INTRODUCTION
Regional anesthesia is an essential part of modern pediatric anesthetic practice, conveying many significant advantages to the patient and to the hospital (Table 1). However, despite a strong body of evidence highlighting the advantages of regional anesthesia, it has been only relatively recently that regional anesthesia has begun to become more common place in anesthetic practice. Large prospective studies by the French-Language Society of Pediatric Anesthesiologists (ADARPEF) have demonstrated no increased risk to children having blocks performed under general anesthesia. However, complications were four times greater in children aged less than 6 months compared to those older than 6 months.
Historically, it was thought that neonates required little or no analgesia. However, inadequate analgesia in the neonate can cause biobehavioral changes that may modulate future responses to pain in childhood. As a consequence, advanced regional anesthesia techniques (eg, epidural analgesia) have become increasingly utilized in children of all ages. Interestingly, the ADARPEF studies identified that there is a now a trend away from the central neuraxial blocks toward peripheral nerve catheter techniques. This change may have been influenced by advances in minimally invasive surgery and the more predictable administration of peripheral catheter techniques in modern regional anesthesia practice.
All regional anesthetic techniques can be safely performed in the pediatric population with the adequate training and mod-ern equipment.
TABLE 1. Advantages of regional anesthesia in children.
| Patient benefits | Superior analgesia: Results in calmer patient and parents/caregivers. |
| Reduced MAC: Reduced risk of deeper GA, smoother emergence, earlier return of appetite. | |
| Neurotoxicity: This potential problem is GA dose–dependent; therefore, a reduced MAC exposure may be beneficial. | |
| Hemodynamic stability: Up to 8 years of age, CNBs rarely cause significant hypotension. | |
| Reduced requirement for postoperative ventilator support: Particularly in neonates and infants undergoing upper abdominal and thoracic surgery. | |
| Obtunds the hormonal stress response. | |
| Reduced intraoperative blood loss: Demonstrated during hypospadias repair, cleft repair, and tonsillectomy. | |
| Improved GI function: Peristalsis better maintained; improved splanchnic perfusion in cases of NEC and gastroschisis | |
| Avoids the need for GA: Premature infants who undergo GA are at risk of postoperative apnea. | |
| Hospital benefits | Easier to nurse: Pain-free children are less labor intensive to care for. |
| Reduced MAC: Rapid discharge from first-stage recovery. | |
| Reduced requirement for postoperative ventilatory support: This is of particular benefit when there is limited PICU support. | |
| Reduced length of stay. |
ANATOMICAL DIFFERENCES BETWEEN CHILDREN & ADULTS
With respect to anatomy, physiology, and pharmacology, adolescents may be considered “little adults”; however, neonates and infants need special consideration. Anatomically, the major difference lies in the spine and its contents; this topic is described in greater detail in Pediatric Epidural en Spinal Anesthesia & Analgesia. Physiologically, there are a number of differences found in the developing paediatric nervous system when compared to adults. Myelination is incomplete at birth, and the process can take 12 years to complete; consequently, lower concentrations of local anesthetic may be effectively utilized in the pediatric population, thereby also reducing the risk of toxicity. Although the nociceptive pathways are fundamentally the same in children as in adults, there are differences that may result in children experiencing greater pain than adults. In children, the receptive field of a neuron may be greater, leading to poor pain localization. The descending inhibitory pathways are immature, and this may allow unmodulated nociceptive inputs to the ascending spinal pain pathways. The physiological immaturities of the neonatal liver in association with a relatively high cardiac output produce pharmacological differences that combine to increase the risk of local anesthetic toxicity in neonates.
PHARMACOLOGY OF LOCAL ANESTHETICS IN PEDIATRIC PATIENTS
There are two main groups of local anesthetic drugs used in pediatric regional anesthesia: the amino esters and the amino amides. (A detailed discussion of these drugs can be found in Clinical Pharmacology of Local Anesthetics.) There are relatively few local anesthetic pharmaco-kinetics in children, and especially in neonates, is limited; unfortunately, it is this age group that is at the greatest risk of local anesthetic drug toxicity.
