADP have been consistently reported when epidural procedures are performed by residents. The risk of ADP also appears to be higher for procedures done at night, strongly suggesting a significant contribution of operator fatigue. A number of procedural details do not appear to influence the rate of development of PDPH, including patient position at the time of meningeal puncture, “bloody tap” during spinal anesthesia, addition of opiates to the spinal block, and volume of CSF removed (for diagnostic purposes).
PREVENTION
Although prophylaxis is most simply thought of as preventing any symptoms of PDPH, in the clinical context this issue is deceptively complex. It is important to appreciate that significant “prevention” may encompass a number of other endpoints, such as a reduced incidence of severe PDPH, a shorter duration of symptoms, or decreased need for EBP. Unfortunately, despite the clear relevance of this issue, the overall quality of evidence for preventive measures is generally weak.
General Measures
As with all regional techniques, appropriate patient selection is crucial in minimizing complications. As age is a major risk factor, indications for spinal anesthesia should be weighed against the risks of PDPH in patients under 40 years of age unless the benefits are sufficiently compelling (such as in the obstetric population). Practitioners (and patients alike) may also wish to carefully consider central neuraxial techniques in those with a previous history of ADP or PDPH (particularly females). Other patient-related factors (eg, obesity) should be considered on a case-by-case basis, weighing the risks of PDPH with the benefits of regional anesthesia. While only recently utilized for neuraxial techniques, the use of ultrasound for regional anesthesia holds some promise in reducing the risk of PDPH. Ultrasound can decrease the number of needle passes required for regional procedures and has been shown to accurately predict the depth of the epidural space. Further study is ongoing to define this potential for ultrasound to reduce the incidence of ADP and PDPH. While multiple pharmacologic agents have been tried and investigated for prevention of PDPH, the efficacy of various strategies remains unclear. As an example, intravenous dexamethasone has recently been shown in randomized controlled trials (RCTs) to decrease, but not influence, and even increase the risk of PDPH.
A recent review of drug therapy for preventing PDPH identified only 10 RCTs for review. The primary outcome, a reduction in the number of patients affected by PDPH of any severity, was affected by administration of intrathecal morphine sulfate or fentanyl, oral caffeine, rectal indomethacin, or intravenous dexamethasone. Although a reduction in the incidence of PDPH was seen with epidurally administered morphine (relative risk [RR] 0.25), intravenous cosyntropin (RR 0.49), and intravenous aminophylline (RR 0.21 at 48 hours), the benefit of each was only demonstrated in a single study. Regardless, despite the paucity of evidence, pharmacologic measures—particularly caffeine—continue to be widely used in hopes of decreasing the incidence or severity of PDPH following meningeal puncture. However, no pharmacologic prophylaxis for PDPH has been independently confirmed, and various regimens used have been associated with adverse events.
A recent survey of US anesthesiologists reported that bed rest and aggressive oral and intravenous hydration continue to be suggested by a sizable majority as prophylactic measures against PDPH. However, a systematic review of the literature regarding bed rest versus early mobilization after dural puncture failed to show any evidence of benefit from bed rest and suggested that the risk of PDPH may actually be decreased by early mobilization. It is notable that the practice of US anesthesiologists regarding bed rest is in contrast to that in UK maternity units, where a survey indicated that 75% of UK consultants encourage mobilization as early as possible following ADP as prophylaxis against PDPH. Likewise, in a randomized prospective trial, increased oral hydration following LP failed to decrease the incidence or duration of PDPH. In summary, at this time there is no evidence to support the common practice of recommending bed rest and aggressive hydration in the prevention of PDPH.
NYSORA Tips
• There is no evidence to support the common practice of recommending bed rest and aggressive hydration in the prevention of PDPH.
Spinal Technique
Needle selection is critical for reducing the incidence of PDPH. Given the strong association between needle gauge and PDPH, spinal procedures should be performed with needles having the smallest gauge reasonably possible. However, it should be acknowledged that needles of extremely small gauge can be more difficult to place, have a slow return of CSF, may be associated with multiple unrecognized punctures of the dura, and can result in a higher rate of a failed block. Attention to needle tip design is another important technical means of reducing the risk of PDPH with spinal anesthesia. If available, noncutting needles should routinely be employed as they appear to be associated with fewer adverse events at a lower overall cost. These factors generally make a 24- to 27-gauge noncutting needle the ideal choice for spinal anesthesia. If cutting-tip needles are used, the bevel should be directed parallel to the long axis of the spine (Figure 7).
Replacing the stylet after CSF collection but prior to needle withdrawal has been shown to be an effective means of lowering the incidence of PDPH after LP. In a prospective, randomized study of 600 patients with procedures using 21-gauge Sprotte needles, replacing the stylet reduced the incidence of PDPH from 16.3% to 5.0% (p < .005). This safe-and-simple maneuver is theorized to decrease the possibility of a wicking strand of arachnoid mater from extending across the dura. However, in a more recent study of 630 patients having spinal anesthesia using 25-gauge Quincke needles, replacing the stylet did not affect the incidence of PDPH. The disparity in these results may be related to the needle gauges used as well as fundamental differences between the techniques of lumbar puncture (drainage of CSF) and spinal anesthesia (injection of anesthetic agent). Continuous spinal anesthesia (CSA) has been reported by some to be associated with surprisingly low incidences of PDPH compared with single-dose spinal techniques using similar-gauge needles.
This observation has been attributed to reaction to the catheter, which may promote better sealing of a breach in the meninges. CSA with small-gauge needles and catheters (“microcatheters”) is an appealing option when titration of spinal drug is desirable and duration of surgery is uncertain, but microcatheters are currently unavailable in the United States, where the risk of PDPH with CSA remains concerning when using about 20-gauge “macrocatheters.” For this reason, although the technique may have clinical advantages, deliberate CSA has been investigated almost exclusively in low-risk populations. As mentioned, aminophylline has been demonstrated in one RCT to reduce the incidence of PDPH. Patients undergoing cesarean delivery under spinal anesthesia were randomized to receive intravenous aminophylline (1 mg/kg) or placebo after cord clamping. At 48 hours after surgery, 3 of 60 patients (5%) receiving aminophylline versus 14 of 60 patients (23.3%) in the control group experienced PDPH. No patients in either group required EBP.
Epidural Technique
Although epidural options are limited, especially with catheter techniques, the risk of PDPH following ADP can be reduced by using the smallest feasible epidural needles. Simply decreasing the size of epidural needles from 16 to 18 gauge has been reported to reduce the incidence of PDPH from 88% to 64%. The issue of air versus liquid for identification of the epidural space with the loss-of-resistance technique has long been a source of controversy. Each method has acknowledged advantages and disadvantages, but neither has been shown convincingly to result in a lower risk of ADP. In this case, operator preference and experience would be expected to strongly influence performance, and the overriding significance of this factor is illustrated in fewer instances of ADP noted when the medium is chosen at the anesthesiologist’s discretion. Bevel orientation for epidural needle insertion remains a matter of debate.
Norris et al found the incidence of moderate-to-severe PDPH after ADP was only 24% when the needle bevel was oriented parallel to the long axis of the spine (compared to 70% with perpendicular insertion). This resulted in fewer therapeutic EBPs administered to patients in the parallel group (p < .05). However, this technique necessitates a controversial 90° rotation of the needle for catheter placement. It appears that a number of concerns regarding parallel needle insertion (lateral needle deviation, difficulties with catheter insertion, and dural trauma with needle rotation) are of greater concern to practitioners. Most respondents (71.3%) to a survey of US anesthesiologists preferred to insert epidural needles with the bevel perpendicular to the long axis of the spine (consistent with the intended direction of catheter travel). Combined spinal-epidural (CSE) techniques have been reported to be associated with a low incidence of PDPH. While providing the advantages of a spinal anesthetic, CSE appears to have no increased incidence of PDPH or need for EBP when compared to conventional epidural analgesia. This observation may be due to several factors, including the ability to successfully use extremely small (eg, 27-gauge) noncutting spinal needles and tamponade provided by epidural infusions.
Measures to Reduce the Risk of PDPH After ADP
The risk-to-benefit ratio of prophylaxis should be most favorable in situations having the greatest likelihood of developing severe PDPH. Therefore, most efforts to reduce the risk of PDPH after ADP have been in the obstetric patient population. Several prophylactic measures, discussed in the material that follows, are worthy of consideration and have been utilized alone or in combination. However, because not all patients who experience ADP will develop PDPH, and only a portion of those who do will require definitive treatment with an EBP, a cautious approach in this regard is still generally warranted. It should be acknowledged that the efficacy of all the measures discussed next is debatable. Therefore, it is critical that in the event of recognized ADP, these patients at the very least be clearly informed of the high risk of PDPH development and be followed daily until discharge (or called at home if discharged within 48 hours).
Stylet Replacement
Although there have not been any studies to support the use of the stylet replacement technique in the setting of ADP, replacing the stylet is a simple and effective means of lowering the incidence of PDPH after LP. Given the innocuous nature of this maneuver, if no other prophylactic measures are taken, there appears to be little reason not to replace the stylet prior to epidural needle removal in the event of ADP.
Subarachnoid Saline
Limited evidence indicates that the subarachnoid injection of sterile preservative-free saline following ADP may be associated with a significant reduction in the incidence of PDPH and need for EBP. In one small study (n = 43), immediate injection of 10 mL saline through the epidural needle substantially reduced the incidence of PDPH (32%, compared with 62% in
a matched control group) and resulted in a significant reduction in the need for EBP (p = 0.004). The injection of saline and the reinjection of CSF have been speculated as important in the prevention of PDPH by maintaining CSF volume.
However, given the relatively rapid rate of CSF regeneration, it may be that the benefit of fluid injection following ADP is actually in preventing a wicking strand of arachnoid (as proposed for stylet replacement after LP). Further investigation into this issue is needed.
Intravenous Cosyntropin
As mentioned, there is no convincing evidence that systemic pharmacologic measures are beneficial in the prevention of PDPH. However, based on a number of theoretical mechanisms, corticotropin (adrenocorticotrophic hormone, ACTH) and its analogs have long been used in the treatment of PDPH. Hakim recently reported randomizing 90 parturients experiencing ADP to receive either 1 mg cosyntropin or saline intravenously 30 minutes after delivery. The incidence of PDPH and EBP was 33% and 11% in the cosyntropin group versus 69% and 30% in the saline group. No serious reactions were associated with cosyntropin use. These limited data are encouraging but will need to be supported through further study.
Limiting/Avoiding Pushing
In the event of ADP, limiting the duration of the second stage of labor (usually to 30–60 minutes) and avoiding pushing at that time may reduce the risk of PDPH. While these measures are not uncommonly recommended in UK maternity units such management is rare in US practice.
