INTRODUCTION
Spinal epidural hematoma (SEH) is an accumulation of blood in the loose areolar tissue between the vertebrae and the dura of the spinal canal. Typically, the hematoma is asymptomatic, but in rare cases it will compress the spinal cord, with potentially devastating neurological consequences. These symptoms include sensory disruption, bowel and bladder incontinence, motor weakness, or, in severe cases, complete paralysis of the affected limbs. This clinical entity was first described in the medical literature in 1682 as spinal hematoma with spinal apoplexy in the Histoire de l’Academie Royale des Science (Volume 2; G.J. Duverney). Nearly 200 years later, in 1869, a report of the first clinical diagnosis of SEH was published in the Lancet.
The SEHs can be spontaneous in nature or may occur in the setting of an invasive procedure, such as lumbar puncture, neuraxial anesthesia, or spine surgery. Hematoma is more likely to be symptomatic in the cervical and thoracic regions, given the constricted spinal canal in this area compared to the greater space available for volume compensation in the lumbar and particularly the cauda equina region.
SPINAL EPIDURAL HEMATOMA
Incidence
Symptomatic SEH accounts for less than 1% of all spinal space-occupying lesions4 and affects only 1 per 1 million people annually. SEHs arise from myriad etiologies but most often from a procedure performed in or near the epidural space. For example, the presence of SEH can be found on postoperative imaging in 33% to 100% of patients after spinal surgery, but patients will rarely show any neurologic deficit. A 2010 systematic review found an overall calculated incidence of symptomatic SEH after spine surgery of 0.2%, with individual study calculations ranging between 0% and 1.0%. Therefore, the incidence of SEH is routinely quoted as the incidence of symptomatic SEH; henceforth in this chapter, the qualifying term symptomatic is implied.
The incidence of SEH after neuraxial anesthesia has historically been approximated to be less than 1 in 150,000 epidural placements and less than 1 in 220,000 spinal anesthetics. However, according to recent epidemiologic studies, the incidence may be increasing. This estimation was confirmed by a large-scale Swedish study that found an incidence of SEH after epidural block of 1:18,000, a figure that results from the average of an obstetric incidence of 1:200,000 and a remarkably high incidence of 1:3600 calculated in a population of elderly female patients undergoing knee arthroplasty. Another study showed an overall SEH incidence of 1:4741 that increased to 1:1000 if the population assessed was narrowed to include only elderly women undergoing lower extremity surgery.
This large disparity can be attributed to the presence or absence of risk factors in these incongruent patient populations. Pregnancy induces a relatively hypercoagulable state and obstetric patients are also younger with a larger and more compliant epidural space than elderly patients. The lower incidence of SEH in obstetric patients was confirmed in a recent study wherein there were no recognized cases of SEH in 709,837 patients evaluated in the peripartum period. This study noted an incidence of 1:9000 (95% confidence interval [CI]: 1:20,189–1:4330) in patients who received perioperative epidural placements.
Risk Factors
Increasing age carries with it an increased risk of SEH. The reduction in the size of the epidural space with age was first reported in 1967 in a study of local anesthetic spread.
This can be extrapolated to account for the higher incidence of SEH in the elderly because an equivalent volume of blood will cause increased pressure in the smaller epidural space of an elderly patient compared to a younger counterpart. No racial predilection has been reported, but SEH is more frequent in females. This could potentially be explained by the higher prevalence of osteoporosis in female patients, which causes vertebral deformities or fractures and enlargement of the vertebral bodies, resulting in narrowing of the spinal canal. Osteoporotic narrowing of the epidural space therefore could account for both the gender and the age predilections of SEH and may contribute to the greater than 50-fold increased likelihood of SEH after epidural anesthesia for knee arthroplasty as compared to epidural labor analgesia. However, thromboprophylaxis is a necessity in the orthopedic surgical population, which also may contribute to their relatively higher incidence of SEH compared to obstetric patients, who do not require routine prophylaxis against deep vein thrombosis.