Amino Amide–type Local Anesthetics
The most commonly used group of local anesthetics in pediat-ric practice are the amino amides: lidocaine, bupivacaine, ropivacaine, and levobupivacaine. The amino amide local anesthetics undergo hepatic metabolism. However, the neonatal liver is immature, with the cytochrome systems maturing at varying rates: the CYP3A4 within the first 9 months of life compared to the CYP1A2, which can take until 8 years of age to mature. The volume of distribution at steady state in infants is greater than in adults. Fluid compartments change drastically with age, 80% of body weight consisting of water in a premature neonate, 75% in a term neonate, 65% in an infant, and 60% in older children. As age increases, the intracellular fluid increases from 20% of body weight in premature neonates to the 30% seen in adults; within this time frame, extracellular fluids are halved. Local anesthetics are water soluble; therefore, the age-related changes in fluid compartment composition are significant. Infants have lower levels of local anesthetic–binding proteins (eg, alpha-1-acid glycoprotein and albumin), which leads to an increased fraction of unbound local anesthetic and therefore to a greater risk of toxicity. However, in the first 48 hours postoperatively, there is an increase in alpha-1-acid glycoprotein that may act to protect the neonate. The clearance of these drugs is decreased in those less than 3 months of age, gradually reaching adult levels by 8 months of age. Consequently, elimination half-lives of local anesthetics are longer in neonates and infants compared to adults.
Bupivacaine
Bupivacaine is an isomer with both l- and d-enantiomer, the d-enantiomer causing most of the adverse effects that are seen in humans. Given that bupivacaine is the most toxic of the amino amide local anesthetics, consideration should be given to using a safer alternative, particularly for neonates and when continuous infusion techniques through indwelling catheters are administered. The pharmacokinetics and pharmacodynamics of bupivacaine have been well documented in the literature. The preferred concentration for children is 0.25% for peripheral nerve blocks and 0.1% for continuous infusions. Older children can tolerate a higher dose of local anesthetic solution (0.4 mg/kg/h) compared with neonates and infants (0.2 mg/kg/h). The dosage of bupivacaine is limited to 2–4 mg/kg for a single-dose injection and 0.2–0.4 mg/kg for a continuous infusion.
Ropivacaine
Ropivacaine is a newer amide local anesthetic that is being used more frequently in pediatric surgery. It is an l-enantiomer with less cardiovascular and central nervous system side effects compared with bupivacaine. Ropivacaine has slight vasoconstrictive properties that may explain the longer Tmax when administered caudally compared to bupivacaine. Pharmacokinetic data are available in children on the use of ropivacaine in continuous infusions as well as for single-shot injections. Pediatric trials have demonstrated a longer duration of action with ropivacaine than with mepivacaine when used for peripheral nerve block. Caution should be exercised while using ropivacaine in children as well, as cases of cardiovascular toxicity has been reported.
Levobupivacaine
Levobupivacaine is a newer l-enantiomer with potentially less risk of severe cardiovascular toxicity. Pharmacokinetic data are available in children, and the dosage interval is similar as that of bupivacaine. Animal experiments have shown that levobupivacaine causes less myocardial depression and a decreased incidence of inducing fatal dysrhythmias compared with bupivacaine. Although this drug provides the practitioner with the option of a drug that is less cardiotoxic, caution should still be exercised.
Ester-type Local Anesthetics
Due to their short duration of action and propensity to cause allergic reactions, the amino esters (eg, procaine, 2-chloroprocaine, tetracaine) are the least commonly used group of local anesthetics. Unlike amino amide local anesthetics, the amino esters are metabolized by plasma cholinesterases. As a result, the metabolism of ester local anesthetics depends on plasma cholinesterase levels. Hence, in populations with decreased plasma cholinesterase levels, such as neonates and infants, the plasma level of these drugs may be increased, potentially leading to toxic drug levels. The presence of plasma cholinesterase also limits the duration of action of these drugs, leading to a shortened activity. The most common ester local anesthetics used in infants and children are chloroprocaine and tetracaine. These drugs are occasionally used in children as an adjuvant to spinal anesthesia in formerly premature infants undergoing spinal anesthesia or as the sole anesthetic solution for caudal analgesia. Tetracaine has been reported in spinal anesthesia, especially in premature infants, as the sole anesthetic for inguinal hernia repair. 2-chloroprocaine has been used extensively in children for analgesia in the central neuraxial space.
