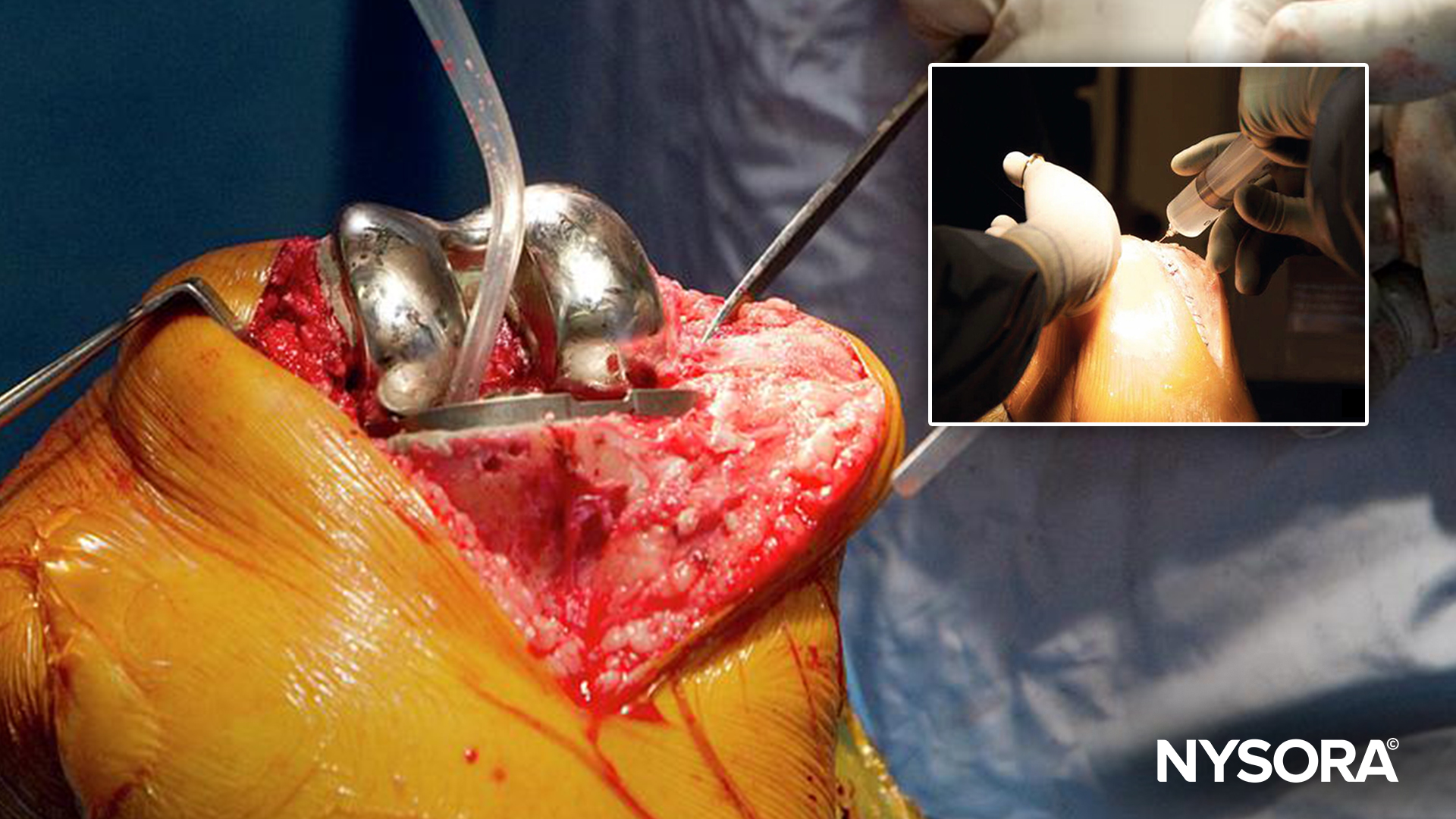
Cardiopulmonary bypass (CPB) remains an indispensable tool in pediatric cardiac surgery, but it introduces significant challenges to coagulation management. While modern perfusion techniques have advanced, coagulopathy, a condition of impaired blood clotting, remains a stubborn complication. This is particularly concerning in children, whose hemostatic systems are already underdeveloped compared to adults.
A new study led by Dr. Elena Ashikhmina Swan and colleagues at the Mayo Clinic explores the perioperative dynamics of thrombin generation, a central component of the coagulation cascade. This comprehensive investigation sheds light on how surgical procedures involving CPB alter the body’s ability to produce thrombin and how this change correlates with clinical outcomes.
Why thrombin matters in coagulation
Thrombin plays a pivotal role in hemostasis. It converts fibrinogen to fibrin, activates platelets, and helps stabilize the blood clot. The body’s ability to generate thrombin quickly and sufficiently is crucial during and after surgical procedures, especially those involving extracorporeal circulation like CPB.
The endogenous thrombin potential (ETP)
The ETP reflects the total amount of thrombin that can be generated in plasma over time. This parameter provides a more comprehensive understanding of coagulation status than traditional tests like prothrombin time (PT) or activated partial thromboplastin time (aPTT), which measure only the initiation of clot formation.