Intrathecal Catheters
Following ADP in an obstetric setting, Russell noted a 41% incidence of at least two additional attempts at epidural placement and a 9% risk of a second dural puncture. Immediately placing an intrathecal catheter (ITC) after ADP has the advantages of being able to rapidly provide spinal analgesia as well as eliminate the possibility of another ADP under challenging clinical circumstances. However, the potential benefits of ITC use must be weighed against the readily appreciated risks involved (accidental use, misuse, and infection). Although evidence is extremely limited, ITC use has also been proposed to reduce the risk of PDPH after ADP. The mechanism of benefit from ITCs is unclear but may be due to reaction to the catheter, with inflammation or edema preventing further CSF loss after removal. Ayad and colleagues placed and maintained an ITC for 24 hours following ADP. In their obstetric population, catheter placement resulted in a PDPH rate of only 6.2%, with an expected incidence of greater than 50% in this setting. However, this impressive reduction in the incidence of PDPH has generally not been duplicated. A recent meta-analysis of nine studies concluded that ITC insertion following ADP failed to statistically decrease the incidence of PDPH (RR = 0.82, 95% CI 0.67–1.01, p = .06) but did, however, significantly reduce the need for EBP (RR = 0.64, 95% CI 0.49-0.84, p = 0.001). It should be noted that benefits have often not been reported in studies where catheters have been left in situ for less than 24 hours. There are also preliminary data to suggest that the incidence of PDPH may be further reduced by the injection of preservative-free saline through an ITC immediately prior to removal. With some accepted and other possible benefits, rates of ITC use following ADP have clearly increased during the past decade. Recent surveys of US, UK, and Australian practice have noted rates of routine intrathecal catheterization following ADP in obstetric patients of 18%, 28%, and 35%, respectively. Although ITC use has become more common, reattempting an epidural at an adjacent interspace remains the preferred action following ADP. Provided an epidural catheter can be successfully placed, several epidural approaches have been used in hope of reducing the incidence and severity of PDPH.
Epidural Saline
Efforts regarding epidural saline have included both bolus (usually around 50 mL as a single or repeated injection) and continuous infusion techniques (commonly 600–1000 mL over 24 hours). As these measures are resource intensive and may only serve to delay the inevitable onset of symptoms, they have generally not been continued beyond 36 hours. In one large analysis (n = 241), Stride and Cooper reported a reduction in the incidence of PDPH from 86% in a conservatively treated control group to 70% with epidural saline infusion. Trivedi and colleagues noted a similar reduction in PDPH (from 87% to 67%) in 30 patients who received a single prophylactic “saline patch” (40–60 mL) following completion of an obstetric procedure. Other studies of epidural saline have noted this modest decrease in the incidence of PDPH. Stride and Cooper also reported a lower incidence of severe headache (from 64% to 47%), but this effect has been inconsistently seen by other investigators, and there is no convincing evidence that epidural saline reduces the eventual need for EBP.
Epidural Opiates
Epidural opiates (especially morphine), while long utilized for the treatment of PDPH, have been thought unlikely to influence the natural history of the disorder. However, recently revisiting the issue of opiates as prophylaxis after ADP, Al-metwalli found two epidural injections of morphine (3 mg in 10 mL), compared with epidural injections of an equal volume of saline, resulted in fewer episodes of PDPH (p = 0.014) and decreased the need for EBP (p = 0.022). Due to the small number of patients involved (n = 25), a further prospective investigation is warranted.
Prophylactic Epidural Blood Patch
The impressive efficacy of the EBP, when used asa treatment for PDPH, has fueled interest in the technique for prophylaxis. Research into the efficacy of the EBP for prophylaxis has yielded mixed results, and closer scrutiny indicates that optimism should be guarded. The strongest investigation to date has been by Scavone and colleagues, who performed a prospective, randomized, double-blind study in 64 parturients comparing the prophylactic EBP (PEBP) to a sham EBP. In this study, an identical 56% of patients in each group went on to develop PDPH. Although there was a trend toward fewer therapeutic EBPs recommended and performed in the prophylactic group, the difference was not statistically significant (p = 0.08). The primary benefit of the PEBP was a shorter total duration of symptoms (from a median of approximately 5 days to 2 days) and, consequently, a reduction in the overall pain burden.
While there are studies that have shown greater benefit from PEBP, systematic reviews of the evidence have repeatedly noted the inferior methodology of these other studies when compared with that of Scavone et al. With such inconclusive support, the PEBP is not currently recommended as a routine measure based on available evidence. Due to concerns of exposing patients to a potentially unnecessary and marginally beneficial procedure, prophylactic application of the EBP has declined substantially in recent years. If used for prophylaxis, the EBP should be performed only after any spinal or epidural local anesthetic has worn off, as premature administration has been associated with excessive cephalad displacement of local anesthetic. Residual epidural local anesthetic may also inhibit coagulation of blood, further decreasing the efficacy of the EBP.
DIAGNOSTIC EVALUATION
Postdural puncture headache remains a diagnosis of exclusion. Although headache following meningeal puncture will naturally be suspected to be PDPH, it remains critical to rule out other etiologies (Table 1). Fortunately, a careful history with a brief consideration of other possible diagnoses is usually all that is necessary to differentiate PDPH from other causes of headache. While numerous clinical variations have been reported, most cases of PDPH will have (a) a history of known or possible meningeal puncture, (b) delayed onset of symptoms (but within 48 hours), and (c) bilateral postural headache (possibly accompanied by associated symptoms if moderate or severe). Importantly, most non-MPHs will not have a strong positional nature. Laboratory studies are usually not necessary for the diagnosis of PDPH and, if obtained, are generally unremarkable (most commonly, MRI may show meningeal enhancement and LP may reveal low opening pressures and increased CSF protein).
TABLE 1. Differential diagnosis of non-PDPH following meningeal puncture.
| Benign etiologies |
| Nonspecific headache |
| Exacer bation of chronic headache (eg, tension-type headache) |
| Hypertensive headache |
| Pneumocephalus |
| Sinusitis |
| Drug-related side effect |
| Spontaneous intracranial hypotension |
| Other |
| Serious etiologies |
| Meningitis |
| Subdural hematoma (SDH) |
| Subarachnoid hemorrhage |
| Preeclampsia/eclampsia |
| Intracranial venous thrombosis (ICVT) |
| Other |
Physical examination plays a limited role in the diagnosis of PDPH. Vital signs (normal blood pressure and absence of fever) and a basic neurologic exam (gross motor and sensory function plus ocular and facial movements) should be documented. Firm bilateral jugular venous pressure, applied briefly (10–15 seconds), tends to worsen headaches secondary to intracranial hypotension. Conversely, the “sitting epigastric pressure test” may result in transient relief of PDPH symptoms.101 For this test, the patient is placed in a sitting position until headache symptoms become manifest. Firm, continuous abdominal pressure is applied with one hand, while the other hand is secure against the patient’s back. In cases of PDPH, some improvement is usually noted within 15–30 seconds with prompt return of symptoms on release of abdominal pressure. It must be appreciated that benign headaches are frequently encountered in the perioperative setting, even in the absence of meningeal puncture, but have generally been noted to be less severe than PDPH (common etiologies include dehydration, hypoglycemia, anxiety, and caffeine withdrawal). With spinal anesthesia, the specific local anesthetic used and the addition of dextrose or epinephrine may influence the occurrence of nonspecific headache but do not affect the rate of true PDPH. The majority of headaches following meningeal puncture will be benign, nonspecific headaches. In a careful analysis of headache following spinal anesthesia for ambulatory surgery in the general population using strict criteria for PDPH, Santanen and colleagues found an incidence of non-MPH of 18.5%, with an incidence of true PDPH of only 1.5%. Headaches and neck/shoulder pain are also common in the postpartum period.37 In one study, 39% of postpartum patients were noted to be symptomatic, but over 75% of these issues were determined to be primary headaches (migraine, tension type, cervicogenic, and cluster). In this analysis, while 89% of patients received neuraxial anesthesia, only 4.7% of postpartum headaches were PDPH. Benign headaches can often be differentiated from PDPH by their characteristic features. Exacerbation of chronic headache (eg, tension-type, cluster, or migraine) is usually notable for a history of similar headaches. In the study cited immediately in the preceding paragraph, a previous headache history was a significant risk factor for postpartum headache (adjusted odds ratio = 2.25, if > 12 episodes per year). Significant hypertension may cause headaches and should be detected through routine vital sign assessment. Stella et al studied severe and unrelenting postpartum headaches with onset more than 24 hours from the time of delivery and found that 39% were tension-type headaches, 24% were due to preeclampsia/eclampsia, and only 16% were PDPHs (despite neuraxial anesthesia in 88% of patients).
Based on this observation, they recommended treatment of tension/migraine headache prior to consideration of PDPH. Pneumocephalus can produce a positional headache that can be difficult to distinguish from PDPH and does not respond to EBP but is readily diagnosed with computerized tomography (CT). Sinusitis may be associated with purulent nasal discharge and tenderness over the affected sinus and is often improved with assuming an upright position. It should be kept in mind that headache is also a side effect of some commonly utilized pharmacologic agents, such as ondansetron.106 Although certainly unusual, classic PDPH symptoms may even conceivably represent a coincidental case of spontaneous intracranial hypotension (SIH). A number of other benign etiologies are possible. Serious causes of headache will be rare but must be excluded. It is important to remember that lateralizing neurologic signs (with the exception of cranial nerve palsies), fever/chills, seizures, or changes in mental status are not consistent with a diagnosis of PDPH. Meningitis tends to be associated with fever, leukocytosis, changes in mental status, and meningeal signs (such as nuchal rigidity). Subdural hematoma (SDH) is a recognized complication of dural puncture and is believed under these circumstances to be due to intracranial hypotension resulting in excessive traction on cerebral vessels, leading to their disruption.
Practitioners must maintain a high index of suspicion for SDH, which is often preceded by typical PDPH symptoms but progresses to lose its postural component and may evolve to include disturbances in mentation and focal neurologic signs. It has been proposed that early definitive treatment of severe PDPH may serve to prevent SDH. Subarachnoid hemorrhage, most commonly due to rupture of a cerebral aneurysm or arteriovenous malformation, is usually associated with the sudden onset of excruciating headache followed by a decreased level of consciousness or coma.110 Preeclampsia/eclampsia often presents with headache and may only become evident in the postpartum period. Intracranial venous thrombosis (ICVT) is most often seen in the postpartum obstetric population, where headache symptoms are easily confused with PDPH but may progress to seizures, focal neurologic signs, and coma. Predisposing factors for ICVT include hypercoagulability, dehydration, and inflammatory and infectious diseases. Reports of other intracranial pathology (intracranial tumor, intracerebral hemorrhage, etc.) misdiagnosed as PDPH are extremely uncommon and will be detected with a thorough neurological evaluation.
Diagnosis of PDPH can be particularly challenging in patients who have undergone LP as part of a diagnostic workup for headache. In these situations, a change in the quality of headache, most commonly a new postural nature, points toward PDPH. Occasionally, if the benign diagnostic possibilities cannot be narrowed down with certainty, a favorable response to EBP can provide definitive evidence for a diagnosis of PDPH.
TREATMENT
Once a diagnosis of PDPH has been made, patients should be provided a straightforward explanation of the presumed etiology, anticipated natural course (factoring in the time from meningeal puncture), and a realistic assessment of treatment options (with consideration of needle gauge). Treatment considerations are presented individually next. Although surveys indicate that formal protocols for management of PDPH are common practice in the United Kingdom, such plans remain the exception in North American practice. A treatment algorithm, based primarily on the severity of symptoms, can serve as a useful guide for management (Figure 8).