DOSING OF LOCAL ANESTHETICS IN PEDIATRIC PATIENTS
Most drug doses in pediatric patients are based on the weight of the patient (Table 2), though it is often debated whether total body weight or lean body mass is more appropriate for drug calculations. However, this may not be applicable to local anesthetic considerations; studies done on infants under-going spinal anesthesia found a larger requirement of local anesthetic solution (weight-scaled) compared with their adult counterparts using bupivacaine or tetracaine.
TABLE 2. Maximum recommended doses and approximate duration of action of commonly used local anesthetic agents.
| Local Anesthetic | Class | Maximum Dose (mg/kg)a | Duration of Action (min) | Infusion (mg/kg/h) |
|---|---|---|---|---|
| Procaine | Ester | 10 | 60–90 | - |
| 2-chloroprocaine | Ester | 20 | 30–60 | - |
| Tetracaine | Ester | 1.5 | 180–600 | - |
| Lidocaine | Amide | 5 | 90–200 | - |
| Bupivacaine | Amide | 2.5 | 180–600 | 0.2–0.4 |
| Ropivacaine | Amide | 2.5 | 180–600 | 0.2–0.5 |
| Levobupivacaine | Amide | 2.5 | 180–600 | 0.2–0.5 |
The concentration of local anesthetic administered should also be carefully considered. A lower concentration of long-acting local anesthetic (eg, 0.25% levobupivacaine) is often used because the child is also receiving a general anesthetic; therefore, the block is used for analgesia only. However, in certain scenarios, lower or higher concentrations of local anesthetic should be administered. Lower concentrations (eg, 0.125% levobupivacaine) are useful in decreasing the risk of toxicity in neonates and are less likely to mask compartment syndrome or delay ambulation. Higher concentrations of local anesthetic (eg, 0.5% levobupivacaine) should be considered where a profound motor block is desirable (eg, lower limb tendon transfer surgery in children with cerebral palsy.
TOXICITY OF LOCAL ANESTHETIC DRUGS
The toxicity of local anesthetics in children include cardiovascular and central nervous system toxicity (Table 3) and allergic reactions to ester local anesthetic solutions. The already discussed pharmacokinetic differences, together with the immaturity of the blood–brain barrier, may make central nervous system toxicity more likely in neonates. However, the co-administration of general anesthesia may mask early signs and symptoms of systemic toxicity. The local anesthetic dose for children is always calculated on a milligram-per-kilogram basis, rather than predicted volumes as in adult regional anesthesia. While it is recognized that, of pediatric patients, infants are at a higher risk of systemic toxicity, caution should be the norm for children of all ages, as higher concentrations of some local anesthetics have been recorded in adolescents compared to adults. Toxic plasma levels have also been reported following safe doses of local anesthetic used for caudal and ilioinguinal blocks, so the minimum effective dose is recommended.
TABLE 3. Systemic toxicity of local anesthetic solution.
| Central nervous systema |
| Dizziness and lightheadedness |
| Visual and auditory disturbances |
| Muscle twitching and tremors |
| Generalized convulsions |
| Cardiovascular |
| Direct cardiac effects |
| Depressed rapid phase of repolarization of Purkinje fibers |
| Depressed spontaneous firing of the sinoatrial node |
| Negative inotropic effect on cardiac muscle |
| Calcium influx altered, leading to decreased myocardial contractility |
| Effects on vascular tone |
| Low concentrations: vasoconstriction |
| High concentrations: vasodilatation |
| Increased pulmonary vascular resistance |
Interestingly, Weintraud et al. found that US-guided ilioin-guinal blocks resulted in higher plasma concentrations when compared to landmark-based techniques. This may be due to a greater surface area of absorption created by placing the local anesthetic precisely in a fascial plane compared to the multiple deposits within muscle that often occur with a landmark-based method.
NYSORA Tips
Preventing Systemic Toxicity in the Pediatric Population
- Choose a less toxic drug (eg, levobupivacaine or ropivacaine).
- Do not exceed the maximum dose (in neonates, it may be prudent to halve the maximum dose).
- Inject small aliquots and aspirate repeatedly.
- US allows for lower doses of drugs to be used and for injection visualization.
- Beware of repeated doses, and limit infusions in neonates to 48 hours.
- An epinephrine-containing test dose may be used to identify intravascular injection but is limited by which general anesthetic is administered.
- No matter which technique is used, all children, given any local anesthetic solution, particularly when given as continuous infusions, should be monitored continuously for adverse effects.
