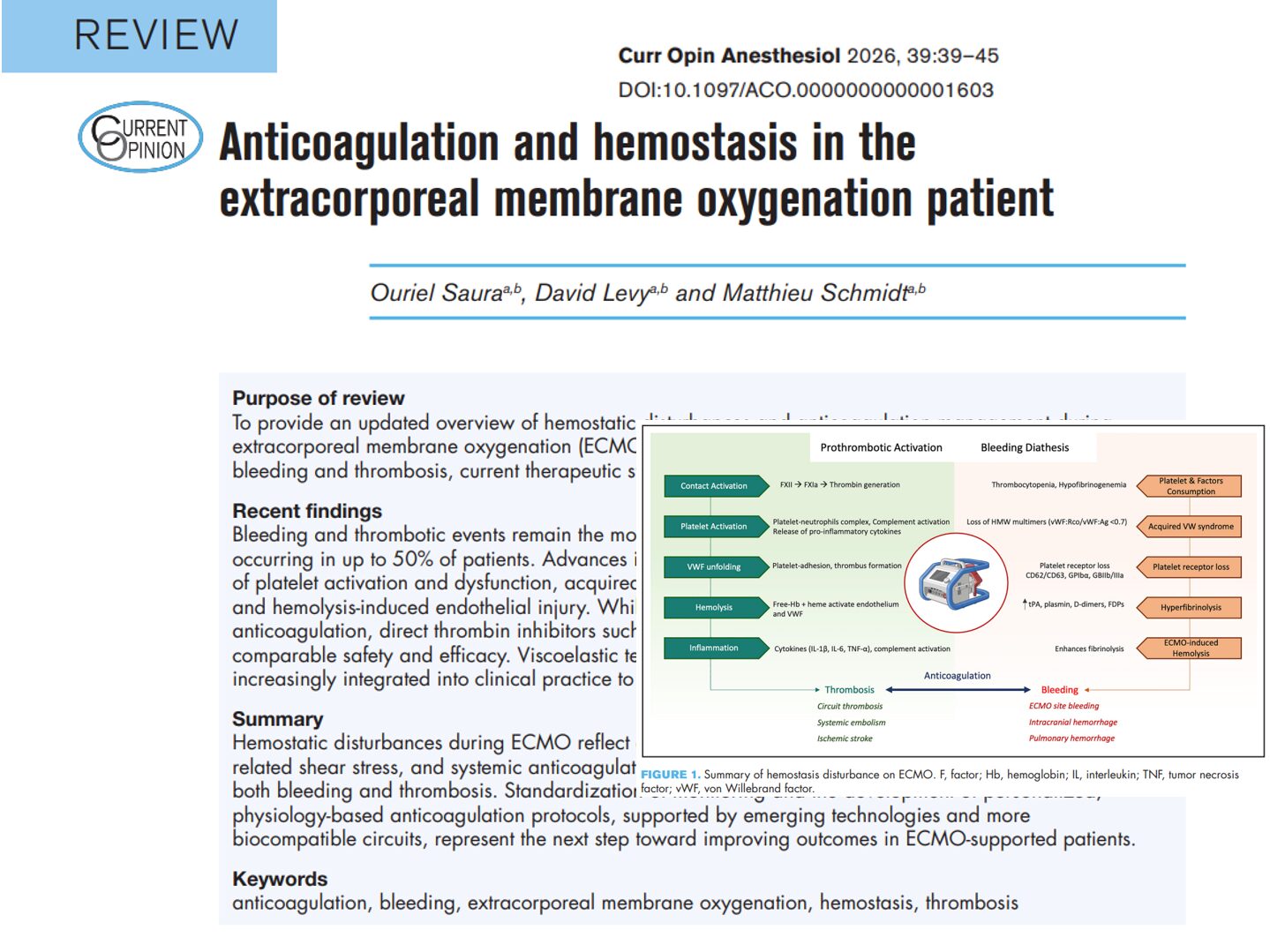
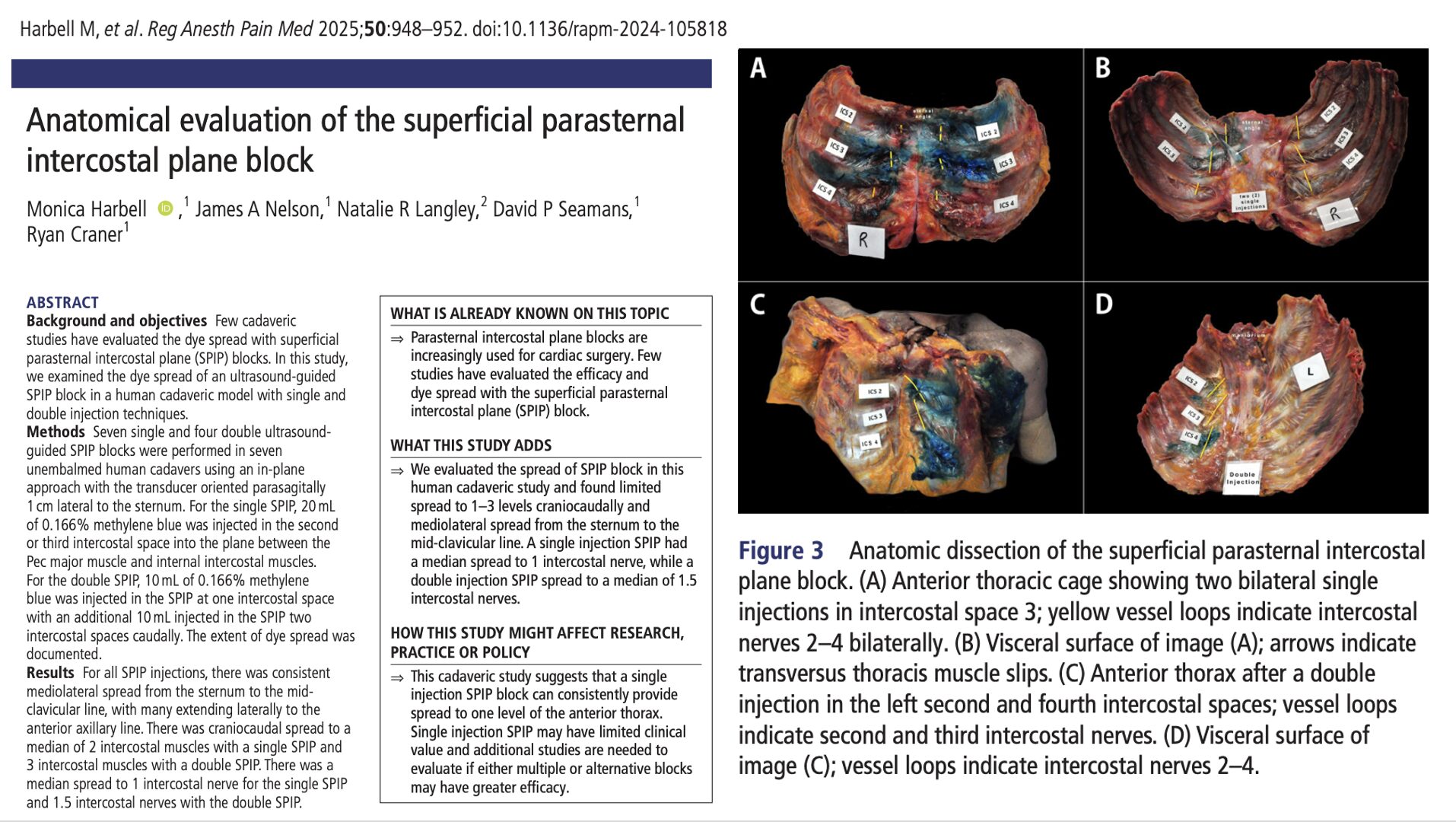
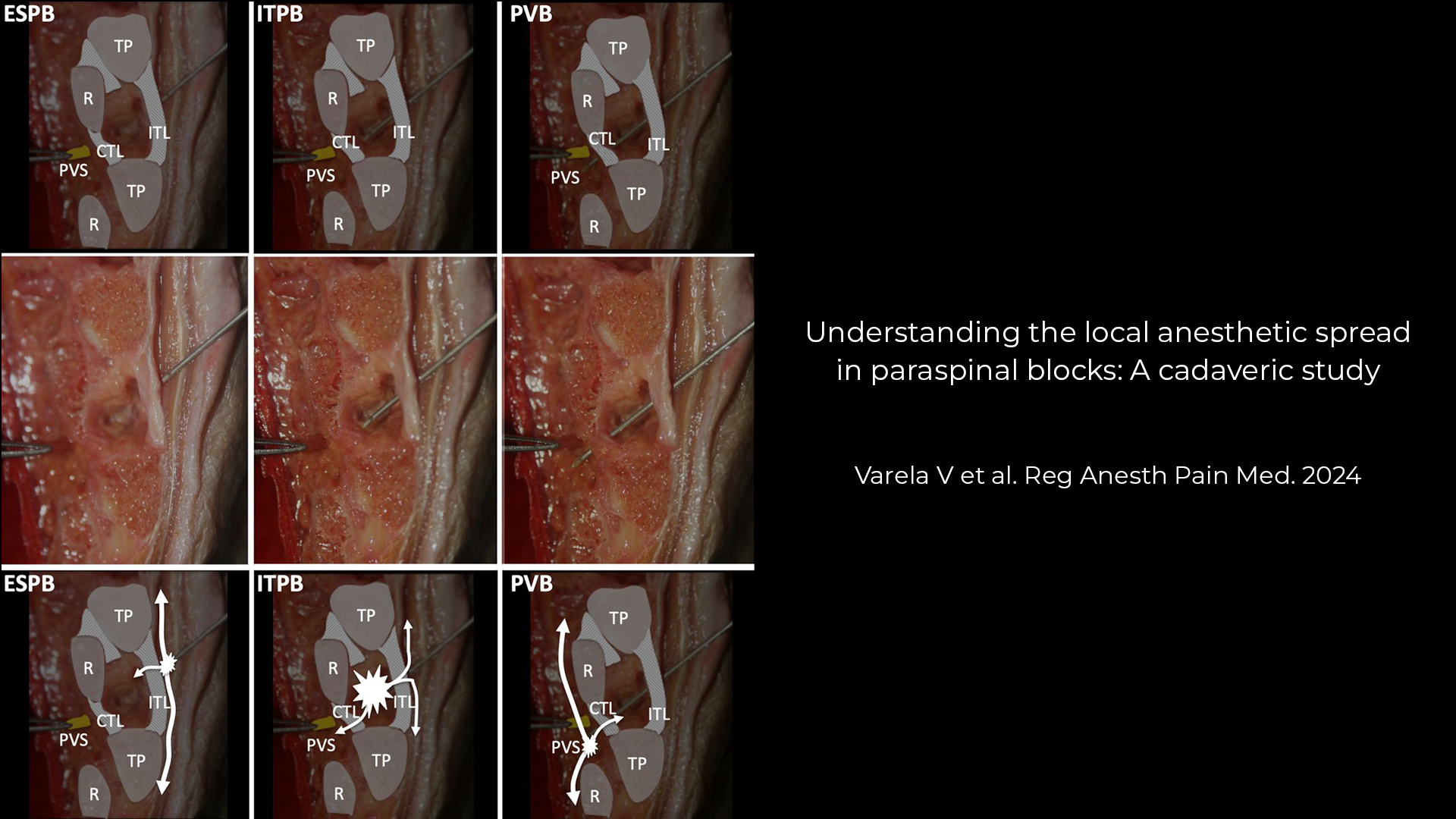
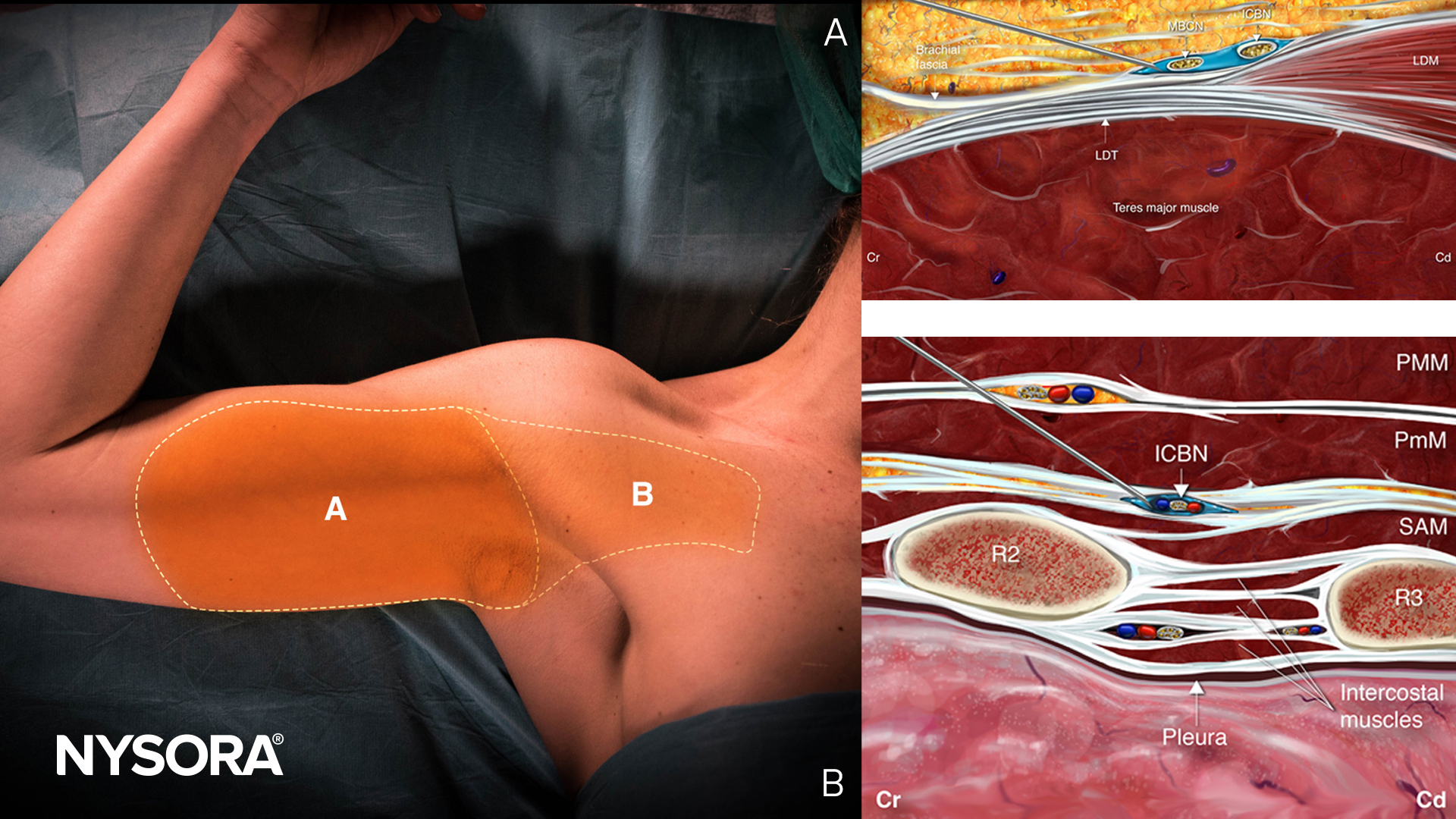
Peripheral nerve blocks (PNBs) are a cornerstone of modern regional anesthesia, offering excellent pain control with reduced opioid exposure. But as patient populations evolve—older, frailer, and more comorbid—this question arises: Are our go-to local anesthetics and vasopressor additives still the best choices?
A recent review by Kendall et al. 2026 in Anesthesiology challenges long-standing conventions in PNB pharmacology, proposing a re-evaluation of the default use of bupivacaine and the routine addition of epinephrine. Backed by a critical review of clinical and translational evidence, this