Miguel A. Reina, Xavier Sala-Blanch, Fabiola Machés, Riánsares Arriazu, and Alberto Prats-Galino
INTRODUCTION
A better understanding of some features of the fine structure of peripheral nerves may provide us with essential information that can be helpful in anesthetic clinical practice. This chapter reviews the ultrastructure of connective tissues of peripheral nerves to facilitate understanding of its role as the perineurial diffusion barrier and implication in regional anesthesia.
FASCICLES
Nerves and their principal branches (Figures 1 to 3) consist of parallel bundles of nerve fibers (nerve fascicles, fasciculi). The size, number, and pattern of fasciculi vary among nerves and at different distances from their origin. When the connective tissue of the peripheral nerve is removed, 20 or more tubular structures or fascicles are typically seen.
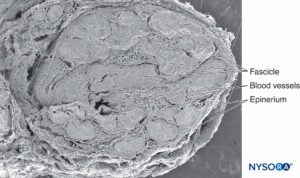
FIGURE 1. Sciatic nerve at the level of popliteal fossa. Scanning electron microscopy. Magnification ×25. (Reproduced with permission from Reina MA, Arriazu R, Collier CB, et al: Electron microscopy of human peripheral nerves of clinical relevance to the practice of nerve blocks.
A structural and ultrastructural review based on original experimental and laboratory data, Rev Esp Anestesiol Reanim. Dec 2013;60(10):552-562.)
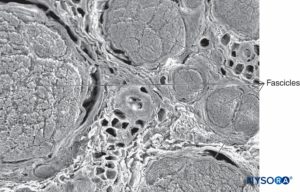
FIGURE 2. Scanning electron microscopy image of the human tibial nerve fascicles and adipose tissue that between fascicles.
Magnification ×75. (Reproduced with permission from Wikinski J, Reina MA, Bollini C, et al: Diagnóstico, prevención y tratamiento de las complicaciones neurológicas asociadas con la anestesia regional periférica y central. Buenos Aires: Panamericana Ed; 2011.)
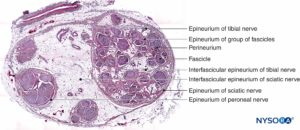
FIGURE 3. Sciatic nerve at the level of popliteal fossa. Hematoxylin-eosin. (Reproduced with permission from Reina MA, De Andres JA, Hernández JM, et al: Successive changes in extraneural structures from the subarachnoid nerve roots to the peripheral nerve, influencing anesthetic block, and treatment of acute postoperative pain. Eur J Pain. Suppl 2011;5(2):377-385.)
Inside each nerve, the axons form an intraneural plexus in such a fashion that one axon can contribute to different fascicles along the nerve length (Figure 4). In other words, an axon can travel from a peripheral position to a more central position as well as swap the fascicles altogether along its descent more peripherally. Indeed, cross-sectional anatomy of nerves at close distance from each other demonstrates that the location and number of fascicles within nerves are highly variable (see Figure 3) with the presence of intraneural plexuses (Figures 5 and 6). The number, size, and location of fascicles in peripheral nerves are also variable even within a single nerve and can vary as much as times along a 4- to 5-cm length of nerve.
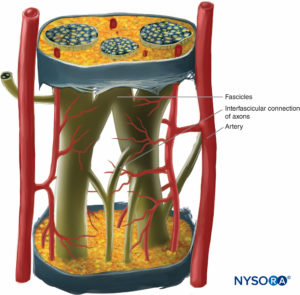
FIGURE 4. Diagram of intraneural plexus enclosed in a peripheral nerve. (Reproduced with permission from De Andrés JA, Reina MA,
López A, et al: Blocs nerveux périphériques, paresthésies et injections intraneurales, Le Practicien en Anesthésie Réanimation 2010;14:213-221.)
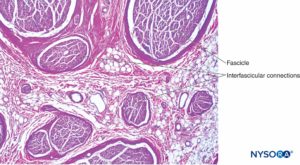
FIGURE 5. Intraneural plexus within a peripheral nerve, from the brachial plexus. Hematoxylin-eosin. (Reproduced with permission from Reina MA, Arriazu R, Collier CB, et al: Electron microscopy of human peripheral nerves of clinical relevance to the practice of nerve blocks. A structural and ultrastructural review based on original experimental and laboratory data, Rev Esp Anestesiol Reanim. 2013 Dec;60(10):552-562.)