Study design and objectives
Patient cohort
- Population: 54 pediatric patients with congenital heart disease
- Age: Median 6.3 months (range from neonates to toddlers)
- Weight: 68.5% weighed under 10 kg
- Procedure: All patients underwent CPB as part of their cardiac surgery
Time points for blood sampling
- T1: Pre-CPB, before heparin administration
- T2: Post-CPB, approximately 8–10 minutes after protamine administration
- T3: At the time of sternal closure
Key findings
-
Significant and persistent ETP reduction
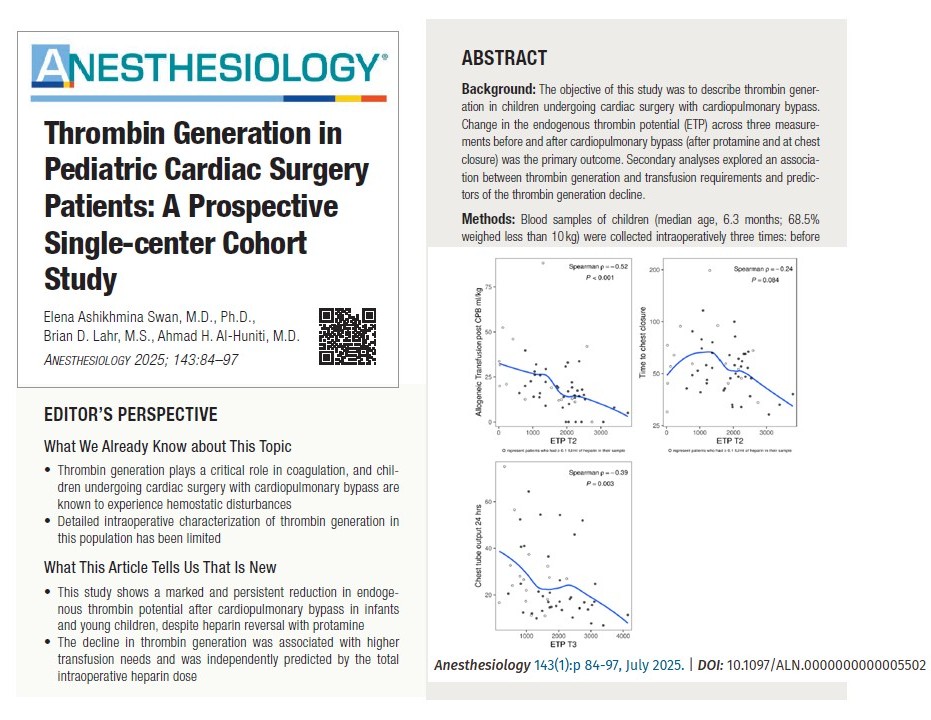
Mean ETP decreased by 1,911 nM·min after protamine and remained low at chest closure (T3), with a mean drop of 1,865 nM·min from baseline.
Other parameters, like peak thrombin and velocity index, also showed significant declines. This suggests incomplete hemostatic recovery despite standard transfusion protocols.
-
Heparin dosage predicts thrombin suppression
Total intraoperative heparin dose was the only independent predictor of ETP decline in multivariable analysis.
Heparin doses >1,000 units/kg showed no additional ETP decline, suggesting a plateau effect.
-
Clinical impact of low ETP
ETP at T2 negatively correlated with transfusion volume after CPB (p = −0.52).
Lower ETP at T3 was moderately associated with increased chest tube output over the first 24 hours post-op (p = −0.39).
Step-by-step implications for clinicians
- Use heparin judiciously: Aim for the lowest effective dose to achieve adequate anticoagulation.
- Reevaluate protamine protocols: Consider individualized reversal strategies based on heparin concentration.
- Incorporate thrombin generation data: As tools become available, use TGAs to guide transfusion and anticoagulation decisions.
- Monitor post-CPB coagulation dynamically: Relying solely on ACT or TEG may overlook important deficits.
Limitations and strengths of the study
Limitations
- Small cohort from a single center
- The use of platelet-poor plasma excluded cellular elements that influence thrombin formation
- Residual heparin activity may have confounded results despite anti-Xa screening
Strengths
- Rigorously designed with each patient as their own control
- Simultaneous heparin quantification enhanced data validity
- Comprehensive statistical modeling identified clear predictors of ETP change
Future directions
- Multicenter trials to validate findings and assess generalizability
- Point-of-care thrombin generation testing to bring this research into clinical practice
- Studies on thrombin dynamics in specific subgroups, such as neonates or those requiring mechanical circulatory support
- Investigation of long-term outcomes associated with perioperative thrombin suppression
Conclusion
This study represents a landmark in understanding the hemostatic impact of CPB in pediatric cardiac surgery. It shows that even with meticulous heparin reversal and transfusion protocols, thrombin generation remains significantly impaired through the conclusion of surgery. This raises important questions about the adequacy of current hemostatic practices.
By identifying a strong link between thrombin suppression and transfusion needs, and highlighting the predictive role of heparin dose, this research sets the stage for more personalized and effective coagulation management in vulnerable pediatric populations.
For more information, refer to the full article in Anesthesiology.
Swan EA, Lahr BD, Al-Huniti AH. Thrombin Generation in Pediatric Cardiac Surgery Patients: A Prospective Single-center Cohort Study. Anesthesiology. 2025;143(1)
For more literature updates in pediatric anesthesia, read NYSORA’s Pediatric Anesthesia Updates 2025!