Indeed, the most important risk factor for SEH is the presence of a physiologic or iatrogenic disorder of the coagulation system, such as liver disease, alcoholism, thrombocytopenia, or pharmacologic anticoagulation. A recent retrospective study also identified a significant increase in SEH after spine surgery in patients with Rh+ blood types, intraoperative blood loss greater than 1 L, hemoglobin level less than 10 g/dL, and an international normalized ratio greater than 2.0 in the first 48 hours. Anticoagulant therapy in association with neuraxial analgesia as well as the length and intensity of anticoagulation have been identified as the most significant risk factors for epidural hematoma. Approximately one-quarter to one-third of all SEH cases are associated with anticoagulation therapy. In an extensive review of every case of SEH associated with neuraxial anesthesia, 87% of patients had either a hematologic abnormality or a procedure complicated by technical difficulty. Cases of spontaneous hematoma are rare, but when they do occur are often associated with anticoagulation, thrombolysis, blood dyscrasias, coagulopathies, thrombocytopenia, neoplasms, vascular malformations, or vertebral hemangioma.
Decreased patient weight, which may exaggerate the anticoagulant response, represents a theoretical concern for bleeding tendency and has been suggested as the explanation for the increased risk in women and the elderly. However, in Sweden, a gender-specific reduced dose of low molecular weight heparin for prophylaxis against deep vein thrombosis has not been shown to improve the well-described increased incidence of SEH in women. Thrombolytic therapy imposes the greatest risk of bleeding complications, and neuraxial procedures should be diligently avoided in patients who have recently undergone thrombolysis. Learn more about Neuraxial Anesthesia and Peripheral Nerve Blocks in Patients on Anticoagulants.
Clinical Pearl
- Recent studies on the incidence of the risk of spinal hematoma in patients without overt risk factors showed an increase to 1:18,000 after epidural and 1:3600, even 1:1000, in elderly patients undergoing lower extremity surgery.
Etiology and Location of Hematoma