NYSORA Tips
Managing Local Anesthetic Toxicity
In pediatric practice, early warning signs and symptoms of toxicity may be masked by the concurrent administration of general anesthesia. This means the first sign may be arrhythmia or cardiovascular collapse. Consider the following when managing local anesthetic toxicity:
- Stop the local anesthetic injection.
- Institute basic life support, and call for assistance.
- Secure the airway, ventilate with 100% oxygen, and gain intravenous access.
- Seizures can be managed with a benzodiazepine or anesthetic induction agent.
- If cardiac arrest has occurred, commence advanced life support.
- Note that arrhythmias are often refractory, and resuscitation should therefore be prolonged.
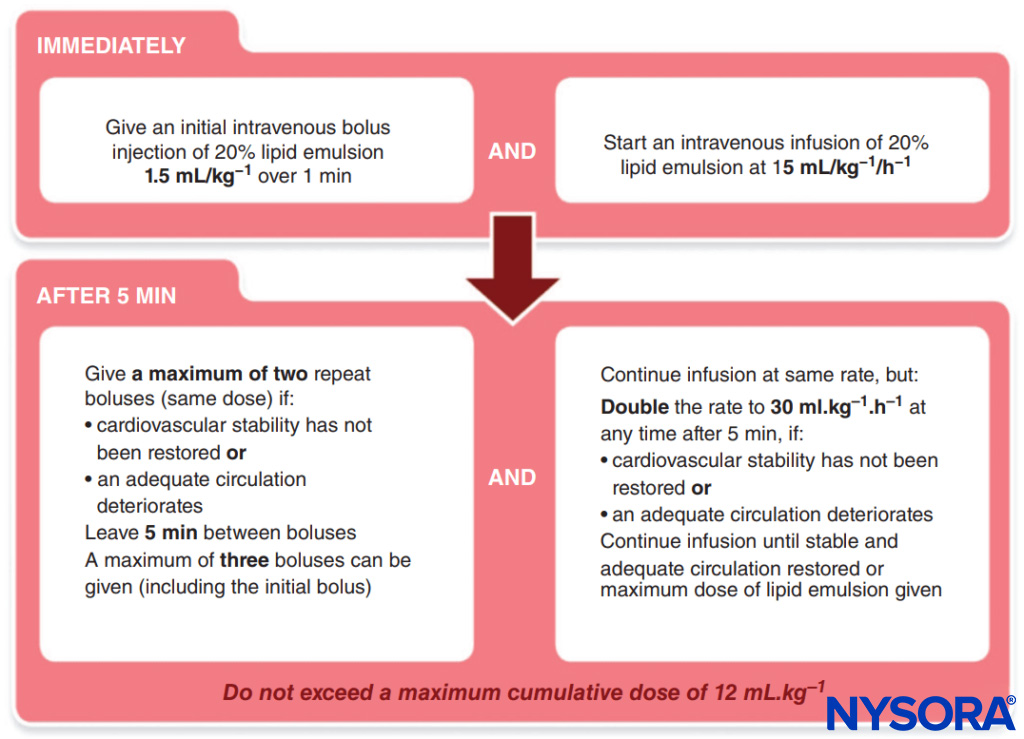
- Lipid administration. The Association of Anaesthetists of Great Britain and Ireland has published a simple protocol to follow (Figure 1).

FIGURE 1. Association of Anaesthetists of Great Britain and Ireland (AAGBI) Protocol for Local Anesthetic Toxicity.
TOPICAL ANESTHESIA
It is important to discuss the use of topical anesthesia in children because it is commonly used in clinical practice to provide analgesia for intravenous catheter placement, lumbar puncture, and other invasive procedures (eg, circumcision in neonates). The most common preparations include lidocaine, tetracaine, benzocaine, and prilocaine. The topical anesthetic solution permeates through the skin to provide analgesia. The three most common preparations available include eutectic mixture of local and anesthetics (EMLA), LMX-4 (4% liposomal lidocaine solution), and Ametop (4% amethocaine gel). EMLA contains lidocaine and prilocaine and must be applied at least one hour before cannulation. Its duration of action is only 30–60 minutes, though it can be left on for 4–5 hours. LMX-4 requires only 30 minutes to take effect and can also be left on for 4–5 hours. Ametop requires 45 minutes to take effect and must be removed within an hour of application; its duration of effect is up to 3 hours, as it binds to proteins in the stratum corneum. Ametop is vasodilatory, which may aid cannulation. However, Ametop can cause erythema and edema, which may obscure veins.