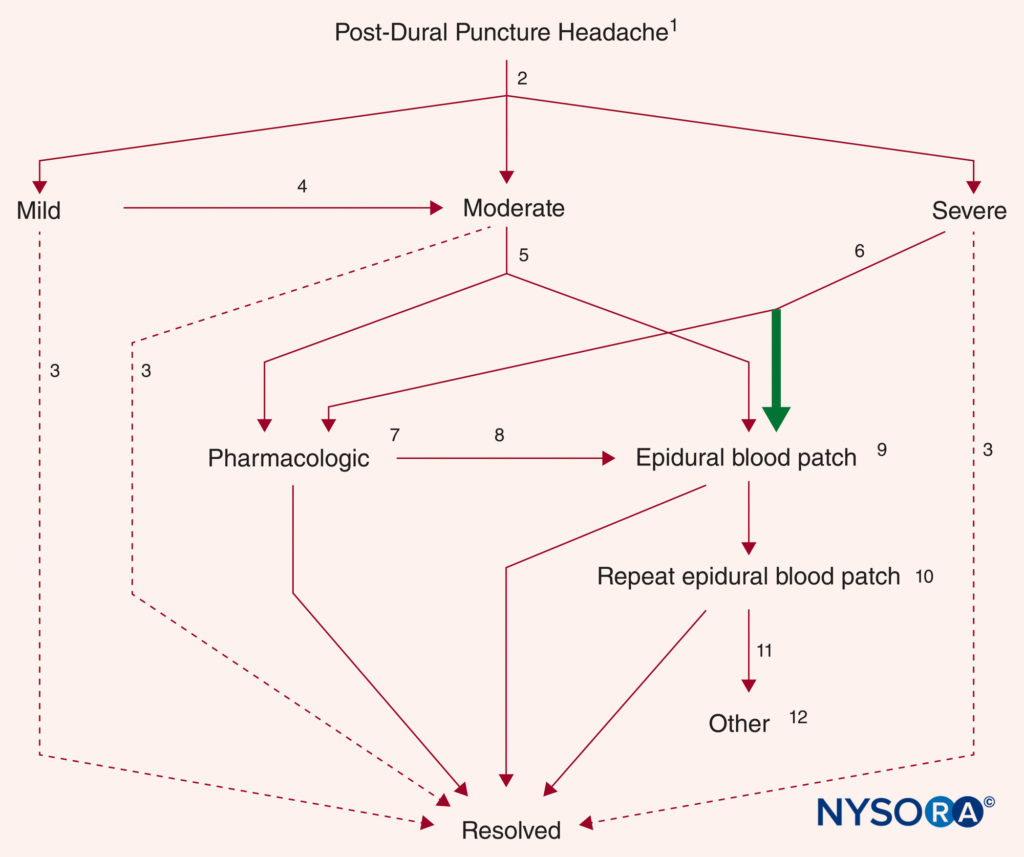
FIGURE 8. Treatment algorithm for established PDPH (see text for further details). (1) Patient education, reassurance, and supportive measures. (2) Triage by severity of symptoms. (3) Resolution over time without further treatment. (4) Worsening symptoms or failure to improve substantially within 5 days. (5) Choice of EBP or pharmacologic measures based on patient preference. (6) Definitive treatment (EBP) is recommended (bold arrow). (7) Caffeine or other agents. (8) Failure, worsening of symptoms, or recurrence. (9) Patch materials other than blood remain preliminary. (10) Generally performed no sooner than 24 hours after a first EBP. (11) Serious reconsideration of diagnosis. (12) Radiologic guidance is recommended if another epidural blood patch (EBP). (Reproduced with permission from Neal JM, Rathmell JP: Complications in Regional Anesthesia and Pain Medicine, 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2013.)
Time
Because PDPH is a complication that tends to resolve spontaneously, the simple passage of time plays an important role in the appropriate management of this disorder. Prior to the introduction of the EBP as definitive therapy, the natural history of PDPH was documented by Vandam and Dripps as they followed 1011 episodes of PDPH after spinal anesthesia using cutting needles of various sizes. While their analysis was flawed by a lack of information regarding duration in 9% of patients, if one considers their observed data, spontaneous resolution of PDPH was seen in 59% of cases within 4 days and 80% within 1 week.
More recently, Lybecker et al closely followed 75 episodes of PDPH after spinal anesthesia and, while providing an EBP to 40% of their patients (generally to those having the most severe symptoms), observed in the untreated patients a median duration of symptoms of 5 days with a range of 1–12 days. van Kooten et al, in a small but prospective, randomized, blinded study of patients with moderate or severe PDPH following LP primarily using 20 gauge needles, noted 18 of 21 patients (86%) in the control treatment group (24-hour bed rest, at least 2 L of fluids by mouth daily, and analgesics as needed) still having headache symptoms at 7 days, with over half of these still rating symptoms as moderate or severe115 (Figure 9).
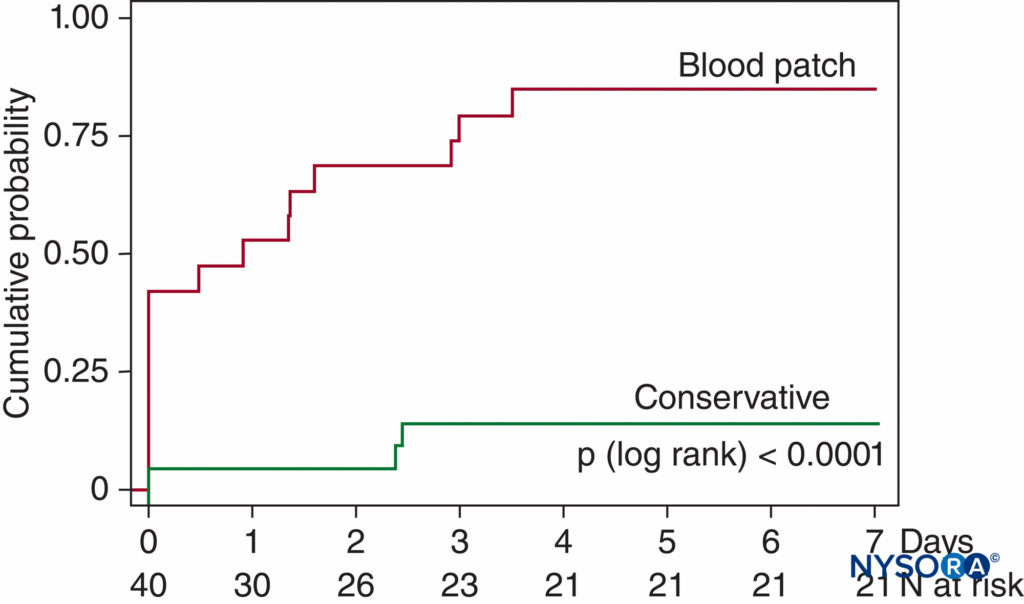
FIGURE 9. Cumulative probability of recovery from PDPH. Recovery from moderate-to-severe PDPH after diagnostic LP in 40 patients. At 7 days, only 3 of 21 conservatively treated patients had fully recovered (no headache symptoms) versus 16 of 19 patients treated with EBP (p < .0001). (Reproduced with permission from van Kooten F, Oedit R, Bakker SL, et al: Epidural blood patch in post-dural puncture headache: a randomized, observer-blind, controlled clinical trial. Neurol Neurosurg Psychiatry. 2008 May;79(5):553–558.)
These data serve to illustrate the unpredictable and occasionally prolonged duration of untreated PDPH. Indeed, Vandam and Dripps reported 4% of patients still experiencing symptoms 7–12 months after spinal anesthesia. Given this reality, it is not surprising that there are a number of case reports of successful treatment of PDPH months and even years after known or occult meningeal puncture. Largely due to the self-limited nature of PDPH, the optimal time course of treatment has not been well defined. Clinically, the practical issue is how long definitive therapy (ie, the EBP) can appropriately be delayed. Many practitioners currently advocate a trial, most commonly 24–48 hours, of conservative management. However, the rationale behind this approach is questionable given the often severely disabling nature of symptoms, particularly in the postpartum period when newborn care may be significantly impaired.
Supportive Measures
Reassurance and measures directed toward minimizing symptoms, while not expected to alter the natural course of the disorder, are advised for all patients. By definition, the majority of patients with moderate-to-severe PDPH will naturally seek a recumbent position for symptomatic relief. Despite a lack of supportive evidence, aggressive hydration continues to be the most frequently recommended practice utilized in treatment of PDPH. Although aggressive hydration does not appear to influence the duration of symptoms,74 patients should and often must be encouraged to avoid dehydration. Analgesics (acetaminophen, nonsteroidal anti-inflammatory drugs [NSAIDs], opiates, etc.) may be administered by a number of different routes and are commonly used, yet the relief obtained is often unimpressive, especially with severe headaches. Antiemetics and stool softeners should be prescribed when indicated. Abdominal binders have been advocated but are uncomfortable and seldom used in modern practice. Alternative measures that have been suggested in the management of PDPH include acupuncture and bilateral greater occipital nerve block.
Pharmacologic Therapies
Many pharmacologic agents have been advocated as treatments for PDPH. Reports of successful use of pharmacologic agents for the treatment of PDPH are intriguing, but their proper place in the management of PDPH awaits further study of efficacy and safety. While appealing, these options have generally been poorly studied and are of questionable value due to the small number of patients treated, methodological flaws in published reports, publication bias, and the self-limited nature of the disorder. A recent review of RCTs assessing the effectiveness of any pharmacological drug used for treating PDPH only included118 seven studies with a total of 200 participants (primarily parturients). Given the initial optimistic but eventually disproven role for so many treatments through the years, practitioners are advised to have guarded expectations in this regard, especially when dealing with severe PDPH. A detailed review of pharmacologic therapies for PDPH is beyond the scope of this section, but some popular or recently investigated options include the following:
1. Methylxanthines. Due to known cerebral vasoconstrictive effects, this class of drugs has become the most commonly used pharmacologic approach to PDPH. These agents include aminophylline, theophylline, and—the most familiar—caffeine. Experimentally, caffeine has been used intravenously (usually 500 mg caffeine sodium benzoate, which contains 250 mg caffeine) and orally (eg, 300 mg). Published studies of caffeine for PDPH consistently demonstrated improvement at 1–4 hours in over 70% of patients treated. However, a single oral dose of 300 mg
caffeine for treatment of PDPH is statistically no better than placebo at 24 hours. With a terminal half-life usually less than 6 hours, repeated doses of caffeine would seem necessary for treatment of PDPH, yet few studies have evaluated more than 2 doses for efficacy or safety (of particular concern in the nursing parturient). Furthermore, there is no convincing evidence that caffeine, or any pharmacologic agents, reduce the eventual need for EBP. Overall, the use of caffeine for PDPH does not appear to be supported by the available literature. Nevertheless, surveys indicated that it continues to be widely used in the treatment of PDPH. Clinically, encouraging unmonitored caffeine intake is of extremely uncertain value, especially considering the widespread lack of awareness of caffeine content in readily available beverages and medications. The temporary benefit often observed with caffeine would indicate that, if used, it is perhaps most appropriate for the treatment of PDPH of moderate (and possibly mild or severe) intensity while awaiting spontaneous resolution of the condition. While the familiarity of caffeine for nonmedical purposes would argue for its general safety, practitioners should note that its use is contraindicated in patients with seizure disorders, pregnancy-induced hypertension, or a history of supraventricular tachyarrhythmias.
