Complex regional pain syndrome (CRPS) is a challenging chronic pain condition that typically affects one limb after injury or surgery. Its hallmarks, severe pain, swelling, temperature changes, and motor dysfunction, are often disproportionate to any initial trauma. Despite decades of research, the underlying pathophysiology of CRPS remains largely unclear, leaving patients with limited treatment options.
A new international study led by Dr. Emmanuel Gonzalez and Dr. Tali Sahar explores how the gut microbiome – specifically, the bacteria and metabolites in our intestines – differs in people with CRPS. This prospective cohort study suggests that gut microbiome composition and function could be intimately linked to CRPS symptoms and hold diagnostic value.
Why gut microbes matter in chronic pain
The gut microbiome plays a crucial role in regulating inflammation, immune function, and pain processing. Research has identified altered gut microbial profiles in several chronic pain conditions, like fibromyalgia and neuropathic pain. CRPS, classified as a nociplastic pain condition, shares several pathophysiologic features with these disorders, making it a prime candidate for microbiome research.
Understanding SCFAs and bacterial balance
Short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate are important microbial metabolites that maintain intestinal health, regulate immune responses, and modulate pain pathways. They are produced by bacterial fermentation of dietary fiber.
Reduced levels of SCFA-producing bacteria may impair these protective mechanisms, potentially contributing to pain persistence and immune dysregulation seen in CRPS.
Study design and objectives
Patient cohorts
- Population: 53 patients diagnosed with CRPS and 52 matched controls (age, sex, ethnicity)
- Sites: Rambam Health Campus (Haifa, Israel) and McGill University Health Centre (Montreal, Canada)
- Design: Multicenter observational cohort study using 16S rRNA gene sequencing and targeted metabolomics
Sample collection and analysis
- Stool and plasma samples were collected and frozen for microbiome and metabolite analysis.
- 16S rRNA sequencing was used to identify bacterial exact sequence variants (ESVs).
- Metabolomics: Targeted analysis of SCFAs in stool and plasma using mass spectrometry.
- Machine learning: Models were trained to classify CRPS based on microbiome data.
Key findings
- Unique gut microbial signature in CRPS
- CRPS patients exhibited reduced microbial diversity (lower Shannon index).
- 87 ESVs differed significantly between patients and controls.
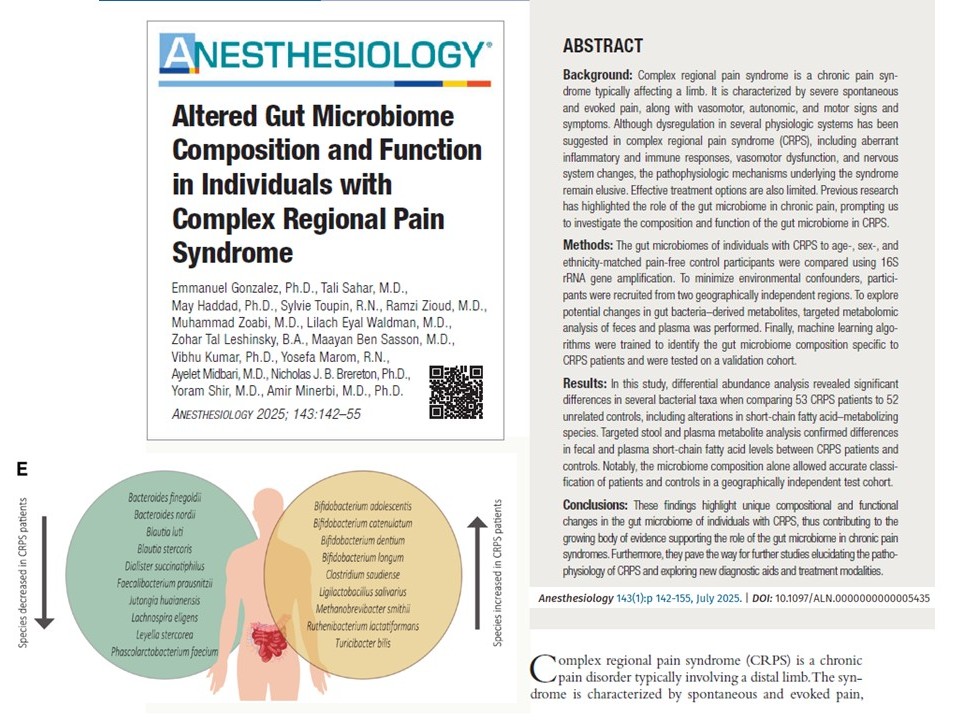
- Bacteria that metabolize SCFAs, especially propionate, were depleted in CRPS.