NYSORA Tips
The following are considerations for children undergoing regional anesthesia:
- Regional anesthesia is mostly commonly performed with the patient under general anesthesia.
- The dose of local anesthetic used is much lower than for adults and is calculated in milligrams per kilogram).
- Use a local anesthetic at the lowest effective concentration.
- If moderate or severe pain is expected, intense physiotherapy required post operatively, or there is a history of chronic pain, consider using a catheter technique.
- Far fewer regional anesthesia complications are reported in children than in adults.
- Always obtain patient assent or consent if the child is older.
- Always explain postoperative paresthesia to patients.
PREOPERATIVE ASSESSMENT
The preoperative visit is an opportunity to assess and prepare the patient and family for the proposed surgery and anesthetic technique. It is important that the anesthesiologist understand the intended surgery so that a decision can be made to perform either a single-injection or continuous regional anesthetic technique. Generally, the more peripheral block should be chosen, as this typically has the lowest risk and fewest side effects and will therefore have greater acceptability to the patient and parents or caregivers alike. A catheter technique is recommended for moderate to major surgery, procedures that will require prolonged postoperative physiotherapy, and for children with chronic pain. It is also important to ensure there will be nursing staff trained to manage continuous regional anesthetic techniques.
The anesthesiologist should seek potential contraindications (eg, comorbidities) to a given regional anesthetic technique. A thorough pain history should be taken, looking specifically for a history of chronic pain or muscle spasticity. Children with special needs can be particularly challenging, and the anesthesiologist needs to know how the child copes with and expresses pain. The standard preoperative anesthetic examination should include an examination of the proposed block insertion site to identify things such as anatomical difficulties (eg, scoliosis) and local infection. In children with neurological disease, their pre-operative neurological deficit should be documented.The anesthesiologist should explain the advantages, side effects, and potential complications of the proposed anesthetic plan to the child and the parents/caregivers; it is prudent also to discuss a “plan B” should the proposed technique fail. Alternative analgesic strategies should be discussed so that informed consent can be given. In particular, the postoperative paresthesiae of the regional technique should be explained to children in terms they can understand, as this is often an experience they find difficult to cope with. If a continuous regional anesthetic technique will be used, reassure the child that removal of the catheter will not be painful. Parents or caregivers typically provide consent for a procedure for their child. However, if the child has the cognitive ability to discern right from wrong, it is suggested that the child’s assent for a regional technique be obtained as well. There is debate as to when or what this age may be. If a child refuses to have a regional procedure despite the parents’ or caregivers’ insistence, it is important for the anesthesiologist to provide an alternative modality of pain relief.
Analgesic premedication with paracetamol (acetaminophen) and a nonsteroidal anti-inflammatory drug (NSAID) should be considered, especially where it is known that the block will not provide total analgesia (eg, a transverse abdominis plane [TAP] block for appendectomy). Postoperative instructions should be given preoperatively and reinforced postoperatively on discharge.
REGIONAL ANESTHESIA: AWAKE OR ASLEEP?
Whether it is best for the patient to be awake or asleep during regional anesthesia has been a controversial issue in adults, and this debate once permeated the realm of pediatric regional anesthesia practice. Placing a regional block in an awake child is difficult due to the inability of the child to cooperate as well as the cognitive inability of the child to relate to symptoms such as paresthesia or pain. Therefore, the child is best provided with a regional technique under deep sedation or after the induction of general anesthesia; this practice has been the consensus of pediatric anesthesiologists in the U.S. as well as abroad for some time.
However, there are two scenarios in which awake regional techniques are used in children. First, it was thought that by avoiding general anesthesia in premature infants undergoing minor surgery, the incidence of postoperative apnea could be reduced. This is probably less of an issue now that premature neonatal lungs are better protected and with the availability of newer inhalational agents that provide a more rapid emergence.