2. Serotonin type 1d receptor agonists. These agents cause cerebral vasoconstriction and are commonly used for migraine headache. Despite anecdotal reports of success, sumatriptan was ineffective for treatment of severe PDPH in a small randomized, prospective study.
3. Ergot alkaloids. These cerebral vasoconstrictive agents are also commonly used for migraine headache. A small, uncontrolled pilot study suggested that methylergonovine (0.25 mg orally three times daily for 24–48 hours) may hasten resolution of PDPH.
4. Corticosteroidogenics (corticotropin [ACTH] and its synthetic analogues [ie, cosyntropin/tetracosactin]). Although the mechanism of action remains speculative, ACTH is known to have multiple physiologic effects that could theoretically improve symptoms of PDPH.87 However, a synthetic ACTH analog was ineffective for treatment of severe PDPH in a small randomized, prospective study.
5. Corticosteroids. Similar to corticosteroidogenics, corticosteroids have multiple physiologic effects that could theoretically improve symptoms of PDPH. In a randomized, prospective study of 60 patients with severe PDPH after spinal anesthesia using 25-gauge Quincke needles for cesarean delivery, addition of hydrocortisone (200 mg IV initially, followed by 100 mg every 8 hours for 6 doses) resulted in significantly lower headache intensity. Only one patient in this study (in the conventionally treated group) required EBP. A similar randomized study in 60 nonobstetric surgery patients experiencing PDPH after spinal anesthesia showed significant reductions in headache intensity in the hydrocortisone group.
6. Anticonvulsants. Several membrane-stabilizing agents are widely used for various pain syndromes. Some reports have suggested that gabapentin may be useful in the setting of PDPH. In an uncontrolled case series of 17 postpartum patients with severe PDPH, 9 (53%) experienced “excellent” (visual analog scale [VAS] < 2 of 10 plus resumption of normal activity) relief with gabapentin (200 mg initially, followed by 100–300 mg three times daily, with dose adjusted to tolerance and efficacy).
In a randomized, placebo-controlled study, pregabalin (75 mg twice a day for 2 days, then 150 mg twice a day for 2 days) was demonstrated to result in lower pain scores and analgesic consumption in patients with PDPH following spinal anesthesia or LP.129 It is interesting to note that, despite starting with mean VAS scores of greater than 8 of 10, none of the 40 patients in this study required EBP.
Epidural Therapies
While not a contraindication to epidural treatments, a history of significant technical difficulties with attempted neuraxial techniques should naturally encourage a trial of less-invasive measures. However, the appeal of epidural approaches is evident if access to the epidural space is deemed reasonable or if the patient already has a correctly placed catheter in situ.
Epidural Saline
Epidural saline, as bolus and infusion, has a long history of use for treatment of PDPH. Bolus injections of epidural saline (usually 20–30 ml, repeated as necessary if a catheter is present) have been reported to produce prompt and virtually universal relief of PDPH, yet the practice is plagued by an extremely high rate of headache recurrence. This transient effect is not surprising as increases in epidural pressure following bolus administration of saline have been demonstrated to return to baseline within 10 minutes.130 Favorable results achieved with this approach have been speculated to represent the mechanical reapproximation of a dural flap (the “tin-lid” phenomenon). However, bolus administration of saline for treatment of PDPH has been convincingly shown to be inferior to the EBP, especially when headaches are secondary to large-bore needle punctures. Overall, epidural saline appears to be of limited value for established PDPH. Nevertheless, the successful use of epidural saline, administered as bolus or infusion, continues to be reported occasionally under exceptional circumstances.
Epidural Blood Patch
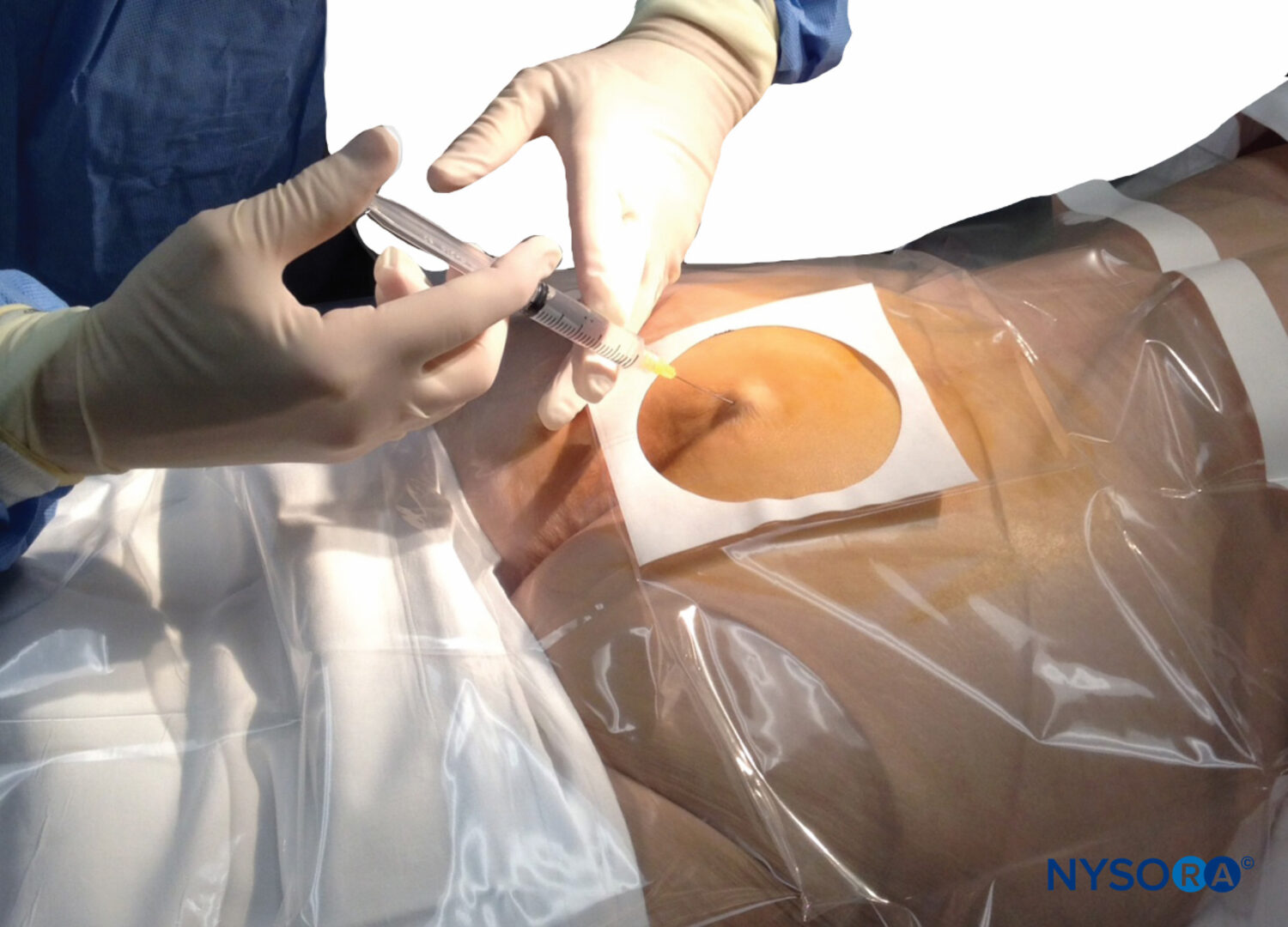
During the past several decades, the EBP has emerged as the “gold standard” for treatment of PDPH (Figure 10). A Cochrane review (a systematic assessment of the evidence) regarding the EBP concluded that the procedure now has proven benefit over more conservative treatment.
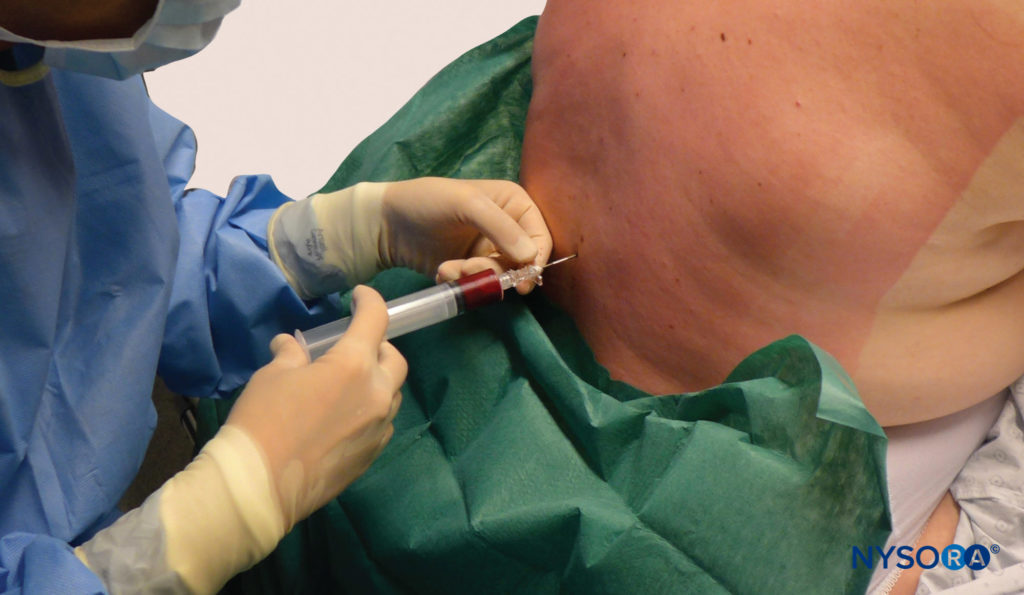
FIGURE 10. Blood patch. Administration of an epidural blood patch using 20 mL of freshly drawn blood. The blood is injected until 20 mL are reached or the patient perceives significant pain or pressure in the back, whichever comes first.
The mechanism of action of the EBP, while not entirely elucidated, appears to be related to the ability to stop further CSF loss by the formation of clot over the defect in the meninges as well as a tamponade effect with cephalad displacement of CSF (the “epidural pressure patch”). The appropriate role of the EBP in individual situations will depend on multiple factors, including the duration and severity of headache and associated symptoms, type and gauge of original needle used, and patient wishes. The EBP should be encouraged in patients experiencing ADP with an epidural needle and those whose symptoms are categorized as severe (ie, pain score > 6 on a 1–10 scale). Informed consent for the EBP should include a discussion with the patient regarding the common as well as serious risks involved, true success rate, and anticipated side effects. Finally, patients should be provided with clear instructions for the provision of timely medical attention should they experience a recurrence of symptoms. A number of controversies surround the EBP, reflecting the scarcity of adequately powered, randomized trials. The procedure itself has been well described and consists of the sterile injection of fresh autologous blood near the previous dural puncture (Table 2). An MRI study of the EBP in 5 young patients (ages 31–44) using 20 mL blood noted a spread of 4.6 ± 0.9 intervertebral spaces (mean ± SD), averaging 3.5 levels above and 1 level below the site of injection. This and other observations of a preferential cephalad spread of blood in the lumbar epidural space has led to the common recommendation to perform the EBP “at or below” the meningeal puncture level. However, the influence of the level of placement and use of an epidural catheter (often situated considerably cephalad to a meningeal puncture) on efficacy for EBP has never been clinically evaluated. The optimal timing of the EBP is a matter of debate. After diagnosis, most practitioners prefer to delay performing the EBP, possibly to further confirm the diagnosis as well as to allow an opportunity for spontaneous resolution. A 1996 survey of UK neurological departments found that only 8% would consider the EBP before 72 hours had passed following LP. A recent survey of UK maternity units reported that 71% would perform the EBP only “after the failure of conservative measures.”