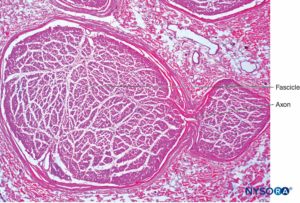
FIGURE 6. Intraneural plexus inside a peripheral nerve. Interfascicular connection from axons between two fascicles, obtained from the brachial plexus. Hematoxylin-eosin. (Reproduced with permission from Reina MA, Arriazu R, Collier CB, et al: Electron microscopy of human peripheral nerves of clinical relevance to the practice of nerve blocks. A structural and ultrastructural review based on original experimental and laboratory data. Rev Esp Anestesiol Reanim. 2013 Dec;60(10):552-562.)
NYSORA Tips
Inside each nerve, the axons form an intraneural plexus in such way that an axon can occupy different fascicles.
In a cross section of a sciatic nerve, fascicles comprise 25%–75% of the cross-sectional area (see Figures 1 and 3). This proportion varies in different nerves and at different levels of the same nerve. Up to 50% of the cross-sectional area is made up of non-neural tissue, including endoneural fluid and connective stroma. The number of fascicles increases at the level of nerve branching. In the proximity of joints, the fascicles are thinner, more numerous and have a thicker perineurium, which may confer better protection against pressure and stretching.
CONNECTIVE TISSUE SHEATHS OF PERIPHERAL NERVES
The connective tissue inside nerves functions to support and protect nerves blood and lymphatic vessels (see Figure 1 and 2). The connective tissue of peripheral nerves takes different names according to its location. On the outside of each peripheral nerve, there is collagenous tissue: epineurium. Surrounding every fascicle within the nerve is the perineurium. Individual nerve fibers within the fascicles are embedded in endoneurium, which fills the space bound by the perineurium. As the peripheral nerve divides and the number of fascicles decreases, the connective tissue sheaths become progressively thinner. For instance, in monofascicular nerves the epineurium is absent, distributed irregularly, or appears integrated with the perineurium. Connective tissue that connect nerves to the surrounding structures are thinner and disperse, often losing any distinguishable feature from that of general connective tissue.
ENDONEURIUM
The endoneurium (Figures 7 and 8) intimately surrounds Schwann cells and fills the space bounded externally by the perineurium. Endoneurium contains collagen fibers, fibroblasts, capillaries, and a few mast cells and macrophages. Collagen fibers are permeable and concentrated in a zone beneath the perineurium and around nerve fibers and blood vessels. The collagen fibers surround both myelinated and unmyelinated nerve fibers. However, the endoneurial sheaths around smaller myelinated fibers and around some unmyelinated axons are less well organized (Figure 9).
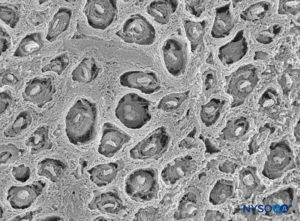
FIGURE 7. Endoneurium shaped as multiple canaliculi, enclosing fascicles of tibial nerve. Scanning electron microscopy. Magnification ×900. (Reproduced with permission from Reina
MA: Atlas of Functional Anatomy for Regional Anesthesia and Pain Medicine. New York: Springer; 2015.)
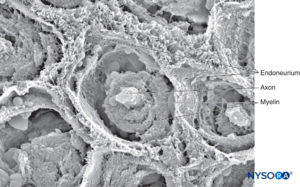
FIGURE 8. Endoneurium envelops myelinated axons in fascicles in a peripheral nerve. Scanning electron microscopy. Magnification ×3300. (Reproduced with permission from Reina MA, Arriazu R, Collier CB, et al: Electron microscopy of human peripheral nerves of clinical relevance to the practice of nerve blocks. A structural and ultrastructural review based on original experimental and laboratory data. Rev Esp Anestesiol Reanim. 2013 Dec;60(10):552-562.)
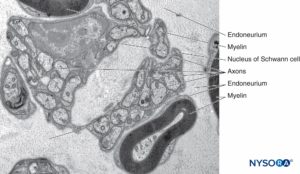
FIGURE 9. Unmyelinated and myelinated axons enclosed by endoneurium. Transmission electron microscopy. Magnification ×20000. (Reproduced with permission from Reina MA, Arriazu R, Collier CB, et al: Electron microscopy of human peripheral nerves of clinical relevance to the practice of nerve blocks. A structural and ultrastructural review based on original experimental and laboratory data. Rev Esp Anestesiol Reanim. 2013 Dec;60(10):552-562.