Second, the more mature child may be considered suitable for an awake regional technique when undergoing a minor surgery if they prefer or when a general anesthetic is considered too risky (eg, lymph node biopsy in a teenager with a mediastinal mass). For older patients, it is essential to prepare the child and parents or caregivers for the whole operating room visit, not just the insertion of the block. The block insertion can be made more comfortable by applying a topical anesthetic cream (EMLA has the best penetration) over the proposed injection site. During the operating room visit, the child should be sup-ported and distracted by a nurse or play specialist. The child may also find the use of a DVD or MP3 player a useful distraction. It may be necessary to use nitrous oxide (Entonox) or anxiolytic doses of propofol or remifentanil to ease proceedings. Marhofer et al. have shown that in the pediatric trauma scenario, brachial plexus blocks are more comfortably inserted with US than with nerve stimulation. In pediatric hospitals, the operating room staff and surgeon must be reminded that the patient will be awake, and the drugs and equipment needed to convert to general anesthesia must be ready.
PERIOPERATIVE BLOCK MANAGEMENT
Some simple rules need to be followed when performing any regional anesthetic technique in children. A skilled assistant should be present at all times, and this individual should understand the basic principles of regional anesthesia, in particular the need to aspirate regularly prior to injection, the need to warn the anesthesiologist about injection resistance. Further, the assistant should be able to make basic adjustments to the peripheral nerve stimulator (PNS) and US machines.
The child should have a secure airway, intravenous access, and full monitoring prior to commencing the block. As part of the World Health Organization (WHO) Surgical Safety Checklist, consent and side of surgery are checked, and just before block insertion, the site should again be confirmed with the anesthesiologist’s assistant.
The child, US machine (when used), equipment, and anesthesiologist should be positioned ergonomically.
There is some debate as to the minimal standards for asepsis for regional anesthesia. For single-injection techniques, it is sufficient to scrub one’s hands, wear sterile gloves, apply an alcoholic solution to the patient’s skin, and cover the US probe. For catheter techniques, a more rigorous aseptic technique is advised. When performing any catheter technique, it is useful when draping the child to have a large area of anatomy visible; this allows the anesthesiologist sufficient room to perform a mapping/scout scan and also a better view, and thus appreciation of, the patient’s anatomy (particularly important when placing epidurals in children with scoliosis).It may be beneficial to tunnel the catheter, as this aids fixation, and in some cases may decrease the risk of infection (eg, with a caudal catheter, the catheter can be tunneled away from the diaper area.
There is no ideal test dose; therefore, repeated, frequent aspiration during injection is suggested.When the block is not expected to cover all aspects of surgical pain (eg, a rectus sheath block for pyloromyotomy), consideration should be given to the administration of paracetamol intravenously and/or an NSAID per rectum to aid analgesia. Other adjuvants may prove beneficial (eg, intraoperative magnesium sulphate 50 mg/kg may decrease postoperative muscle spasms in cerebral palsy patients undergoing lower-limb surgery).
It is the author’s opinion that, where possible, the regional anesthetic technique’s efficacy should not be obscured by the administration of opioids or nitrous oxide. It is important in the intraoperative assessment of the block that any changes in heart rate and blood pressure are noted and related to the specific surgical proceedings at the time. This allows for the planning of rescue blocks at the end of the procedure. Even with successful blocks, occasional cardiovascular responses to surgical stimuli may occur, as the blocks are generally performed using a lower concentration of local anesthetic (eg, 0.25% levobupivacaine), and, if a tourniquet is used, a gradual increase in heart rate and blood pressure will occur after the first 30–40 minutes.
The degree of intraoperative cardiovascular stability helps the anesthesiologist decide whether any patient distress during the first stage of recovery is due to pain or another cause (eg, emergence delirium).In first-stage recovery, if there is doubt as to why a child is upset, then it should be assumed that the patient is in pain, and this should swiftly be dealt with by administering a fast-acting opiate (eg, fentanyl).
POSTOPERATIVE CARE
Postoperative advice pertaining to protecting the anesthetized area should be given verbally preoperatively and repeated post-operatively, and written instructions should also be given to the family. The family and child should be warned of muscle weakness and diminished sensation. Slings should be provided to pediatric patients with upper-limb blocks. Ambulatory children with lower-limb blocks should have the means to mobilize and travel home arranged. Every institution must have guidelines for in-patient nursing care and monitoring of regional anesthetic techniques, and the staff must receive regular education on managing regional blocks postoperatively and care of the anesthetized extremity. It is generally more economical and safer to place patients receiving regional anesthesia and analgesia on specific wards. (For more information on this topic, see Acute and Chronic Pain Management in Children.)