TABLE 2. Epidural blood patch procedure.
| Obtain written informed consent. |
| Establish intravenous access. An 18-gauge or larger saline lock is sufficient. |
| Position the patient for epidural needle placement (mindful that a lateral decubitus position may be more comfortable than sitting for the patient). |
| Using standard sterile technique, place an epidural needle into the epidural space at or below the level of previous meningeal puncture. |
| Collect 20 mL fresh autologous venous blood using strict sterile technique (this is usually readily accomplished using the previously placed saline lock). |
| Without delay, steadily inject blood through the epidural needle until the patient reports fullness or discomfort in the back, buttocks, or neck. |
| Maintain the patient in a recumbent position for a period of time (1–2 hours may result in more complete resolution of symptoms). Intravenous infusion of 1 L crystalloid during this interval is often helpful. |
| Instructions for discharge: |
| Encourage over-the-counter analgesics (eg, acetaminophen, ibuprofen) as needed for any mild residual discomfort. |
| Prescribe stool softeners or cough suppressants if indicated. |
| Avoid lifting, straining, or air travel for 24 hours. |
| Provide clear instructions on how to contact anesthesia personnel for inadequate relief or recurrence of symptoms. |
Likewise, the majority of respondents to recent surveys of practice in the United States and Nordic countries usually waited at least 24 hours from the onset of symptoms before performing the EBP. Several studies have suggested that the EBP procedure may become more effective with the passage of time. Safa-Tisseront et al. found a delay of less than 4 days from meningeal puncture before performing an EBP to be an independent risk factor for failure of the procedure. Yet, these authors were careful to state that failure of the EBP may be primarily related to the severity of the CSF leak (with larger, harder-to-treat situations demanding earlier attention), and that their study should not be grounds for delaying the EBP. Sandesc and colleagues performed a prospective, randomized, double-blind study of the EBP versus conservative management (intravenous or oral fluids up to 3 L/d, NSAIDS, and caffeine sodium benzoate 500 mg IV every 6 hours) in 32 patients with severe PDPH symptoms (mean pain intensity = 8.1). At the time treatment was initiated, none of these patients had experienced symptoms for longer than 24 hours. While all patients in the EBP group had satisfactory resolution of symptoms at 24-hour follow-up, the control group was essentially unchanged (mean pain intensity = 7.8). Notably, 14 of 16 patients in the conservatively treated group then elected for EBP treatment. These investigators concluded that there was no reason to delay the EBP for more than 24 hours after making a diagnosis of severe PDPH. This recommendation was further supported by a prospective analysis of 79 patients with PDPH that determined early EBP in those with moderate-tosevere symptoms minimized overall patient suffering. The ideal volume of blood for EBP has been an evolving issue that is becoming more clearly understood. Conceptually, the volume of blood used should be sufficient to form an organized clot over the meningeal defect as well as produce some degree of epidural tamponade. When performing the EBP, anesthesiologists commonly inject as much blood as was drawn (usually around 20 mL), stopping when the patient complains of discomfort or fullness in the back, buttocks, or neck. There appear to be geographic preferences regarding blood volume. The largest analysis of the EBP to date (n = 504) utilized a blood volume of 23 ± 5 ml (mean ± SD). Importantly, this French study found no significant difference in blood volumes between successful and failed EBP. Notably, they reported “discomfort” in 78% of injections with 19 ± 5 mL and “pain” in 54% with 21 ± 5 mL, with the only independent risk factor for pain during EBP being age less than 35 years. A recent survey of US anesthesiologists reported general unanimity for a smaller blood volume, with two-thirds (66.8%) most commonly using between 16 and 20 mL. As previously mentioned, there may be some experimental support for using a blood volume of 15–20 mL, as early studies of CSF drainage in volunteers reported consistently producing positional headache symptoms with loss of 10% of total CSF volume (approximately 15 mL). Furthermore, the reduction in CSF pressures produced by this degree of fluid loss would be expected to reduce or eliminate transmeningeal driving pressure, resulting, at that point, in a relative CSF volume homeostasis (in the supine position).
Two RCTs have been performed to determine the optimum volume of blood for EBP in obstetric patients with PDPH following ADP. The first, which compared 7.5 to 15 mL in 33 Taiwanese women, reported similar efficacy with the two volumes and failed to find any advantage with the larger volume. The second was a larger, multicenter study that looked at three volumes of blood for EBP (15, 20, and 30 mL). This trial found that patients receiving 15 mL had less-complete relief of symptoms than those receiving 20 or 30 mL, with no difference in efficacy between 20 and 30 mL. These investigators also found that only 54% of patients randomized to the highest volume were able to tolerate the full 30 mL (compared with 81% in the 20-mL group). While these two studies failed to definitively determine the ideal volume of blood for EBP, they both indicated that it does not appear necessary to use volumes greater than 20 mL. It is notable that although the utility of the EBP in the treatment of SIH is uncertain, much larger blood volumes (up to 100 mL) are commonly recommended for this indication. However, recent case reports highlighted some potential complications, such as severe radiculopathy, from large-volume EBP and practitioners are therefore generally encouraged to use the smallest effective blood volume. To allow for clot organization and regeneration of CSF (approximately 0.35 mL/minute), it is common practice to have patients remain recumbent for a period of time following the EBP. While the optimal duration of bed rest immediately following an EBP remains unknown, one small study suggested that maintaining the decubitus position for at least 1 and preferably 2 hours may result in a more complete resolution of symptoms. Patients are also usually advised to avoid lifting, Valsalva maneuvers (eg, straining with bowel movement), and air travel for 24–48 hours after EBP to minimize the risk of patch disruption. Modifications have been made to the standard EBP technique in special circumstances. To accommodate the religious beliefs of Jehovah’s Witness patients, techniques have been described that keep autologous blood within a continuous circuit. The EBP has been repeatedly demonstrated to be safe and effective for treatment of PDPH in the pediatric population.
A blood volume of 0.2–0.3 mL/kg appears appropriate for young children as well as adolescents. The EBP is also performed with decreased blood volumes at extralumbar sites (eg, cervical spine). Contraindications to the EBP are similar to those of any epidural needle placement: coagulopathy, systemic sepsis, fever, infection at the site, and patient refusal. Theoretical concerns have been expressed regarding the possibility of neoplastic seeding of the central nervous system in patients with cancer. It has been suggested that special care, in the form of slower injections of smaller blood volumes, may be prudent in patients whose central nervous system may be vulnerable to injury produced by increased epidural pressures generated with EBP, such as those with multiple sclerosis. Although not free from concern and controversy, the EBP has been safely provided to patients with HIV infection and acute varicella. Minor side effects are common following the EBP. Patients should be warned to expect aching in the back, buttocks, or legs (seen in approximately 25% of patients). While usually short-lived, backache was noted to be persistent in 16% of patients following EBP and lasted 3–100 days (with a mean duration in this subgroup of 27.7 days). Despite these lingering symptoms, patient satisfaction with the EBP is high. Other frequent but benign aftereffects of the EBP include transient neckache, bradycardia, and modest temperature elevation. Largely through extensive clinical experience, the EBP has been sufficiently proven to be safe. Risks are essentially the same as with other epidural procedures (infection, bleeding, nerve damage, and ADP).
Although some patients may develop temporary back and lower extremity radicular pain as mentioned, such complications are uncommon. With proper technique, infectious complications are vanishingly rare. In general, a previous EBP does not appear to significantly influence the success of future epidural interventions, but case reports suggest that the EBP may occasionally result in clinically significant scarring. Serious complications secondary to the EBP do occur but have usually consisted of isolated case reports and have often been associated with significant deviations from standard practice.
Alternative Treatment Options to EBP
A number of alternatives to blood have been promoted as patch materials. The various rationales for using alternative agents include situations for which the use of blood has been ineffective or is contraindicated. The most commonly proposed materials (dextran 40, hydroxyethylstarch, gelatin, and fibrin glue) have been adapted for a perceived ability to provide prolonged epidural tamponade or result in sealing of a meningeal rent. In a rat model, experimental support for a “blood-like” effect was best shown for fibrin glue. Yet, clinical use of these alternatives is limited to case reports and small series, and their use is uncommon in the United States. While not necessarily without merit, these options remain poorly defined and are not without potential for serious risk (eg, allergic reactions to dextran), and reports of their use should still be considered preliminary.
PERSISTENT OR RECURRENT PDPH
Early reports of the EBP frequently cited success rates between 90% and 100% but often did not include a strict definition of “success,” had little or no follow-up, and failed to consider the influence of such confounding factors as needle size and tip design, severity of symptoms, or natural history of PDPH. The true efficacy of the EBP procedure is now known to be significantly lower than once thought. Persistent or recurrent headaches following the EBP, while not necessarily requiring consultation, warrant follow-up and thoughtful reevaluation. The EBP is associated with nearly immediate symptomatic relief in greater than 90% of cases, but appropriate follow-up reveals a number of patients experiencing incomplete relief, failure, or recurrence of symptoms. In an uncontrolled, prospective, observational study of 504 consecutive patients treated with EBP following meningeal puncture with needles of various sizes, Safa-Tisseront et al reported that some relief of symptoms occurs in 93% of patients. On closer analysis, however, complete relief of symptoms was seen in only 75% of patients, with 18% experiencing incomplete relief. They also found that the EBP was more likely to fail if the original meningeal puncture was made with needles larger than 20 gauge. For needles larger than 20 gauge, the unqualified success rate of the EBP was only 62%, with 17% of patients reporting incomplete relief of symptoms and 21% experiencing failure. Not surprisingly, the majority of these large needles were Tuohy epidural needles.
Expectations of success with the EBP must be further tempered in obstetric patients (all young and female) following ADP with epidural needles. Under these circumstances, Williams et al noted complete relief of symptoms with EBP in only 34% of patients, partial relief in 54%, and no relief in 7% (results unknown in 5%).160 If performed, a second EBP resulted in complete relief in 50%, partial relief in 36%, and no relief in 14%. In a similar patient population, Banks and colleagues, despite initially observing complete or partial relief with EBP in 95% of patients, reported the return of moderate-to-severe symptoms in 31%, with a mean time to development of recurrent headache of 31.8 hours (range 12–96 hours).137 The rates of repeat EBP for the Williams and Banks studies were 27% and 19%, respectively. These studies clearly demonstrated the reduced efficacy of the EBP following meningeal punctures made with large needles, which not uncommonly make it necessary to consider repeating the procedure. Overall, success rates of a second EBP appear to be approximately equal to that of a first. The ideal timing and blood volume for repeat EBP are even more uncertain than for a primary procedure. A majority of US anesthesiologists would wait at least 24 hours after recurrence of PDPH symptoms before performing a second EBP. If more than one EBP is performed within a short period of time, practitioners should remain cognizant of the cumulative amount of blood used as excessive volumes under these circumstances have been implicated in adverse outcomes. Insufficient evidence exists to guide management following a second failed EBP. Given the frequency of PDPH and significant failure rate of the EBP, instances of sequential EBP failure are not unheard of, especially following large-gauge meningeal punctures. In an analysis of outcomes following ADP with 18-gauge Tuohy needles in an obstetric unit, Sadashivaiah reported 3 of 48 patients (6.25%) requiring a third EBP to relieve the headache. Obviously, each failure of the EBP necessitates an even more critical reconsideration of the diagnosis.