Fibroblasts are among the most abundant cell types of the endoneurium. They are responsible for fiber formation and production of ground substance. When sectioned transversely, endoneurial fibroblasts have triangular or rectangular perikaria. The appearance of fibroblasts varies depending on their functional activity. When the cell is metabolically active, as is the case with growth and in tissue regeneration after injury, the nucleus is larger and nucleoli are more prominent. The cytoplasm also stains more deeply and is basophilic in contrast with the lightly staining, slightly acidophilic cytoplasm of a relatively inactive cell. Like those in the epineurium, fibroblasts in the endoneurium lack a basal lamina.
Mast cells are especially numerous along the course of blood vessels. Mast cell granules are soluble in water and are therefore not easily revealed in sections routinely prepared with hematoxylin and eosin stain. After adequate fixation, the granules stain with most basic dyes and become metachromatic after certain dyes, such as toluidine blue. Electron microphotographs show that the secretory granules are membrane bound, and the granule matrices have varying densities and characteristic helical-type patterns (Figure 10). Macrophages are also found frequently around perivascular endoneurium (Figure 11). The endoneurium contributes to the stability of the internal medium where the Schwann cells and axons are located. The endoneurium of cutaneous nerves contains more collagen fibers than deep nerves; this probably is related to its protective role. The endoneural collagen is believed to originate from the Schwann cells, which are 9:1 more prominent than fibroblasts. Schwann cells represent 90% of intrafascicular cells, whereas fibroblasts account for less than 5% of the remaining number. The endoneurium along with the epineurium and perineurium contribute to nerve protection against elongation under strain. The sinuous trajectories of axons confer additional protection on nerves. Endoneurial sheaths around axons is demonstrated in figures 7, 8 and 9. Instead of individually shaped endoneurial layers, the endoneurium appears rather as a continuum, forming several canaliculi into which axons are embedded.
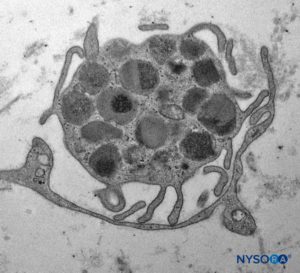
FIGURE 10. Mastocyte inside fascicles from tibial nerve. Transmission electron microscopy. Magnification ×7000. (Reproduced with permission from Reina MA: Atlas of Functional Anatomy for Regional Anesthesia and Pain Medicine. New York: Springer; 2015.)
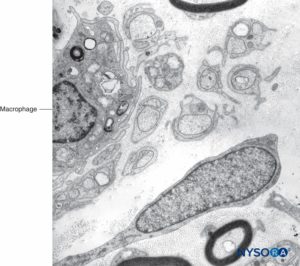
FIGURE 11. Macrophage within fascicles as seen on Transmission electron microscopy. Magnification ×7000.
NYSORA Tips
The endoneurium surrounds Schwann cells and fills the space within perineurium.
PERINEURIUM
Each fascicle is surrounded by a connective tissue sheath, the perineurium. The perineurium consists of concentric layers of flattened cells separated by layers of collagen (Figures 12 to 16). The number of perineurial cell layers depends on the size of the fascicle. As many as 8–16 concentric layers may be present around large nerve fascicles, but a single layer of perineurial cells surrounds small distal fascicles. In larger peripheral nerves, concentric cell layers alternate with layers of collagen fibers arranged longitudinally, similar to those of the epineurium. Collagen fibers are thinner than those of the epineurium, and only a few elastic fibers are scattered among them. Perineurial cells have a basal lamina on each side that may be considerably dense. At sites known as hemidesmosomes, the perineurial cell plasma membrane strongly adheres to the basal lamina.
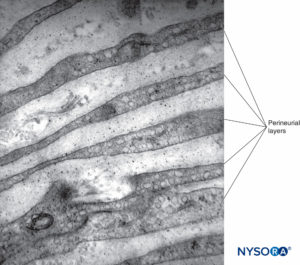
FIGURE 12. Concentric perineurial layers. Transmission electron microscopy. Magnification ×30,000. (Reproduced with permission from Reina MA, Arriazu R, Collier CB, et al: Electron microscopy of human peripheral nerves of clinical relevance to the practice of nerve blocks. A structural and ultrastructural review based on original experimental and laboratory data. Rev Esp Anestesiol Reanim. 2013 Dec;60(10):552-562.)
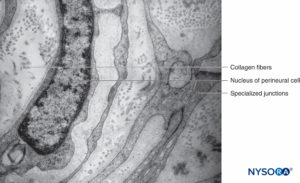
FIGURE 13. Perineurial layers and specialized junctions. Transmission electron microscopy. Magnification ×20,000. (Reproduced with permission from Reina MA, Arriazu R, Collier CB, et al: Electron microscopy of human peripheral nerves of clinical relevance to the practice of nerve blocks. A structural and ultrastructural review based on original experimental and laboratory data. Rev Esp Anestesiol Reanim. 2013 Dec;60(10):552-562.)