The proposed factors that cause SEH include trauma, anticoagulation, thrombolysis, lumbar puncture, epidural or spinal anesthesia, interventional spine procedures or surgeries, coagulopathy or bleeding diathesis, hepatic disease with portal hypertension, vascular malformation, disk herniation, Paget disease of the vertebral bones, Valsalva maneuver, and hypertension. The most important causes of spontaneous SEH are clotting disorders, which may be acquired (eg, anticoagulant therapy, malignancies) or congenital (eg, hemophilia). Vascular malformations are rarely responsible for spontaneous epidural hematomas; only 4% in a series of 158 cases and 6.5% in a series of 199 cases were reported to be due to vascular malformation. Less-common predisposing factors include systemic lupus erythematosus, ankylosing spondylitis, rheumatoid arthritis, Paget disease, disk herniation, and hypertension.
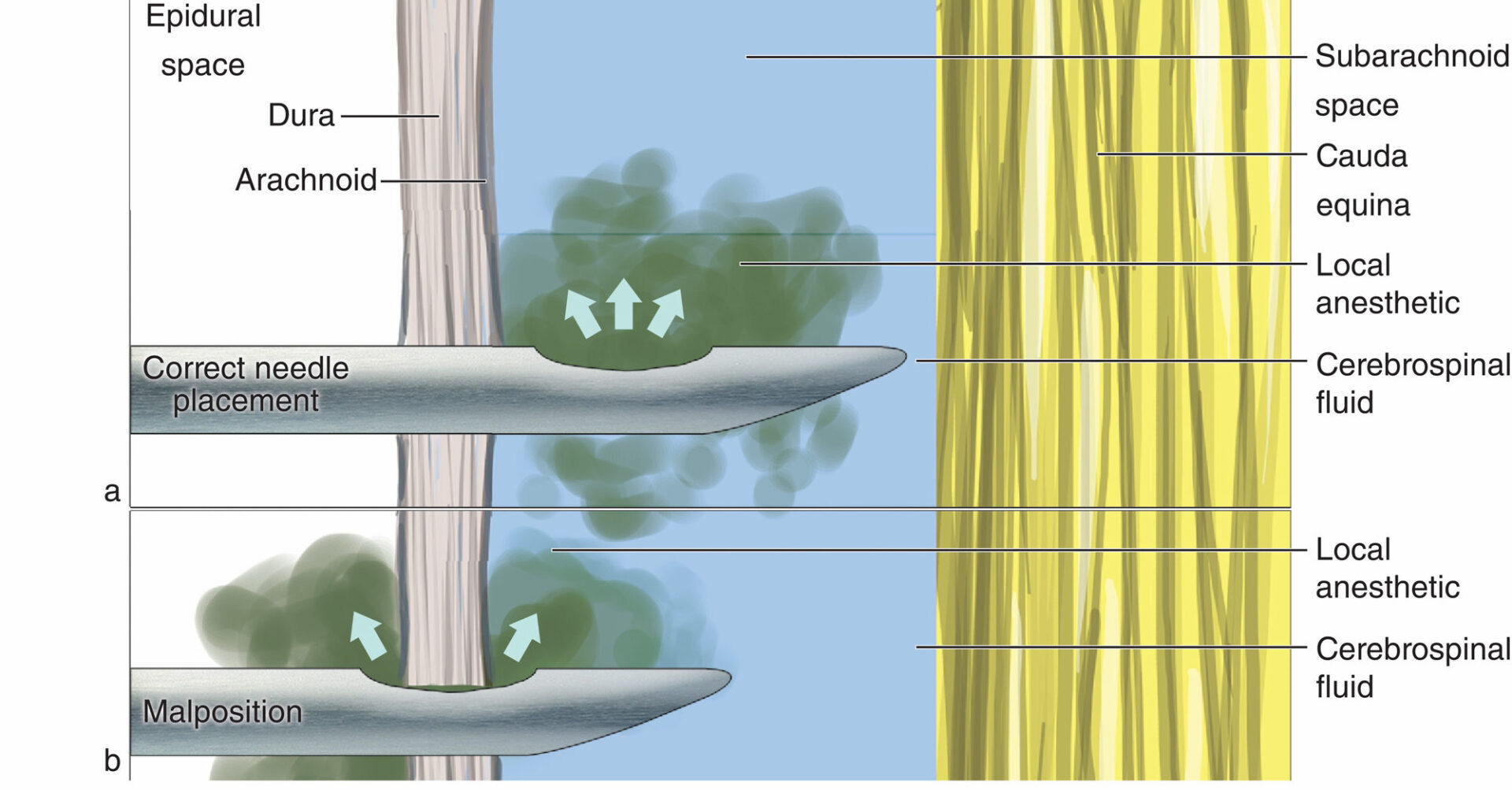
The dorsal venous plexus is the most commonly implicated source of hemorrhage because this plexus lacks valves and permits reversal in blood flow during increased intravascular pressure from physical activity. These veins lack protection as they are surrounded only by loose areolar tissue and are therefore vulnerable to sudden increases in intra-abdominal or intrathoracic pressure, leading to rupture and hemorrhage. The epidural venous plexus is most prominent in the thoracic spine, and spontaneous SEH most often occurs in the thoracic and cervicothoracic region, followed by the thoracolumbar area. SEH is typically posterior or posterolateral to the thecal sac (Figure 1) because the firm adherence of the dural sac to the posterior longitudinal ligament in the ventral aspect of the spinal canal prevents the accumulation of hematoma. The dorsal aspect of the thoracic or lumbar region is commonly involved, and expansion is typically limited to only a few vertebral levels.
In pregnant women, it has been proposed that increased venous pressure due to uterine enlargement, in association with the hemodynamic changes of pregnancy, may predispose to rupture of a preexisting pathologic epidural venous plexus wall. Although a venous source is the most widely accepted, debate continues regarding a potential arterial source of SEH, with proponents of this theory stating that venous blood pressure is less than intrathecal pressure; therefore, although forward flow is possible into the low-pressure epidural space, venous blood could not cause compression of the spinal cord.
NYSORA Tips
- Hemorrhage into the spinal canal most commonly occurs in the epidural space because of the prominent epidural venous plexus.
- SEH may be spontaneous or may follow minor trauma, such as lumbar puncture or neuraxial anesthesia.
- SEH occurs primarily in anticoagulated or thrombocytopenic patients.