While experiences with managing repeated EBP failure have been published,162 such sporadic case reports are insufficient to guide others. However, one frequently cited and logical recommendation regarding repeat EBP, and particularly a third EBP, is to use some form of radiologic guidance to ensure accurate epidural blood placement (eg, fluoroscopy). Other measures under these difficult circumstances may include any of the aforementioned “treatments,” with open surgical repair constituting a last resort.
WHEN TO SEEK FURTHER CONSULTATION
Because PDPH tends to improve even without specific treatment and the EBP has a relatively high rate of success, many practitioners reasonably seek neurological consultation if symptoms have failed to resolve after an arbitrary duration (eg, 7–10 days) or number of EBPs (usually two or three). Consultation is always indicated if serious non-PDPH is suspected or cannot reasonably be ruled out. As previously mentioned, lateralizing neurologic signs, fever/chills, seizures, or change in mental status are not consistent with a diagnosis of PDPH or benign headache. Consultation is also appropriate for any headaches with atypical features. Proceeding with treatment measures directed toward PDPH under uncertain circumstances may hinder a correct diagnosis, cause critical delays in proper treatment, and can prove harmful. The EBP, for example, has occasionally been reported to produce detrimental increases in intracranial pressure. Because PDPH can be anticipated to resolve spontaneously, headaches that worsen over time and no longer have a positional nature should be strongly suspected to be secondary to SDH (especially if there are focal neurologic signs or decreases in mental status). Under these circumstances, a neurological consultation should be obtained and diagnostic radiologic studies performed. Although headache and most associated symptoms, including auditory symptoms, resolve quickly following EBP, cranial nerve palsies generally resolve slowly (within 6 months) and may appropriately prompt a neurology consult for ongoing management and reassurance. Although there are no accepted treatments for cranial nerve palsy associated with PDPH, it seems reasonable to treat these conditions similar to idiopathic facial nerve (CN VII) palsy (“Bell’s palsy”). There is some evidence, for example, to suggest that corticosteroids administered early (within 72 hours of onset) may hasten resolution of symptoms from Bell’s palsy, and similar treatment has been suggested for cranial nerve palsy following meningeal puncture.
SUMMARY
Over a century after being first described, PDPH remains a significant clinical concern for a number of medical specialties. As with any complication, prevention is preferable to treatment. Identification and consideration of the risk factors for PDPH have resulted in an impressive reduction in the incidence of this persistent iatrogenic problem. Accidental meningeal puncture with epidural needles continues to be a major concern and challenge. The consequent PDPH symptoms tend to be more severe, of longer duration, and more difficult to treat than those seen with smaller-gauge needles. It should be noted that there is no evidence to support the two most commonly practiced prophylactic measures in this setting: aggressive hydration and encouraging bed rest. Although some prophylactic measures have shown promise, none to this point appears to be a definitive measure. Many episodes of PDPH, especially those of mild-to-moderate severity, will resolve in a timely manner without specific treatment. Despite being commonly advised, hydration, bed rest, and caffeine are all of questionable value in the treatment of established PDPH. Although alternatives have been proposed, the EBP remains the only proven treatment for PDPH and therefore can be encouraged and performed early (within 24 hours of diagnosis) if symptoms are severe. Unfortunately, the published literature concerning PDPH has generally been of poor quality. Many questions remain regarding the optimal means of preventing and treating this troublesome complication. Even much of what is “known” to this point has not been confirmed in follow-up studies. It is anticipated that these issues will be resolved in the future through well-designed clinical investigations.
REFERENCES
- Van Zundert AAJ, Reina MA, Lee RA. Prevention of post-dural puncture headache (PDPH) in parturients. Contributions from experimental research. Acta Anaesthesiol Scand. 2013;57:947–9.
- Reina MA, Prats-Galino A, Sola RG, Puigdellívol-Sánchez A, Arriazu Navarro R, De Andrés JA. Structure of the arachnoid layer of the human spinal meninges: a barrier that regulates dural sac permeability. Rev Esp Anestesiol Reanim. 2010;57:486–92. Spanish.
- Reina MA, López A, Badorrey V, De Andrés JA, Martín S. Duraarachnoid lesions produced by 22 gauge Quincke spinal needles during a lumbar puncture. J Neurol Neurosurg Psychiatry. 2004;75893–7.
- Reina MA, de Leon-Casasola OA, Lopez A, De Andres J, Martin S, Mora M. An in vitro study of dural lesions produced by 25-gauge Quincke and Whitacre needles evaluated by scanning electron microscopy. Reg Anesth Pain Med. 2000;25:393–402.
- Dittmann M, Reina MA, López García A. New results in the visualization of the spinal dura mater with scanning electron microscopy. Anaesthesist. 1998;47:409–13. German.
- Reina MA, Dittmann M, López Garcia A, van Zundert A. New perspectives in the microscopic structure of human dura mater in the dorsolumbar region. Reg Anesth. 1997;22:161–6.
- Reina MA, López-García A, de Andrés-Ibáñez JA, Dittmann M, Cascales MR, del Caño MC, Daneri J, Zambrano O. Electron microscopy of the lesions produced in the human dura mater by Quincke beveled and Whitacre needles. Rev Esp Anestesiol Reanim. 1997;44:56–61. Spanish.
- Reina MA, López A, van Zundert A, De Andrés JA. Ultrastructure of dural lesions produced in lumbar punctures. In: Reina MA. Atlas of functional anatomy of regional anesthesia and pain medicine. New York: Springer; 2015. p.767–794.
- Reina MA, Castedo J, López A. Postdural puncture headache. Ultrastructure of dural lesions and spinal needles used in lumbar punctures. Rev Arg Anestesiol 2008;66:6–26.
- Tourtellotte WW, Haerer AF, Heller GL, Somers JE: Post-Lumbar Puncture Headaches. Thomas, 1964.
- Moore DC: Headache. In Complications of Regional Anesthesia. Thomas, 1955, pp 177–196.
- Gormley JB: Treatment of postspinal headache. Anesthesiology 1960; 21:565–566.
- DiGiovanni AJ, Dunbar BS: Epidural injections of autologous blood for postlumbar-puncture headache. Anesth Analg 1970;49:268–271.
- Crawford JS: Experiences with epidural blood patch. Anaesthesia 1980;35:513–515.
- Harrington BE, Schmitt AM: Meningeal (postdural) puncture headache, unintentional dural puncture, and the epidural blood patch. A national survey of United States practice. Reg Anesth Pain Med 2009;34:430–437.
- Choi PT, Galinski SE, Takeuchi L, et al: PDPH is a common complication of neuraxial block in parturients: A meta-analysis of obstetrical studies. Can J Anesth 2003;50:460–469.
- Paech M, Banks S, Gurrin L: An audit of accidental dural puncture during epidural insertion of a Tuohy needle in obstetric patients. Int J Obstet Anesth 2001;10:162–167.
- Webb CA, Weyker PD, Zhang L, et al: Unintentional dural puncture with a Tuohy needle increases risk of chronic headache. Anesth Analg 2012;115:124–132.
- Stendell L, Fomsgaard JS, Olsen KS: There is room for improvement in the prevention and treatment of headache after lumbar puncture. Dan Med J 2012;59:1–5.
- Vercauteren MP, Hoffmann VH, Mertens E, et al: Seven-year review of requests for epidural blood patches for headache after dural puncture:referral patterns and the effectiveness of blood patches. Eur J Anaesth 1999;16:298–303.
- Davies JM, Posner KL, Lee LA, et al: Liability associated with obstetric anesthesia. A closed claims analysis. Anesthesiology 2009;110:131–139.
- Lee LA, Posner KL, Domino KB, et al: Injuries associated with regional anesthesia in the 1980s and 1990s: A closed claims analysis. Anesthesiology 2004;101:143–152.
- Fitzgibbon DR, Posner KL, Domino KB, et al: Chronic pain management: American Society of Anesthesiologists Closed Claims Project. Anesthesiology 2004;100:98–105.
- Brull R, McCartney CJL, Chan VWS, et al: Disclosure of risks associated with regional anesthesia: A survey of academic regional anesthesiologists. Reg Anesth Pain Med 2007;32:7–11.
- Levine DN, Rapalino O: The pathophysiology of lumbar puncture headache. J Neurol Sci 2001;192:1–8.
- Kunkle EC, Ray BS, Wolff HG: Experimental studies on headache. Analysis of the headache associated with changes in intracranial pressure. Arch Neurol Psychiatry 1943;49:323–358.
- Larrier D, Lee A: Anatomy of headache and facial pain. Otolaryngol Clin N Am 2003;36:1041–1053.
- Day CJE, Shutt LE: Auditory, ocular, and facial complications of central neural block. A review of possible mechanisms. Reg Anesth 1996; 21:197–201.
- Pogodzinski MS, Shallop JK, Sprung J, et al: Hearing loss and cerebrospinal fluid pressure: Case report and review of the literature. Ear Nose Throat J 2009;87:144–147.
- Nishio I, Williams BA, Williams JP: Diplopia. A complication of dural puncture. Anesthesiology 2004;100:158–164.
- Yaman ME, Ayberk G, Eylen A, et al: Isolated abducens nerve palsy following lumbar puncture: Case report and review of the mechanism of action. J Neurosurg Sci 2010;54:119–123.
- Fang JY, Lin JW, Li Q, et al: Trigeminal nerve and facial nerve palsy after combined spinal-epidural anesthesia for cesarean section. J Clin Anesth 2010;22:56–58.
- Lybecker H, Djernes M, Schmidt JF: Postdural puncture headache (PDPH): Onset, duration, severity, and associated symptoms. An analysis of 75 consecutive patients with PDPH. Acta Anaesthesiol Scand 1995;39:605–612.
- Aida S, Taga K, Yamakura T, et al: Headache after attempted epidural block: The role of intrathecal air. Anesthesiology 1998;88:76–81.
- Reamy BV: Post-epidural headache: how late can it occur? J Am Board Fam Med 2009;22:202–205.
- Amorim JA, Gomes de Barros MV, Valenca MM: Post-dural (post-lumbar) puncture headache: Risk factors and clinical features. Cephalalgia 2012;32:916–923.
- Chan TM, Ahmed E, Yentis SM, et al: Postpartum headaches: Summary report of the National Obstetric Anaesthetic Database (NOAD) 1999. Int J Obstet Anesth 2003;12:107–112.
- Sprung J, Bourke BA, Contreras MG, et al: Perioperative hearing impairment. Anesthesiology 2003;98:241–257.