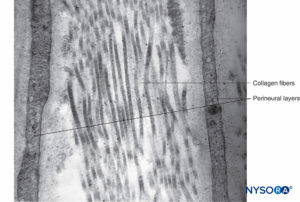
FIGURE 14. Collagen fibers among perineurial layers. Transmission electron microscopy. Magnification ×30,000. (Reproduced with permission from Reina MA: Atlas of Functional Anatomy for Regional Anesthesia and Pain Medicine. New York: Springer; 2015.)
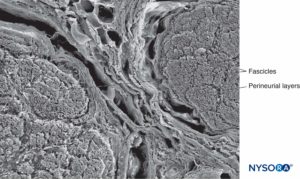
FIGURE 15. Perineurium and fascicles: three-dimensional features of perineural layers. Scanning electron microscopy. Magnification ×150. (Reproduced with permission from Reina MA, Arriazu R, Collier CB, et al: Electron microscopy of human peripheral nerves of clinical relevance to the practice of nerve blocks. A structural and ultrastructural review based on original experimental and laboratory data. Rev Esp Anestesiol Reanim. 2013 Dec;60(10):552-562.)
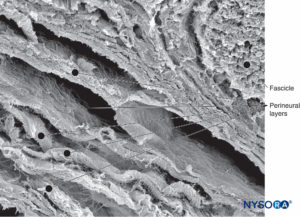
FIGURE 16. Three-dimensional image of perineural layers. Scanning electron microscopy. Magnification ×500. (Reproduced with permission from Reina MA, Arriazu R, Collier CB, et al: Electron microscopy of human peripheral nerves of clinical relevance to the practice of nerve blocks. A structural and ultrastructural review based on original experimental and laboratory data. Rev Esp Anestesiol Reanim. 2013 Dec;60(10):552-562.)
With electron microscopy, perineurial cells are seen as thin sheets of cytoplasm containing small amounts of endoplasmic reticulum, filaments, and numerous endocytic vesicles. Tight junctions and gap junctions between adjacent cells within the same layer of perineurium are also observed. Similar tight junctions may also appear between successive layers of the perineurium when their cells are in close proximity. Tight junctions in the inner layers of the perineurium and tight junctions in endoneurial capillaries form a blood-nerve barrier structure (Figures 17 and 18). The blood-nerve barrier is not equivalent to the blood-brain barrier as the blood-brain barrier astrocytes help regulate the flow of compounds between blood and the brain. The perineural cells are metabolically active, and their cytoplasms contain enzymes like ATPase (adenosine triphosphatase), 5-nucleotidase, and so on. These cells probably play a role in maintaining electrolyte and glucose balance around the nerve cells.
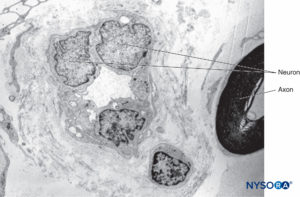
FIGURE 17. Endoneurium and capillaries inside fascicles of peripheral nerves. Transmission electron microscopy. Magnification ×3000. (Reproduced with permission from Reina MA, Arriazu R, Collier CB, et al: Electron microscopy of human peripheral nerves of clinical relevance to the practice of nerve blocks. A structural and ultrastructural review based on original experimental and laboratory data. Rev Esp Anestesiol Reanim. 2013 Dec;60(10):552-562.)
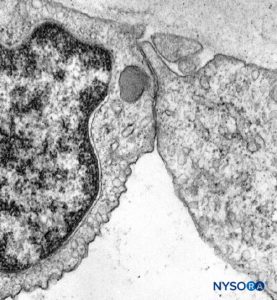
FIGURE 18. Endothelial cell from intrafascicular capillary. Transmission electron microscopy. Magnification ×20,000. (Reproduced with permission from Reina MA, López A, Villanueva MC, et al: The blood-nerve barrier in peripheral nerves. Rev Esp Anestesiol Reanim. 2003 Feb;50(2):80-86.)
The perineurium forms a tubular wrapping that allows some axonal movement inside the fascicles. The thickness of the epineurium varies between 1 and 100 μm. As the number of fascicles within a nerve increases, the thickness of the perineurium generally decreases. For instance, along the trajectory of the median nerve, the epineurium appears proportionally thicker in the wrist than in the axilla. There are three areas where the perineurium is absent and the epineurium becomes in contact with the endoneurium:nerve endings, around blood vessels, and in areas where reticular fibers penetrate the perineurium.