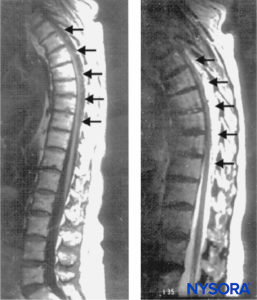
FIGURE 1. Sagittal magnetic resonance images of the thoracolumbar spine. A large complex epidural hematoma extending from T3 to T10 through T11 is seen with hypo- and isodense signal characteristics on a T1-weighted image (left; arrows) and hyperintense signal characteristics on a T2-weighted image (right; arrows). At the center of the hematoma, the spinal cord abuts the posterior aspect of the thoracic vertebral
History and Physical Examination
Classically, the presenting symptom of SEH is acute axial back pain that radiates to corresponding dermatomes and evolves to focal neurologic deficit with signs of nerve root or spinal cord compression. The pain is generally described as a severe, localized constant back pain with or without a radicular component that may mimic disk herniation, especially in the lumbar spine.
Back pain is amplified by percussion over the spine, as well as maneuvers that increase intraspinal pressure, such as coughing, sneezing, or straining. However, a 2010 analysis demonstrated that lower extremity weakness is the most common presenting sign, although back pain is still a common early symptom. Associated symptoms may include numbness, weakness, and urinary or fecal incontinence. The onset of pain is occasionally related to minor strain, such as with lifting, coughing, sneezing, or Valsalva maneuvers, although in the majority of cases the onset of pain is spontaneous.
Depending on the level and the size of the hematoma, physical findings may include unilateral or bilateral weakness, sensory deficits with unilateral or bilateral radicular paresthesias, various alterations in deep tendon reflexes, and alterations of bladder or anal sphincter tone. Signs of spinal cord and nerve root dysfunction appear rapidly and may progress to paraparesis or, in high thoracic or cervical locations, quadriparesis. In cases of SEH related to neuraxial anesthesia or lumbar puncture, the presence of new or progressive postoperative neurologic symptoms should alert the physician to a possible epidural hematoma.
NYSORA Tips
- The patient may present with a severe, localized, constant back pain with or without a radicular component that may mimic disk herniation.
- Associated symptoms may include weakness, numbness, and urinary or fecal incontinence.
- Return of sensory or motor deficit several hours after spinal or epidural block has worn off (with or without back pain) is highly pathognomonic and should be considered and treated as spinal or epidural hematoma until proven otherwise.
- Neurologic recovery after conservative management has been reported in patients with back pain and leg weakness without paralysis.
- Neurologic recovery can occur if surgery and decompression is performed within 36 hours of a complete motor deficit and within 48 hours of a partial deficit.
Diagnosis
As noted previously, the clinical findings of SEH usually include back pain and motor/sensory deficits that may rapidly progress to paraplegia, quadriplegia, or autonomic dysfunction.
An epidural hematoma ordinarily presents within the first 24–48 hours after a procedure. Any new or progressive neurologic symptoms warrant immediate clinical evaluation and diagnostic workup to rule out any space-occupying lesion, including epidural hematoma. A neurologic deficit occurring in the presence of epidural analgesia mandates immediate discontinuation of the infusion, with the catheter left in place, to rule out any contribution from the local anesthetic.
If the epidural infusion is the cause of the neurologic manifestation, a return of sensory and motor function will be noted. Otherwise, an immediate workup and radiographic imaging studies should be obtained and a consultation with a spine surgeon sought to rule out an evolving epidural lesion.
In a patient presenting with acute axial back pain with progression of neurologic deficits, immediate assessment for the presence of pathologic entities associated with nerve root and spinal cord compression is required to differentiate miscellaneous lesions simulating SEH. The clinical presentation of a patient with suspected epidural hematoma may resemble presentation for epidural abscess, spinal cord disease, neoplasm, or acute disk herniation. The differential diagnosis of new or progressive postoperative neurologic symptoms includes surgical neuropraxia, prolonged or exaggerated neuraxial block, anterior spinal artery syndrome, exacerbation of a preexisting neurologic disorder, and presentation of a previously undiagnosed neurologic condition.
A complete blood cell count with platelets should be ordered to assess the extent of bleeding and to determine the presence of infection. Prothrombin time and activated partial thromboplastin time determine the presence of bleeding diathesis.
Rapid radiologic evaluation is essential to minimize delay in treatment of SEH. Currently, magnetic resonance imaging (MRI) is the diagnostic imaging method of choice for spinal emergencies because it allows rapid, noninvasive evaluation of the vertebral column and the spinal cord in all planes. Spinal MRI may delineate the location of an epidural hematoma and identify an associated vascular malformation; it will also provide information regarding the extent of the hematoma as well as the degree of cord compression. MRI can also aid in assessment of the age of the hematoma (Figure 1).