- Increased levels of Bifidobacterium spp. and Turicibacter bilis were seen in CRPS, suggesting altered metabolic pathways.
- Decreased SCFA concentrations
- CRPS patients had lower fecal and plasma SCFA levels, particularly butyrate, acetate, and propionate.
- These changes correlated with the loss of SCFA-producing bacteria.
- Machine learning enables accurate diagnosis
- A microbiome-based model classified CRPS with up to 90.5% accuracy, 90.0% sensitivity, and 88.9% specificity.
- This was validated in a geographically distinct cohort in Canada.
- Half of the patients with pain but not meeting CRPS criteria were classified as CRPS-positive, hinting at potential subclinical gut patterns.
- CRPS microbiome signature persists after recovery
- Three patients underwent limb amputation and achieved clinical remission.
- Despite symptom resolution, their gut microbiome remained unchanged, suggesting a stable systemic signature.
Step-by-step implications for clinicians and researchers
- Screen for gut microbial dysbiosis in CRPS patients to explore novel diagnostic pathways.
- Investigate SCFA supplementation or microbial therapy (e.g., probiotics, FMT) as adjuncts to conventional CRPS treatment.
- For long-term disease surveillance, consider microbiome profiles in recurrent CRPS, even post-amputation.
- Collaborate in multicenter studies to validate microbiome biomarkers across diverse populations.
- Integrate microbiome-based diagnostics into pain clinics as tools become more accessible.
Limitations and strengths of the study
Limitations
- Observational design limits conclusions on causality.
- Microbiome data reflects associations, not mechanisms.
- Small cohort for post-amputation follow-up.
- Potential confounders like medication use may influence gut microbiota.
Strengths
- Bi-national, multi-cohort design enhances generalizability.
- The machine learning model was validated across sites.
- In-depth analysis of both bacterial taxa and microbial metabolites.
- Integration of clinical, dietary, and psychological variables in statistical modeling.
Future directions
- Interventional studies using microbiome modulation (e.g., diet, prebiotics) to reduce CRPS symptoms.
- Longitudinal microbiome tracking before and after pain onset to explore causality.
- Expansion to other nociplastic conditions like migraine and fibromyalgia for shared microbial patterns.
- Exploration of gut-brain axis mechanisms, particularly in neuroimmune signaling and SCFA-driven epigenetic regulation.
Conclusion
This study provides compelling evidence that CRPS is associated with a stable, measurable alteration in gut microbiome composition and function. It links gut dysbiosis to immune and neurologic pathways implicated in chronic pain and proposes microbial markers as potential diagnostic tools.
The research challenges the idea that CRPS is purely a localized disorder and opens new avenues for systemic, microbiome-based diagnostics and therapies. With additional validation, these findings could transform how we understand, diagnose, and manage CRPS.
For more information, refer to the full article in Anesthesiology.
Gonzalez E, Sahar T, Haddad M, et al. Altered Gut Microbiome Composition and Function in Individuals with Complex Regional Pain Syndrome. Anesthesiology. 2025;143(1):142–155.
Read more about the latest impactful research in pain medicine in Pain Medicine Updates 2025!