The role of the perineurium is to maintain intrafascicular pressure and to contribute to the barrier effect. Pressure exerted on the perineurium is transmitted to the endoneurium and ultimately the nerve fibers (axons). The perineurium increases in thickness around points of nerve branching to provide additional protection. The perineurium may be also protective in limiting the extension of infection and inflammatory reactions. For instance, when a nerve with intact perineurium crosses an infected area, the nerve responds generally by thickening of its perineurial layer. Conversely, when perineurium is not intact, the infection easily spreads across nerve fascicles. Injury to the epineurium, however, does not compromise the axonal safety to the same extent. Söderfelt demonstrated that the barrier effect of the epineurium is preserved for up to 22 hours postmortem in conditions of ischemia. Olsson has studied the loss of the nerve barrier effect in vivo after nerve lesion. He also has demonstrated recovery of the effect between 2 and 30 days postinjury.
NYSORA Tips
- Fascicles within the nerve are surrounded by perineurium which confers structural protection against penetration and overstretching injury.
- A blood-nerve barrier is formed by tight junctions in the inner layers of the perineurium and tight junctions in endoneurial capillaries.
EPINEURIUM
The outermost sheath of the epineurium consists of moderately dense connective tissue that binds nerve fascicles (Figures 3, 19, and 20). Epineurium merges with adipose tissue surrounding peripheral nerves, particularly in subcutaneous tissue. The amount of epineurial tissue varies along a nerve and is more abundant around joints. The thickness of the epineurium varies in different nerves and in different locations of the same nerve. For instance, the average thickness of the epineurium is 22% of the ulnar nerve at the level of the elbow and 88% of the sciatic nerve at the gluteal level.
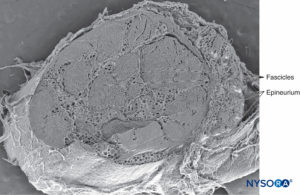
FIGURE 19. Epineurium in human tibial nerve. Scanning electron microscopy. Magnification ×20. (Reproduced with permission from Reina MA, Arriazu R, Collier CB, et al: Electron microscopy of human peripheral nerves of clinical relevance to the practice of nerve blocks. A structural and ultrastructural review based on original experimental and laboratory data. Rev Esp Anestesiol Reanim. 2013 Dec;60(10):552-562.)
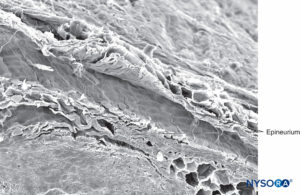
FIGURE 20. Epineurium in human tibial nerve. Scanning electron microscopy. Magnification ×180. (Reproduced with permission from Reina MA, Arriazu R, Collier CB, et al: Electron microscopy of human peripheral nerves of clinical relevance to the practice of nerve blocks. A structural and ultrastructural review based on original experimental and laboratory data. Rev Esp Anestesiol Reanim. 2013 Dec;60(10):552-562.)
In general, the epineurium represents between 30% and 75% of the cross-sectional area of a nerve. The proportion of epineurium is higher in larger nerves with increasing numbers of nerve fascicles. However, the epineurium is absent around monofascicular nerves and at nerve endings.The epineurium contains adipocytes, fibroblasts, connective tissue fibers, mast cells, small blood and lymph vessels, and small nerve fibers innervating the vessels. Epineurium is a permeable structure, and its fibroblasts are ultrastructurally identical to fibroblasts elsewhere in the body. Scattered throughout the epineurium, fibroblasts form the epineurial collagen, the most prominent component of this layer. As collagen is a protein stained by most acid dyes, collagen fibers turn weak pink wiht eosin in preparations stained with hematoxylin-eosin. Under the electron microscope, fibers of mature collagen have frecuent cross bandings. Elastic fibers are also present, and these are considerably more compact than collagen fibers. They stain weak pink in sections stained with hematoxylin and eosin, brown with orcein, and blue-purple with resorcin-fuchsin. In electron micrographs, elastin fibers typically appear more stained (darker) at the periphery and are embedded in a sub-stance containing thinner elastin filaments.The epineurium of some nerves contains a considerable amount of fat, as is the case with the sciatic nerve. The common peroneal and tibial nerves, however, contain less fat than the sciatic nerve, and usually the former contains less fat than the latter.