- Lybecker H, Moller JT, May O, et al: Incidence and prediction of postdural puncture headache: A prospective study of 1021 spinal anesthesias. Anesth Analg 1990;70:389–394.
- Wu CL, Rowlingson AJ, Cohen SR, et al: Gender and post-dural puncture headache. Anesthesiology 2006;105:613–618.
- Angle P, Thompson D, Halpern S, et al: Second stage pushing correlates with headache after unintentional dural puncture in parturients. Can J Anesth 1999;46:861–866.
- Vallejo MC: Anesthetic management of the morbidly obese parturient. Curr Opin Anaesthesiol 2007;20:175–180.
- Kuntz KM, Kokmen E, Stevens JC, et al: Post-lumbar puncture headaches: Experience in 501 consecutive procedures. Neurology 1992; 42:1884–1887.
- Faure E, Moreno R, Thisted R: Incidence of postdural puncture headache in morbidly obese parturients. Reg Anesth 1994;19:361–363.
- Dodge HS, Ekhator NN, Jefferson-Wilson L, et al: Cigarette smokers have reduced risk for post-dural puncture headache. Pain Physician 2013;16:e25–e30.
- Hannerz J: Postlumbar puncture headache and its relation to chronic tension-type headache. Headache 1997;37:659–662.
- van Oosterhout WPJ, van der Plas AA, van Zwet EW, et al: Postdural puncture headache in migraineurs and nonheadache subjects. A prospective study. Neurology 2013;80:941–948.
- Echevarria M, Caba F, Rodriguez R: The influence of the menstrual cycle in postdural puncture headache. Reg Anesth Pain Med 1998;23: 485–490.
- Blanche R, Eisenach JC, Tuttle R, et al: Previous wet tap does not reduce success rate of labor epidural analgesia. Anesth Analg 1994;79: 291–294.
- Halpern S, Preston R: Postdural puncture headache and spinal needle design. Metaanalysis. Anesthesiology 1994;81:1376–1383.
- Kovanen J, Sulkava R: Duration of postural headache after lumbar puncture: Effect of needle size. Headache 1986;26:224–226.
- Lambert DH, Hurley RJ, Hertwig L, et al: Role of needle gauge and tip configuration in the production of lumbar puncture headache. Reg Anesth 1997;22:66–72.
- Reina MA, de Leon-Casasola OA, Lopez A, et al: An in vitro study of dural lesions produced by 25-gauge Quincke and Whitacre needles evaluated by scanning electron microscopy. Reg Anesth Pain Med 2000; 25:393–402.
- Richman J, Joe E, Cohen S, et al: Bevel direction and postdural puncture headache. A meta-analysis. Neurologist 2006;12:224–228.
- Reina MA, Dittmann M, Garcia AL, et al: New perspectives in the microscopic structure of human dura mater in the dorsolumbar region. Reg Anesth 1997;22:161–166.
- Seeberger MD, Kaufmann M, Staender S, et al: Repeated dural punctures increase the incidence of postdural puncture headache. Anesth Analg 1996;82:302–305.
- De Almeida SM, Shumaker SD, LeBlanc SK, et al: Incidence of postdural puncture headache in research volunteers. Headache 2011;51:1503–1510.
- Singh S, Chaudry SY, Phelps AL, et al: A 5-year audit of accidental dural punctures, postdural puncture headaches, and failed regional anesthetics at a tertiary-care medical center. TheScientificWorldJournal 2009;9: 715–722.
- Goldszmidt E, Kern R, Chaput A, et al: The incidence and etiology of postpartum headaches: A prospective cohort study. Can J Anesth 2005; 52:971–977.
- Aya AGM, Manguin R, Robert C, et al: Increased risk of unintentional dural puncture in night-time obstetric epidural anaesthesia. Can J Anesth 1999;46:665–669.
- Paech MJ, Whybrow T: The prevention and treatment of post dural puncture headache. ASEAN J Anaesthesiol 2007;8:86–95.
- Warwick WI, Neal JM: Beyond spinal headache: Prophylaxis and treatment of low-pressure headache syndromes. Reg Anesth Pain Med 2007;32:455–461.
- Apfel CC, Saxena OS, Cakmakkaya OS, et al: Prevention of postdural puncture headache after accidental dural puncture: A quantitative systematic review. Br J Anaesth 2010;105:255–263.
- Perlas A: Evidence for the use of ultrasound in neuraxial blocks. Reg Anesth Pain Med 2010;35 (Suppl. 1):S43–S46.
- Hamzei A, Basiri-Moghadam M, Pasban-Noghabi S: Effect of dexamethasone on incidence of headache after spinal anesthesia in cesarean section. A single blind randomized controlled trial. Saudi Med J 2012;33:948–953.
- Doroudian MR, Norouzi M, Esmailie M, et al: Dexamethasone in preventing post-dural puncture headache: A randomized, double-blind, placebo-controlled trial. Acta Anaesth Belg 2011;62:143–146.
- Yousefshahi F, Dahmardeh AR, Khajavi M, et al: Effect of dexamethasone on the frequency of postdural puncture headache after spinal anesthesia for cesarean section: A double-blind randomized clinical trial. Acta Neurol Belg 2012;112:345–350.
- Basurto Ona X, Uriona Tuma SM, Martinez Garcia L, et al: Drug therapy for preventing post-dural puncture headache. Cochrane Database Syst Rev 2013;(2):CD001792.
- Al-metwalli RR: Epidural morphine injections for prevention of post dural puncture headache. Anaesthesia 2008;63:847–850.
- Hakim SM: Cosyntropin for prophylaxis against postdural puncture headache after accidental dural puncture. Anesthesiology 2010;113:413–420.
- Sadeghi SE, Abdollahifard G, Nasabi NA, et al: Effectiveness of single dose aminopylline administration on prevention of post dural puncture headache in patients who received spinal anesthesia for elective cesarean section. World J Med Sci 2012;7:13–16.
- Jacobus CH: Does bed rest prevent post-lumbar puncture headache? Ann Emerg Med 2012;59:139–140.
- Baraz R, Collis R: The management of accidental dural puncture during labor epidural analgesia: A survey of UK practice. Anaesthesia 2005;60: 673–679.
- Sudlow C, Warlow C: Posture and fluids for preventing postdural puncture headache. Cochrane Database Syst Rev 2002;(2):CD001790.
- Dakka Y, Warra N, Albadareen RJ, et al: Headache rate and cost of care following lumbar puncture at a single tertiary care hospital. Neurology 2011;77:71–74.
- Strupp M, Brandt T, Muller A: Incidence of post-lumbar puncture syndrome reduced by reinserting the stylet: A randomized prospective study of 600 patients. J Neurol 1998;245:589–592.
- Sinikoglu NS, Yeter H, Gumus F, et al: Reinsertion of the stylet does not affect incidence of post dural puncture headaches (PDPH) after spinal anesthesia. Rev Bras Anestesiol 2013;63:188–192.
- Moore JM: Continuous spinal anesthesia. Am J Ther 2009;16: 289–294.
- Sadashivaiah J, McClure H: 18-g Tuohy needle can reduce the incidence of severe post dural puncture headache. Anaesthesia 2009;64: 1379–1380.
- Schier R, Guerra D, Aguilar J, et al: Epidural space identification: A meta-analysis of complications after air versus liquid as the medium for loss of resistance. Anesth Analg 2009;109:2012–2021.
- Segal S, Arendt KW: A retrospective effectiveness study of loss of resistance to air or saline for identification of the epidural space. Anesth Analg 2010;110:558–563.
- Norris MC, Leighton BL, DeSimone CA: Needle bevel direction and headache after inadvertent dural puncture. Anesthesiology 1989;70:729–731.
- Duffy B: Don’t turn the needle! Anaesth Intensive Care 1993;21:328–330.
- Simmons SW, Cyna AM, Dennis AT, et al: Combined spinal-epidural versus epidural analgesia in labour. Cochrane Database Syst Rev 2009;(1):CD003401.
- Kuczkowski KM, Benumof JL: Decrease in the incidence of post-dural puncture headache: Maintaining CSF volume. Acta Anaesthesiol Scand 2003;47:98–100.
- Charsley MM, Abram SE: The injection of intrathecal normal saline reduces the severity of postdural puncture headache. Reg Anesth Pain Med 2001;26:301–305.
- Carter BL, Pasupuleti R: Use of intravenous cosyntropin in the treatment of postdural puncture headache. Anesthesiology 2000;92:272–274.
- Russell IF: A prospective controlled study of continuous spinal anesthesia
versus repeat epidural analgesia after accidental dural puncture in labour. Int J Obstet Anesth 2012;21:7–16. - Ayad S, Bemian Y, Narouze S, et al: Subarachnoid catheter placement after wet tap for analgesia in labor: Influence on the risk of headache in obstetric patients. Reg Anesth Pain Med 2003;28:512–515.
- Heesen M, Klohr S, Rossaint R, et al: Insertion of an intrathecal catheter
following accidental dural puncture: A meta-analysis. Int J Obstet Anesth 2013;22:26–30. - Newman M, Cyna A: Immediate management of inadvertent dural puncture during insertion of a labour epidural: A survey of Australian obstetric anaesthetists. Anaesth Intensive Care 2008;36:96–101.
- Stride PC, Cooper GM: Dural taps revisited: A 20-year survey from Birmingham Maternity Hospital. Anaesthesia 1993;48:247–255.
- Trivedi NS, Eddi D, Shevde K: Headache prevention following accidental dural puncture in obstetric patients. J Clin Anesth 1993;5: 42–45.
- Scavone BM, Wong CA, Sullivan JT, et al: Efficacy of a prophylactic epidural blood patch in preventing post dural puncture headache in parturients after inadvertent dural puncture. Anesthesiology 2004;101:1422–1427.
- Bradbury CL, Singh SI, Badder SR, et al: Prevention of post-dural puncture headache in parturients: A systematic review and meta-analysis. Acta Anaesthesiol Scand 2013;57:417–430.
- Agerson AN, Scavone BM: Prophylactic epidural blood patch after unintentional dural puncture for the prevention of postdural puncture headache in parturients. Anesth Analg 2012;115:133–136.
- Boonmak P, Boonmak S: Epidural blood patching for preventing and treating post-dural puncture headache. Cochrane Database Syst Rev 2010;(1):CD001791.
- Baysinger CL, Pope JE, Lockhart EM, et al: The management of accidental dural puncture and postdural puncture headache: A North American survey. J Clin Anesth 2011;23:349–360.
- Leivers D: Total spinal anesthesia following early prophylactic epidural blood patch. Anesthesiology 1990;73:1287–1289.
- Tobias MD, Pilla MA, Rogers C, et al: Lidocaine inhibits blood coagulation: implications for epidural blood patch. Anesth Analg 1996;82:766–769.
- Gutsche BB: Lumbar epidural analgesia in obstetrics: Taps and patches. In: Reynolds F (ed): Epidural and Spinal block in Obstetrics. Balliere Tindall, 1990, pp 75–106.
- Naulty JS, Hertwig L, Hunt CO, et al: Influence of local anesthetic solution on postdural puncture headache. Anesthesiology 1990;72:450–454.