The chronologic characteristics of an MRI of SEH are similar to those seen with intracranial hemorrhage. In the hyperacute stage (first 6 hours), the SEH appears isointense compared to the spinal cord on T1-weighted images and mildly hyperintense and heterogeneous on T2-weighted images, as a result of the presence of intracellular oxyhemoglobin. In the acute stage (7–72 hours), the hematoma is still isointense on T1-weighted images and becomes hypointense on T2-weighted images. This is due to the presence of intracellular deoxyhemoglobin, which causes T2 shortening. As the concentration of methemoglobin increases, the hematoma becomes hyperintense and homogeneous on T1- and T2-weighted images. Gadolinium-enhanced magnetic resonance arteriography (MRA) may further define the extent of an arteriovenous malformation.
Conventional computed tomography (CT) has the potential to diagnose an epidural hematoma but may give false-negative results if the hematoma is isodense to the thecal sac or spinal cord and the image quality is affected by artifacts often seen in the upper thoracic region. Spinal CT scanning may be nondiagnostic in the thoracic spine, where resolution is poorer than in the lumbar and cervical spine because of the high contrast between the lung parenchyma and vertebral bone. Conventional angiography may be required to definitively demonstrate the presence of a vascular alformation.
Myelography, and later CT myelography, were previously the main modalities for diagnosis of epidural hematoma. Combined with spinal CT scanning, myelography will display SEH as an intraspinal biconvex and hyperdense lesion with a density equivalent to blood. Although this may demonstrate an epidural lesion with partial or complete spinal block, the findings are not specific. Moreover, myelography is invasive and may worsen the patient’s clinical status. In addition, although it can demonstrate signs of compression with visualization of nonspecific contrast block or extradural convex compression, myelography cannot determine the nature and the true extent of the lesion. These techniques are now rarely used in the United States as MRI has become the gold standard diagnostic measure.
Prevention, Treatment, and Prognosis
Lumbar puncture or epidural anesthesia should be avoided in individuals who are on anticoagulant therapy, have received thrombolytic therapy, or are suspected to have a bleeding diathesis. Anesthesiologists are urged to be up to date on their knowledge of anticoagulation protocols, new anticoagulant medications, and current guideline recommendations. The decision to perform neuraxial block and the timing of catheter removal in a patient receiving antithrombotic therapy should be made on an individual basis, weighing the risks of spinal hematoma with the benefits of regional anesthesia for a specific patient. The American Society of Regional Anesthesia and the European and Scandinavian Society of Anaesthesiologists have published consensus statements on neuraxial anesthesia and anticoagulation, which provide an up-to-date source for guidelines in the decision-making process in performing neuraxial anesthesia in a patient with risk factors (see Neuraxial Anesthesia and Peripheral Nerve Blocks in Patients on Anticoagulants).
In contrast, no guidelines exist regarding anticoagulation in surgical patients. Recent retrospective studies regarding the incidence of postoperative hematoma after spine surgery concluded that perhaps patients should receive deep vein thrombosis prophylaxis as this does not appear to have an impact on the likelihood of SEH formation. However, further prospective studies are needed prior to widespread institution of thromboprophylaxis after spine surgery given the devastating consequences of SEH. Case reports have described successful conservative management of epidural hematoma. Nonoperative treatment with good outcome was mainly reported in hematomas localized at the cauda equina level and those with mild neurologic deficit. It appears that complete recovery can occur with conservative management when the patient reports back pain and leg weakness or numbness but does not exhibit leg paralysis. Reversal of clotting abnormalities, close observation of neurologic deficits, and, in rare cases, steroid administration may achieve a good outcome without surgery. A practical approach to management of suspected epidural hematoma is displayed in Figure 2.