Seen under the microscope, intraneural adipose cells resemble honeycombs, with empty vacuoles due to fat dissolution, during the fixation process (Figure 21). Mast cells are distributed throughout connective tissue and are often located in the proximity of small blood vessels. Vasa nervorum supplying peripheral nerves arise from branches of regional arteries. Branches from these arteries enter the epineurium to form an vascular plexus (Figures 22 and 23). From the plexus, vessels penetrate the perineurium and enter the endoneurium as arterioles and capillaries. In nerves consisting of several fascicles, arteries, veins, and lymphatics run longitudinally and parallel to nerve fascicles.
Epineurium also projects longitudinal “ondulations” along its trajectory, providing elasticity especially in nerves supplying innervation to the extremities.
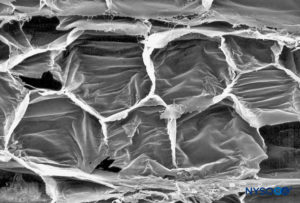
FIGURE 21. Adipocytes in interfascicular tissue of sciatic nerve. Scanning electron microscopy. Magnification ×400. (Reproduced with permission from Reina MA, López A, De Andrés JA: Adipose tissue within peripheral nerves. Study of the human sciatic nerve. Rev Esp Anestesiol Reanim. 2002 Oct;49(8):397-402.)
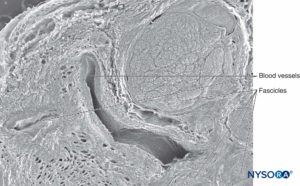
FIGURE 22. Interfascicular tissue and vessels in sciatic nerve. Scanning electron microscopy. Magnification ×50. (Reproduced with permission from Reina MA, López A, De Andrés JA: Adipose tissue within peripheral nerves. Study of the human sciatic nerve. Rev Esp Anestesiol Reanim. 2002 Oct;49(8):397-402.)
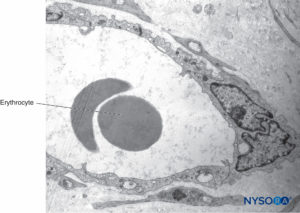
FIGURE 23. Blood vessel inside interfascicular tissue of sciatic nerve. Transmission electron microscopy. Magnification ×7000. (Used with permission from Miguel Angel Reina, MD.)
NYSORA Tips
- Epineurium is the outermost sheath of peripheral nerves.
- Epineurium is permeable and consists of moderately dense connective tissue that binds nerve fascicles.
- The epineurium contains adipocytes, fibroblasts, connective tissue fibers, mast cells, small lymphatics, as well as blood vessels and small nerve fibers innervating the vessels.
PARANEURAL SHEATHS AND COMMON EPINEURAL SHEATH
Whereas gross anatomy descriptions of distal peripheral nerves accurately identify each connective layer enclosing axons (endoneurium), nerve fascicles or bundles of axons (perineurium), and single peripheral nerves (epineurium), this becomes more complex where the connective tissue binds more than one nerve. An example of this is the sciatic nerve at the popliteal fossa. Various terms, such as paraneurium, paraneural sheaths, common epineural sheath, conjuctiva nervorum, or adventitia, are interchangeably used to refer to the same connective tissue. Small nerves formed by a single group of fascicles feature a layer of perineurium enclosing each fascicle along with scarce amounts of adipose tissue. A connective tissue known as epineurium composed of collagenous fibers encloses the nerve. Specific techniques enable identification of perineurium using staining methods positive for EMA (epithelial membrane antigen) and of collagen with Masson trichrome and EMA-negative stain.
Similarly, staining techniques aid in the identification of structures in more complex nerves, such as the sciatic nerve, where groups of variable numbers of fascicles are present. In these types of nerves, EMA staining reveals that perineurium encloses each nerve fascicle, as opposed to connective tissue made up by collagen fibers typically present in epineurium that encloses groups of fascicles (detected with Masson trichromic stain). Microscopic analysis of complex nerve structures such as the sciatic nerve at increasingly proximal locations shows that nerve branches within these nerve structures appear divided by their respective epineurial layers even before physical branch division materializes. Each peripheral nerve at both plexus and terminal sites is encircled by concentric clusters of fat tissue, which appear just prior to the division of the nerve (Figure 24 and 25). The adipose tissue extends alongside its collateral or terminal branches. The amount and shape of fat tissue vary along the nerve, progressively losing their concentric contour and becoming unevenly distributed. The collagen layer, similar to the epineurium, that wrapps together the nerve components and the adipose tissue in between has been refered to as paraneurium, paraneural sheath, common epineural sheath, conjuctiva nervorum or adventitia by different authors.