paper opens a timely discussion on how our pharmacologic decisions may inadvertently increase the risk of neurologic and myotoxic complications—especially in vulnerable patients.
Bupivacaine: gold standard or neurotoxic relic?
For decades, bupivacaine has been the mainstay local anesthetic for long-acting peripheral nerve blocks. Its benefits are well known:
- Reliable and prolonged sensory block duration
- Minimal motor block at lower concentrations
- Widely available and familiar to clinicians
However, the review calls attention to the dark side of bupivacaine—its potential for nerve and muscle toxicity, especially when injected intraneurally, used in large doses, or combined with vasoconstrictive additives.
Key concerns:
- Bupivacaine is more neurotoxic than ropivacaine or levobupivacaine in preclinical models.
- Higher incidence of nerve injury in patients receiving bupivacaine for brachial plexus blocks.
- Prolonged exposure, especially in frail patients or those with diabetes, may increase the risk of neurologic sequelae.
Clinical context:
In upper limb blocks like supraclavicular and infraclavicular approaches, where intrafascicular injection risk is already higher, the additive toxicity of bupivacaine becomes especially relevant.
Revisiting vasopressor additives: Is epinephrine doing more harm than good?
Epinephrine has traditionally been added to local anesthetics for two main reasons:
- Prolongation of block duration.
- Vascular marker to detect intravascular injection.
But new data challenge both assumptions:
Questionable benefits:
- In modern ultrasound-guided PNBs, intravascular injection can be avoided by careful visualization and aspiration.
- Blockade prolongation with epinephrine is minimal or absent, particularly with long-acting agents like bupivacaine.
Emerging harms:
- Epinephrine causes vasoconstriction, which may:
- Reduce perineural blood flow.
- Impair nerve perfusion.
- Exacerbate local anesthetic toxicity.
Key findings from the review:
- Animal studies show that epinephrine significantly worsens nerve injury when added to neurotoxic local anesthetics.
- Case reports in humans suggest epinephrine may contribute to nerve ischemia, especially in patients with microvascular disease.
Ropivacaine and levobupivacaine: safer alternatives?