- Santanen U, Rautoma P, Luurila H, et al: Comparison of 27-gauge (0.41-mm) Whitacre and Quincke spinal needles with respect to postdural puncture headache and non-dural puncture headache. Acta Anaesthesiol Scand 2004;48:474–479.
- Stella CL, Jodicke CD, How HY, et al: Postpartum headache: is your work-up complete? Am J Obstet Gynecol 2007;196:318.e1–318.e7.
- Somri M, Teszler CB, Vaida SJ, et al: Postdural puncture headache: An imaging-guided management protocol. Anesth Analg 2003;96:1809–1812.
- Sharma R, Panda A: Ondansetron-induced headache in a parturient mimicking postdural puncture headache. Can J Anesth 2010;57:187–188.
- Hurlburt L, Lay C, Fehlings MG, et al: Postpartum workup of postdural puncture headache leads to diagnosis and surgical treatment of thoracic pseudomeningocele: A case report. Can J Anesth 2013;60:294–298.
- National Institute of Neurological Disorders and Stroke. Meningitis and encephalitis fact sheet. http://www.ninds.nih.gov/disorders/encephalitis_meningitis/detail_encephalitis_meningitis.htm. Accessed June 28, 2013.
- Machurot PY, Vergnion M, Fraipont V, et al: Intracranial subdural hematoma following spinal anesthesia: Case report and review of the literature. Acta Anaesth Belg 2010;61:63–66.
- Bleeker CP, Hendriks IM, Booij LHDJ: Postpartum post-dural puncture headache: Is your differential diagnosis complete? Br J Anaesth 2004; 93:461–464.
- Matthys LA, Coppage KH, Lambers DS, et al: Delayed postpartum preeclampsia: An experience of 151 cases. Am J Obstet Gynecol 2004;190:1464–1466.
- Lockhart EM, Baysinger CL: Intracranial venous thrombosis in the parturient. Anesthesiology 2007;107:652–658.
- Wittmann M, Dewald D, Urbach H, et al: Sinus venous thrombosis: A differential diagnosis of postpartum headache. Arch Gynecol Obstet 2012;285:93–97.
- Vanden Eede H, Hoffmann VLH, Vercauteren MP: Post-delivery postural headache: Not always a classical post-dural puncture headache. Acta Anaesthesiol Scand 2007;51:763–765.
- van Kooten F, Oedit R, Bakker SLM, et al: Epidural blood patch in post dural puncture headache: A randomized, observer-blind, controlled clinical trial. J Neurol Neurosurg Psychiatry 2008;79:553–558.
- Sharma A, Cheam E: Acupuncture in the management of post-partum headache following neuraxial analgesia. Int J Obstet Anesth 2009;18:417–419.
- Takmaz SA, Kantekin CU, Kaymak C, et al: Treatment of post-dural puncture headache with bilateral greater occipital nerve block. Headache 2010;50:869–872.
- Cohen S, Ramos D, Grubb W, et al. Reg Anesth Pain Med 2014;39:563.
- Basurto Ona X, Martinez Garcia L, Sola I, et al: Drug therapy for treating post-dural puncture headache. Cochrane Database Syst Rev 2011;(8):CD007887.
- Choi A, Laurito CE, Cummingham FE: Pharmacologic management of postdural puncture headache. Ann Pharmacother 1996;30:831–839.
- Camann WR, Murray RS, Mushlin PS, et al: Effects of oral caffeine on postdural puncture headache. A double-blind, placebo-controlled trial. Anesth Analg 1990;70:181–184.
- Halker RB, Demaerschalk BM, Wellik KE, et al: Caffeine for the prevention and treatment of postdural puncture headache: debunking the myth. Neurologist 2007;13:323–327.
- Connelly NR, Parker RK, Rahimi A, et al: Sumatriptan in patients with postdural puncture headache. Headache 2000;40:316–319.
- Hakim S, Khan RM, Maroof M, et al: Methylergonovine maleate (methergine) relieves postdural puncture headache in obstetric patients. Acta Obstet Gynecol Scand 2005;84:100.
- Rucklidge MWM, Yentis SM, Paech MJ, et al: Synacthen Depot for the treatment of postdural puncture headache. Anaesthesia 2004;59: 138–141.
- Noyan Ashraf MA, Sadeghi A, Azarbakht Z, et al: Hydrocortisone in post-dural puncture headache. Middle East J Anesthesiol 2007;19:415–422.
- Rabiul A, Aminur R, Reza E: Role of very short-term intravenous hydrocortisone in reducing postdural puncture headache. J Anaesthesiol Clin Pharmacol 2012;28:190–193.
- Wagner Y, Storr F, Cope S: Gabapentin in the treatment of post-dural puncture headache: A case series. Anaesth Intensive Care 2012;40:714–718.
- Huseyinoglu U, Huseyinoglu N, Hamurtekin E, et al: Efect of pregabalin on post-dural-puncture headache following spinal anesthesia and lumbar puncture. J Clin Neurosci 2011;18:1365–1368.
- Kroin JS, Nagalla SKS, Buvanendran, et al: The mechanisms of intracranial pressure modulation by epidural blood and other injectates in a postdural puncture rat model. Anesth Analg 2002;95:423–429.
- Bart AJ, Wheeler AS: Comparison of epidural saline placement and epidural blood placement in the treatment of post-lumbar-puncture headache. Anesthesiology 1978;48:221–223.
- Liu SK, Chen KB, Wu RSC, et al: Management of postdural puncture headache by epidural saline delivered with a patient-controlled pump—A case report. Acta Anaesthesiol Taiwan 2006;44:227–230.
- Kara I, Ciftci I, Apiliogullari S, et al: Management of postdural puncture headache with epidural saline patch in a 10-year-old child after inguinal hernia repair: A case report. J Pediatr Surg 2012;47:E55–E57.
- Vakharia SB, Thomas PS, Rosenbaum AE, et al: Magnetic resonance imaging of cerebrospinal fluid leak and tamponade effect of blood patch in postdural puncture headache. Anesth Analg 1997;84:585–590.
- Serpell MG, Haldane GJ, Jamieson DR, et al: Prevention of headache after lumbar puncture: Questionnaire survey of neurologists and neurosurgeons in United Kingdom. Br Med J 1998;316:1709–1710.
- Darvish B, Gupta A, Alahuhta S, et al: Management of accidental dural puncture and post-dural puncture headache after labour: A Nordic survey. Acta Anaesthesiol Scand 2011;55:46–53.
- Banks S, Paech M, Gurrin L: An audit of epidural blood patch after accidental dural puncture with a Tuohy needle in obstetric patients. Int J Obstet Anesth 2001;10:172–176.
- Safa-Tisseront V, Thormann F, Malassine P, et al: Effectiveness of epidural blood patch in the management of post-dural puncture headache. Anesthesiology 2001;95:334–339.
- Sandesc D, Lupei MI, Sirbu C, et al: Conventional treatment or epidural blood patch for the treatment of different etiologies of post dural puncture headache. Acta Anaesth Belg 2005;56:265–269.
- Vilming ST, Kloster R, Sandvik L: When should an epidural blood patch be performed in postlumbar puncture headache? A theoretical approach based on a cohort of 79 patients. Cephalalgia 2005;25:523–527.
- Chen LK, Huang CH, Jean WH, et al: Effective epidural blood patch volumes for postdural puncture headache in Taiwanese women. J Formos Med Assoc 2007;106:134–140.
- Paech MJ, Doherty DA, Christmas T, et al: The volume of blood for epidural blood patch in obstetrics: A randomized, blinded clinical trial. Anesth Analg 2011;113:126–133.
- Schievink WI: Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA 2006;295:2286–2296.
- Riley CA, Spiegel JE: Complications following large-volume epidural blood patches for postdural puncture headaches. Lumbar subdural hematoma and arachnoiditis: Initial cause or final effect? J Clin Anesth 2009;21:355–359.
- Desai MJ, Dave AP, Martin MB: Delayed radicular pain following two large volume epidural blood patches for post-lumbar puncture headache: A case report. Pain Physician 2010;13:257–262.
- Martin R, Jourdain S, Clairoux M, et al: Duration of decubitus position after epidural blood patch. Can J Anaesth 1994;41:23–25.
- Jagannathan N, Tetzlaff JE: Epidural blood patch in a Jehovah’s Witness patient with post-dural puncture cephalgia. Can J Anaesth 2005;52:113.
- Janssens E, Aerssens P, Alliet P, et al: Post-dural puncture headaches in children: A literature review. Eur J Pediatr 2003;162:117–121.
- Kokki M, Sjovall S, Kokki H: Epidural blood patches are effective for postdural puncture headache in pediatrics—A 10-year experience. Pediatr Anesth 2012;22:1205–1210.
- Waldman SD, Feldstein GS, Allen ML: Cervical epidural blood patch: A safe effective treatment for cervical post-dural puncture headache. Anesth Rev 1987;14:23–24.
- Bucklin BA, Tinker JH, Smith CV: Clinical dilemma: A patient with postdural puncture headache and acute leukemia. Anesth Analg 1999;88:166–167.
- Koeva V, Bar-Or A, Gendron D, et al: Epidural blood patch in a patient with multiple sclerosis: Is it safe? Can J Anaesth 2013;60:479–483.
- Tom DJ, Gulevich SJ, Shapiro HM, et al: Epidural blood patch in the HIV-positive patient. Anesthesiology 1992;76:943–947.
- Martin DP, Bergman BD, Berger IH: Epidural blood patch and acute varicella. Anesth Analg 2004;99:1760–1762.
- Abouleish E, de la Vega S, Blendinger I, et al: Long-term follow-up of epidural blood patch. Anesth Analg 1975;54:459–463.
- Andrews PJD, Ackerman WE, Juneja M, et al: Transient bradycardia associated with extradural blood patch after inadvertent dural puncture in parturients. Br J Anaesth 1992;69:401–403.
- Hebl JR, Horlocker TT, Chantigian RC, et al: Epidural anesthesia and analgesia are not impaired after dural puncture with or without epidural blood patch. Anesth Analg 1999;89:390–394.
- Collier CB: Blood patches may cause scarring in the epidural space: Two case reports. Int J Obstet Anesth 2011;20:347–351.
- Vassal O, Baud MC, Bolandard F, et al: Epidural injection of hydroxyethyl starch in the management of postdural puncture headache. Int J Obstet Anesth 2013;22:153–155.
- Williams EJ, Beaulieu P, Fawcett WJ, et al: Efficacy of epidural blood patch in the obstetric population. Int J Obstet Anesth 1999;8: 105–109.
- Sadashivaiah J: 18-G Tuohy needle can reduce the incidence of severe post dural puncture headache. Anaesthesia 2009;64:1379–1380.
- Ho KY, Gan TJ: Management of persistent post-dural puncture headache after repeated epidural blood patch. Acta Anaesthesiol Scand 2007;51:633–636.
- Sullivan FM, Swan IRC, Donnan PT, et al: Early treatment with prednisone or acyclovir in Bell’s palsy. N Engl J Med 2007;357: 1598–1607.
- Choi PTL, Galinski SE, Lucas S, et al: Examining the evidence in anesthesia literature: A survey and evaluation of obstetrical post-dural puncture headache reports. Can J Anaesth 2002;49:49–56.