Urgent surgical decompression is the treatment of choice for SEH causing acute compromise of cord function. Laminectomy is followed by evacuation of the hematoma, coagulation of bleeding sites, and inspection of the dura. The dura is then tented to the bone, and, occasionally, epidural drains are employed for as long as 24 hours. Although historically the efficacy of drains has been controversial, recent data suggest that subfascial drains significantly decrease SEH formation. Recovery after prolonged paralysis without surgery is rare, and surgical consultation for consideration of emergent decompressive laminectomy must be obtained as soon as possible. The overall mortality rate is 8%. Ultimately, the surgery team must decide to observe or operate on a case-by-case basis. The critical factors for recovery after SEH are the level of preoperative neurologic deficit and the operative interval. Prognosis for neurologic recovery primarily depends on the patient’s preoperative neurologic status and duration of neurologic dysfunction. The prognosis is worse when there is a delay between the injury and surgical intervention.
Previously, complete neurologic recovery was considered to be unlikely if more than 8 hours had elapsed between the development of paralysis and surgical intervention. However, other authors have noted recovery when surgery is performed within 12 hours of paralysis.
This recommended time frame for intervention has been further delineated by the recent finding that recovery can be achieved when surgery is performed within 48 hours of an incomplete motor deficit and within 36 hours of a complete motor deficit (Table 1). It is interesting to note that functional recovery after 72 hours of symptoms has been reported. While it is reassuring that functional recovery may occur after such intervals, appraisal of the patient’s symptoms and emergency MRI take time. Therefore, consultation with a spine surgeon regarding potential emergent evacuation of the hematoma should be obtained as soon as possible.
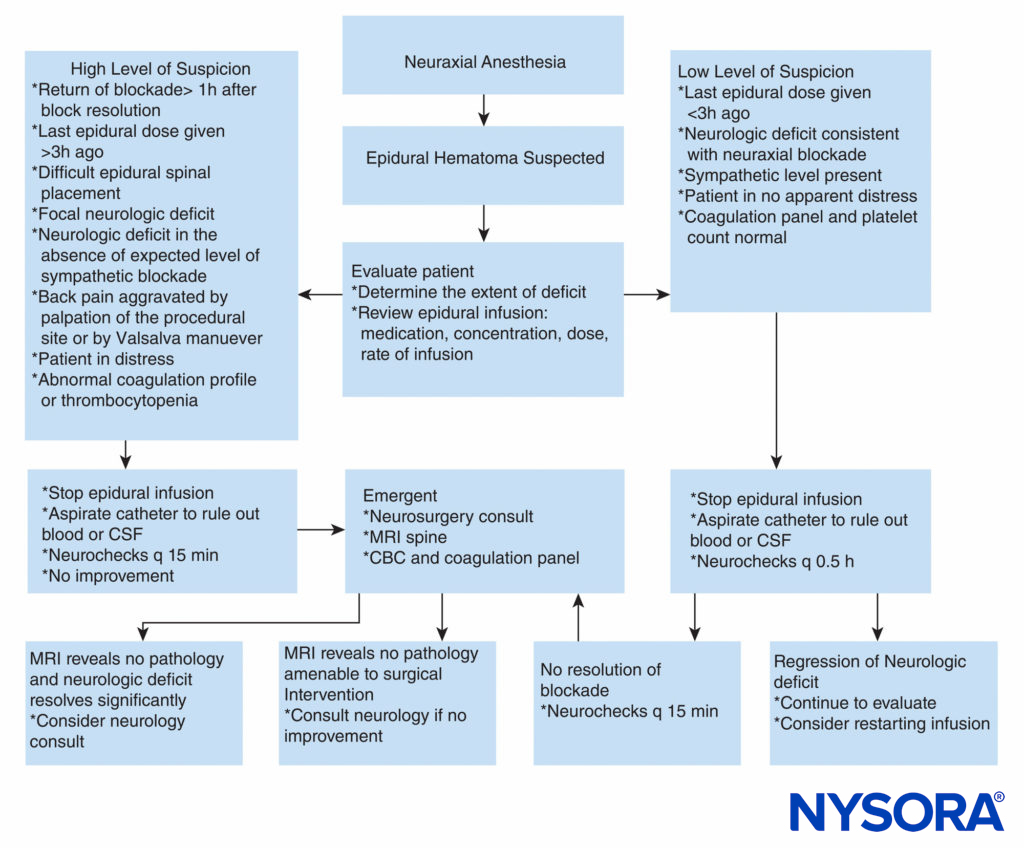
FIGURE 2. Practical approach to decision-making in workup and treatment of a patient with suspected epidural hematoma. CBC = complete blood cell count; CSF = cerebrospinal fluid; MRI = magnetic resonance imaging; q = every.