Both ropivacaine and levobupivacaine offer advantages over bupivacaine:
- Lower cardiotoxicity
- Reduced neurotoxicity in animal and cellular models
- Comparable efficacy for sensory blockade in most clinical scenarios
While neither agent is completely benign, the review supports favoring ropivacaine in patients at higher risk of nerve injury, including:
- Elderly patients with frailty or sarcopenia
- Individuals with diabetes or peripheral vascular disease
- Patients undergoing repeated nerve blocks or catheter-based analgesia
What about block duration?
One of the biggest arguments for sticking with bupivacaine is its long duration of action—a crucial benefit in surgeries with significant postoperative pain. But the review challenges this rationale:
- When comparing equivalent doses, ropivacaine provides comparable analgesia with less motor block.
- For inpatient surgeries, duration can be extended with:
- Continuous catheter techniques.
- Perineural adjuncts like dexmedetomidine or clonidine (which have less vasoconstrictive effect than epinephrine).
- In ambulatory surgery, shorter blocks are safer and facilitate earlier discharge.
Rethinking default combinations: tailoring drugs to patients
The review emphasizes that default drug combinations may not suit all patients. A personalized pharmacologic approach is now essential, especially with:
- Perineural nerves already suffer microvascular compromise.
- Increased risk of prolonged nerve block or permanent damage.
- Older or frail patients
- Decreased muscle mass increases local anesthetic concentration per volume.
- Increased susceptibility to myotoxicity and delayed recovery.
- Peripheral vascular disease
- The risk of vasoconstrictor-induced ischemia is amplified.
- Epinephrine may cause harm in patients with limited perfusion reserve.
Myotoxicity: an overlooked complication
While much of the focus has been on nerve injury, the article also highlights myotoxicity as an under-recognized issue:
- All local anesthetics cause some degree of muscle damage, but bupivacaine is the most myotoxic.
- Myotoxic effects are compounded by:
- Higher concentrations
- Prolonged exposure
- Repeat injections or catheter infusions
In frail patients or those with limited regenerative capacity, muscle damage can contribute to functional decline, particularly in the upper limb.
From evidence to practice: new principles for peripheral nerve block pharmacology
Based on the evidence reviewed, the authors propose a paradigm shift in local anesthetic selection for nerve blocks:
Recommendations:
- Avoid the default use of bupivacaine, especially in high-risk patients.
- Minimize or eliminate the use of epinephrine unless specifically indicated.
- Consider ropivacaine for most single-shot PNBs
- Use adjuncts like dexmedetomidine or clonidine for block prolongation instead of epinephrine.
- Adjust concentration and volume based on patient characteristics (age, weight, comorbidities).
- Employ ultrasound guidance to reduce the risk of intraneural injection.
- Be vigilant for delayed neurologic recovery, especially in patients with diabetes and those aged 65 and older.
What does this mean for regional anesthesia providers?
The implications are significant:
- Patient safety: Reducing neurologic and muscular complications in vulnerable patients.
- Precision anesthesia: Tailoring block agents to surgical context and patient risk factors.
- Education and protocols: Updating training and institutional guidelines to reflect evolving evidence.
As the population of patients undergoing regional anesthesia grows older and more complex, clinicians must move beyond tradition and embrace pharmacologic nuance. This means reassessing not only how blocks are performed, but what drugs we use and why.
The road ahead: research and unanswered questions
The review identifies critical areas for future investigation:
- Dose thresholds for myotoxicity and neurotoxicity in clinical practice.
- Comparative studies on nerve injury rates with different anesthetic-vasopressor combinations.
- The role of genetic and metabolic factors in susceptibility to nerve injury.
- Long-term outcomes in patients receiving repeated or prolonged PNBs.
These questions will help define the next generation of safe and effective regional anesthesia.
Conclusion
The review by Kendall et al. 2026 makes a compelling case for rethinking the selection of local anesthetics and vasopressors for peripheral nerve blocks. As ultrasound and precision techniques become standard, our pharmacologic choices must evolve as well. Abandoning default protocols in favor of individualized, evidence-based combinations will reduce complications and improve recovery—especially in patients most at risk of injury. It’s time to ask not just how we perform nerve blocks—but what we’re injecting and whether it’s the best choice for our patients.
For more information, refer to the full article in Anesthesiology.
Gasteiger L, Lirk P, Marhofer P, Gasteiger E, Hollmann MW, Stundner O. Is It Time to Reassess Local Anesthetic and Adjuvant Mixtures? A Narrative Review of Practice, Evidence, and Risks. Anesthesiology. 2026 Jan 1;144(1):177-190.
Learn more about local anesthetics in our Regional Anesthesiology Module on NYSORA 360 on NYSORA360 – an essential learning resource for residents with practical, up-to-date guidance.