TRAINING
The training of anesthesiologists in pediatric regional anesthesia and anatomy is essential for its successful and safe implementation. Where possible, each department should provide a structured approach to teaching the common simple blocks: caudal, penile, femoral, axillary, ilioinguinal, and rectus sheath. Training should include US scanning and needling techniques. Once basic US needling skills have been learned on phantoms and scanning skills developed on adult volunteers, these skills can then be transferred to the clinical setting. Generally, these techniques are easier and more safely performed on older patients first.
NYSORA Tips
Where possible, each department should provide a structured approach to teaching the common blocks: caudal, penile, femoral, axillary, ilioinguinal, and rectus sheath.
SUMMARY
Regional anesthesia improves the postoperative experience of both children and parents or caregivers and facilitates the efficient use of hospital facilities and it is expected that the use of regional anesthesia in children will continue to grow in popularity. Regional anesthetic techniques should provide the correct balance between risks and benefits for the children and surgery of today. US is allowing a greater array of peripheral blocks to be safer and reliably used in children. Technology is important, but is not a replacement for a solid understanding of anatomy and a high standard of general safe practice.
Clinical updates
Carley et al. (Regional Anesthesia & Pain Medicine, 2025) reported a large retrospective review of 3,627 single-shot mepivacaine spinal anesthetics in children aged 5–18 years undergoing orthopedic surgery. Mepivacaine dosing (mg/kg) decreased with increasing age and weight, plateauing around age 15, while absolute doses increased modestly with surgical duration. Bupivacaine predominated in children under 10 years, whereas mepivacaine was more commonly used in older children and adolescents. Conversion to airway placement was rare (≈1.3%), supporting the feasibility of spinal anesthesia with intermediate-acting agents. These data provide the first pediatric dosing reference for spinal mepivacaine and support its use as a shorter-acting alternative to bupivacaine in ambulatory pediatric surgery.
- Read more about the study HERE.
Reysner et al. (2025, Regional Anesthesia & Pain Medicine) reported a randomized, double-blind, placebo-controlled trial evaluating perineural dexamethasone as an adjuvant to ropivacaine popliteal sciatic nerve block in children aged 2–5 years undergoing foot or ankle surgery. Adding dexamethasone 0.1 mg/kg significantly prolonged the time to first rescue opioid analgesia (≈18.4 h) compared with 0.05 mg/kg (≈16.3 h) and ropivacaine alone (≈8.5 h). Motor block duration was similarly prolonged in a dose-dependent manner, and total opioid consumption within 48 hours was reduced versus control. Pain scores were lower during the early postoperative period with dexamethasone. Blood glucose levels and inflammatory stress markers (NLR, PLR) were unchanged across groups. No nerve injuries or clinically relevant adverse effects were observed, supporting perineural dexamethasone as an effective and metabolically safe pediatric block adjuvant.
- Read more about the study HERE.
Barnett et al. (Best Practice & Research Clinical Anaesthesiology, 2024) reviewed the current state of pediatric regional anesthesia and acute pain management, highlighting major shifts toward ultrasound-guided peripheral nerve blocks. Large registry data demonstrate that regional anesthesia performed under general anesthesia in children has a very low incidence of serious neurologic injury and local anesthetic systemic toxicity. Peripheral blocks are increasingly preferred over neuraxial techniques because of lower complication rates and comparable analgesic efficacy. Regional anesthesia consistently reduces opioid consumption, improves postoperative pain scores, and facilitates recovery within ERAS pathways. Ongoing controversies—including the risk of compartment syndrome, epidural test dosing, and caudal blocks for hypospadias repair—are addressed with evidence supporting the cautious yet continued use of regional techniques.
- Read more about the study HERE.
Baskin et al. (Regional Anesthesia & Pain Medicine, 2023) reviewed the current evidence for ultrasound-guided spinal anesthesia in infants. Ultrasound provides excellent visualization of infant spinal anatomy, including dura, subarachnoid space, and conus medullaris, with image quality superior to that in older children and adults. Evidence directly evaluating ultrasound-guided infant spinal anesthesia is limited to small observational studies and case reports, but suggests feasibility and potential safety benefits. Ultrasound is particularly useful for identifying midline anatomy and low-lying conus, though it does not reliably predict depth to the intrathecal space. Continuous real-time ultrasound guidance may improve needle control and safety, but high-quality studies assessing success rates and outcomes remain limited.
- Read more about the study HERE.