TABLE 1. Neurologic recovery in relation to timing of surgery.
| Author | Interval between paralysis and recovery |
|---|---|
| Wulf68 | 8 hours 36 hours of a complete motor deficit |
| Lawton et al48 | 12 hours 48 hours of an incomplete motor deficit |
| Groen and Van Alphen69 |
Spinal Epidural Hematoma: Summary
Spinal epidural hematoma is a rare source of neurologic deterioration and can result in autonomic, sensory, and motor disturbance of varying degrees depending on the location and size of the hemorrhage. SEH may be acute or chronic, spontaneous, post-traumatic, or iatrogenic. Known risk factors include technically difficult neuraxial procedure, presence of intrinsic or acquired coagulopathy, female gender, and advanced age. Potential risk factors as evidenced by inconsistent data in the literature include low hemoglobin level, presence of Rh+ antibody, and anatomical abnormalities of the spinal cord. Given that SEH is a rare but potentially reversible cause of spinal cord compression, it is essential that the diagnosis be made without delay to enable full recovery. Spinal hematoma may occur even in the absence of identifiable risk factors; therefore, health care providers must maintain a high index of suspicion and vigilance in monitoring for new neurologic symptoms.
Although MRI cannot be conducted with the rapidity of a CT scan, it is the diagnostic modality of choice because it is both sensitive and specific. Swift detection of neurologic deterioration is critical to early diagnostic imaging and prompt intervention. It is reassuring to note that recovery can occur without surgery in the absence of paralysis. When paralysis occurs, surgical decompression of the spinal cord and nerve roots can result in full functional recovery when accomplished within a reasonably rapid interval. If intervention is delayed, permanent sequelae of SEH can include sensory deficits, paraplegia, spasticity, neuropathic pain, and urinary or anal sphincter dysfunction.
PERIPHERAL HEMATOMA AFTER NERVE BLOCKS
Hematoma in the epidural space is certainly the most devastating hemorrhagic sequelae of neuraxial or peripheral regional anesthesia, but hematoma may also occur in the periphery after single-shot or continuous nerve blocks. The most common risk factors appear to be procedural difficulty and concomitant anticoagulation or antiplatelet therapy. This complication is exceedingly rare, with fewer than 30 cases of hematoma after peripheral nerve block (PNB) reported in the literature to date, and the consequences are also less dire as compared to SEH given that hemorrhage occurs in a compressible peripheral space.
In contrast to SEH, the presenting symptom of hematoma after PNB is rarely neurologic dysfunction but more typically visible bruising (Figure 3), local tenderness, decrease in hemoglobin/ hematocrit, or relative hypotension due to blood loss. This is not to say that that the compliant nature of the peripheral space precludes significant morbidity and mortality, as there is one reported case of a patient fatality secondary to retroperitoneal hemorrhage after lumbar sympathetic block in the setting of antiplatelet therapy. The patient was found on autopsy to have lost 3 L of blood in his retroperitoneal space, which speaks to the occult threat presented by the compliant periphery. Indeed, several other cases have been reported where patients suffered significant morbidity due to PNB hematoma, including lengthened hospital stay, requirement for transfusion, or acute renal failure.
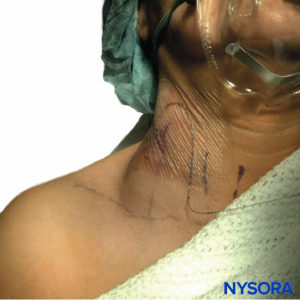
FIGURE 3. Neck hematoma in a patient in whom the external jugular vein was punctured with an 18-gauge Tuohy-style needle during insertion of a catheter in the interscalene groove. The hematoma shown was self-contained and was treated conservatively with local compression.
Given that in every case reported in the literature, the neurologic deficit, if present, had resolved by 1 year, it appears that the more concerning source of morbidity is blood loss into the hematoma. However, due to the rare nature of this complication, it is difficult for experts to make recommendations regarding this procedure in the setting of anticoagulation. This difficulty is exacerbated by the existence of case reports wherein a hematoma occurred despite the practitioners following American Society of Regional Anesthesia and Pain Medicine (ASRA) guidelines. In addition, there are reported cases of spontaneous hematomas that have occurred in patients on enoxaparin or thrice-daily heparin. Taken together, these facts have led to somewhat-contrasting guidelines in different countries. For example, the German Society of Anaesthesiology and Intensive Care has issued recommendations regarding cessation of anticoagulation prior to PNB, but the Austrian Society states that distal PNBs, such as sciatic or axillary blocks, may be performed in an anticoagulated patient.