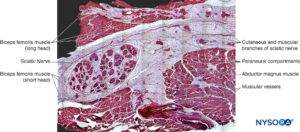
FIGURE 24. Sciatic nerve and its surrounding paraneurium at the level of the popliteal fossa. Hematoxylin-eosin. (Reproduced with permission from Reina MA: Atlas of Functional Anatomy for Regional Anesthesia and Pain Medicine. New York: Springer; 2015.)
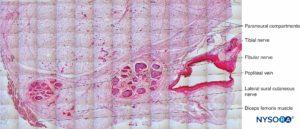
FIGURE 25. Tibial nerve, fibular nerve and its paraneurium at popliteal fossa. Hematoxylin-eosin. (Reproduced with permission from Reina MA: Atlas of Functional Anatomy for Regional Anesthesia and Pain Medicine. New York: Springer; 2015.)
In clinical practice, ultrasound-guided injection of local anesthetics enables the indirect identification of paraneurium as the space between this layer and the nerve expands displaying a concentric shape. Neural layers surrounding fascicles, groups of fascicles, nerves, as well as more complex nerve structures have similar morphology, and they are mainly composed of collagen fibers. Therefore, it may seem reasonable to unify terminology noting that present denominations based on the anatomic location of each neural fascia seems rather confusing. However, epineurium and paraneurium may best to avitted terms to avoid the present confusion. Both epineurium and paraneurium have similar functions, which include insulation and protection of nerves from injury. Paraneural compartments facilitate longitudinal displacement of nerves controlling body movement. This movement is necessary to neutralize lateral compression by changing their shape. If the tissue is exposed to external irritation, it reacts, leading to interfascicular fibrosis.
In relation to the anatomic features of the sciatic nerves, Andersen et al found the sheath surrounding the sciatic nerve to be a thin transparent structure that was clearly distinctive, both macroscopically and microscopically different from the epineurium. The sheath facilitated the spread of the injectate along the nerve. However, its projections did not completely encircle the nerve. The internal layers of the paraneurium around the sciatic nerve had a similar structure of that sheath. Vloka et al used the term common epineural sheath. Tran et al compared the effectiveness of the sciatic nerve block in relation to what they called “subepineural” injection at the “bifurcation,” which fills a common paraneural compartment shared by tibial and peroneal nerves close to their macroscopic division.
Orebaugh et al reported that needle-tip placement in the interscalene region and injection of anesthetic solution took place frequently inside the epineurium. This occurred in approximately 50% of the nerve blocks without evidence of fascicular or axonal damage and no trace of dye within fascicles to suggest that the needle had traversed them Spinner et al demonstrated that intraepineurial injection of dye results in its dissection along paths of least resistance, suggesting the presence of anatomic constraints among epineurial compartments. When Spinner injected the inner epineurium, the dye expanded within the same compartment but did not cross over or extend to the common external epineurial space. Therefore, the concept of “intraneural injection” should be revised for each nerve examined, avoiding extrapolations based on studies of a single nerve due to the considerable anatomic variability among peripheral nerves.
NYSORA Tips
A peripheral nerve at both plexus and terminal sites is encircled by concentric clusters of adipose tissue. This explains why perineural injections result in low opening injection pressure.
PERIPHERAL NERVE BLOCKS
Diffusion of anesthetic into the axons is influenced by the presence and characteristics of the connective tissue sheaths (eg, perineurium, myelin) and the size and location of the axons inside fascicles. During intravenous peripheral anesthesia (Bier block), the local anesthetic most likely reaches the peripheral nerve endings through the intraneural capillary network. Perineurium and endoneural capillary endothelium protect the axons from foreign substances thanks to their tight junctions between endothelial cells and among perineural cells. Local anesthetic injected outside the epineurium of a nerve must traverse both the epineurium and perineurium to reach the axons. Subsequently, only a small proportion of the injected anesthetic comes in direct contact with axons leading to delayed onset, incomplete or failed neural block. For instance, Popitz and collaborators injected 1% lidocaine into the sciatic nerve of rats and found that when the block was complete, the intraneural amount of local anesthetic was around 1.6% of the injected dose.
SUMMARY
The composition and arrangement of the connective tissue of peripheral nerves play a major role in protection of the peripheral nerves and in the practice of regional anesthesia. Characteristics and variability of the connective tissue may substantially influence the spread of the local anesthetic during nerve block injection and therefore the dynamics and quality of the neural block. Supplementary video related to this subject can be found at Anatomy of Nerve block Video.
REFERENCES
- Reina MA, López A, Villanueva MC, De Andrés JA, León GI: Morphology of peripheral nerves, their sheaths, and their vascularization. Rev Esp Anestesiol Reanim 2000;47:464–475.