The ASRA guidelines state that the recommendations regarding neuraxial techniques should be applied to deep plexus blocks and PNBs. Therefore, patients receiving anticoagulation are not candidates for these anesthetic techniques, but some researchers suggested that with the advent of ultrasound guidance, these anticoagulated patients could safely undergo block of peripheral nerves. In addition, studies showed not only a reduced incidence of vascular puncture with the use of ultrasound but also a decreased rate of local anesthetic toxicity. This option has the potential to improve patient safety, given that patients on anticoagulation often have risk factors for general anesthesia and would benefit from avoiding the resultant fluctuations in hemodynamics and fluid status.
A CT scan is currently the most common imaging technique for diagnosis of blood in peripheral tissues, especially the retroperitoneal space. However, ultrasound has been used to demonstrate the presence of renal subcapsular hematoma93 and could potentially be a more easily accessible diagnostic technique than CT in regions of the body amenable to this method of visualization. The increased use of ultrasound in initial placement of PNBs could facilitate diagnosis of suspected cases of post-block hematoma as the ultrasound equipment will be readily available.
Although timely diagnosis is ideal, subsequent treatment of hematoma after PNBs is typically expectant management. A surgical team is ordinarily consulted, blood transfusions are ordered as necessary, and surgical drainage is considered only in critical or rapidly deteriorating patients. Certain case reports of psoas hematoma have resolved without surgical evacuation of the hematoma, and the patients regained their sensory and motor status a few days to 4 months after the diagnosis. As for hematoma concomitant with peripheral nerve catheter, these also are often self-limiting, but there are reports where surgical drainage was performed.
Given the dearth of data available regarding hemorrhage after PNB or continuous nerve block, it may be difficult to accurately determine superiority of a certain anesthetic technique for a specific patient. Anesthesiologists should individualize their decision based on the suitability of PNBs in patients on anticoagulants and, as always, discuss in detail the risks and benefits of the block with the patient and the surgeon. If a block is performed, the patient should be observed closely in the perioperative period for signs and symptoms of peripheral hematoma.
Clinical updates
Schieber et al. (Regional Anesthesia & Pain Medicine, 2025) analyzed 345 reported cases of spinal epidural hematoma (SEH) after neuraxial anesthesia and found that antithrombotic therapy was not associated with larger hematoma size, even after propensity matching. However, antithrombotic use doubled the odds of persistent motor deficit, and non-adherence to guideline timing increased the risk more than threefold, supporting stricter neurologic surveillance and adherence to catheter insertion/removal timing recommendations.
- Read more about the study HERE.
Harris et al. (Canadian Journal of Anesthesia, 2024) report a case of anterior spinal cord syndrome (ASCS) after thoracic epidural catheter removal in a 74-year-old man with a normal coagulation profile following open abdominal aortic aneurysm repair. CT angiography was initially negative; delayed MRI revealed a multilevel (T3–T11) thoracic SEH with cord infarction, and decompression occurred ~7 hours after motor loss, resulting in poor neurologic recovery. This case highlights that SEH can present atypically as isolated motor loss (ASCS pattern), that MRI is the diagnostic modality of choice, and that neurologic checks after catheter removal—especially in patients undergoing vascular surgery—are critical.
- Read more about the study HERE.
Benzon et al. (Regional Anesthesia & Pain Medicine, 2024) reviewed 940 SEH case reports across pediatric, obstetric, and adult populations and found that most hematomas were spontaneous, including in patients on anticoagulants, and that SEH occurred even when ASRA guidelines were followed—particularly in patients on multiple agents. Neurologic recovery correlated more strongly with preoperative ASIA impairment grade than with time-to-surgery; patients with less severe deficits had significantly higher odds of full recovery, and some severe cases improved with conservative management, suggesting careful neurologic monitoring and individualized surgical timing rather than automatic emergent decompression in all cases.
- Read more about the study HERE