- Reina MA, Wikinski J, Prats-Galino A, Machés F: Morfologia del nervio periférico. In: Wikinski J, Reina MA, Bollini C, et al (eds): Diagnóstico, prevención y tratamiento de las complicaciones neurológicas asociadas con la anestesia regional periférica y central. Panamericana Ed, 2011, pp 71–86.
- Reina MA, Arriazu R, Collier CB, Sala-Blanch X: Histology and electron microscopy of human peripheral nerves of clinical relevance to the practice of nerve blocks. Rerv Esp Anestesiol Reanim 2013;60:552–562.
- Reina MA, De Andres JA, Hernández JM, et al: Successive changes in extraneural structures from the subarachnoid nerve roots to the peripheral nerve, influencing anesthetic block, and treatment of acute postoperative pain. Eur J Pain Suppl 2011;5:377–385.
- Sunderland S, Marshall RD, Swaney WE: The intraneural topography of the circumflex musculocutaneous and obturador nerves. Brain 1959;82: 116–129.
- Sunderland S, Ray LJ: The intraneural topography of the sciatic nerve and its popliteal divisions in man. Brain 1948;71:242–258.
- Sunderland S: The intraneural topography of the radial, median and ulnar nerves. Brain 1945;68:243–255.
- Sunderland S: Troncos nerviosos periféricos. Salvat Ed, 1985, pp 31–60.
- Boyd IA, Davey MR: Composition of Peripheral Nerves. Churchill Livingstone, 1968.
- Friede RL, Bischhausen R: The organization of endoneurial collagen in peripheral nerves as revealed with the scanning electron microscopy. J Neurol Sci 1978;38:83–89.
- Reina MA, López A, De Andrés JA: The blood-nerve barrier in peripheral nerves. Rev Esp Anestesiol Reanim 2003;50:80–86.
- De Andrés JA, Reina MA, López A, Sala-Blanch X, Prats A. Blocs nerveux périphériques, paresthésies et injections intraneurales. Prat Anesth Reanim 2010;14:213–221.
- Olsson Y, Kristensson K: The perineurium as a diffusion barrier to protein tracers following trauma to nerves. Acta Neuropath 1973;23:105–111.
- Sunderland S, Bradley KC: The perineurium of the peripheral nerves. Anat Rec 1952;113:125–142.
- Olsson Y, Resse TS: Permeability of vas nervorum and perineurium in mouse sciatic nerve studied by fluorescence and electron microscopy. J Neuropathol Exp Neurol 1971;30:105–119.
- Soderfeldt B, Olsson Y, Kristensson K: The perineurium as a diffusion barrier to protein tracers in human peripheral nerve. Acta Neuropath 1973;25:120–126.
- Llewelyn JG, Thomas PK: Perineurial sodium-potasium ATPase activity in streptozotocin-diabetic rats. Exp Neurol 1987;97:375–382.
- Lundborg G: Structure and function of the intraneural microvessels as related to trauma, edema formation and nerve function. J Bone Joint Surg 1975;57:938–948.
- Sunderland S. The effect of rupture of the perineurium on the contained nerve fibres. Brain 1946;69:149–152.
- Sunderland S: The connective tissues of peripheral nerves. Brain 1965;88: 841–854.
- Sunderland S: The adipose tissue of peripheral nerves. Brain 1945;68: 118–122.
- Reina MA, López A, De Andrés JA: Adipose tissue within peripheral nerves. Study of the human sciatic nerve. Rev Esp Anestesiol Reanim 2002;49:397–402.
- Krstic R. Die Gewebe des Menschen und der Seaugetiere. Springer, 1978.
- Andersen HL, Andersen SL, Tranum-Jensen J. Injection inside the paraneural sheath of the sciatic nerve: Direct comparison among ultrasound imaging, macroscopic anatomy, and histologic analysis. Reg Anesth Pain Med. 2012;37:410–414.
- Vloka JD, Hadzic A, Lesser JB, et al: A common epineural sheath for the nerves in the popliteal fossa and its possible implications for sciatic nerve block. Anesth Analg 1997;84:387–390; The division of the sciatic nerve in the popliteal fossa: anatomical implications for popliteal nerve block. Vloka JD, Hadzić A, April E, Thys DM. Anesth Analg. 2001 Jan;92(1):215-217.
- Lang J. On connective tissue and blood vessels of the nerves. Z Anat Entwicklungsgesch 1962;123:61–79.
- Van Beek A, Kleinert HE: Practical neurorrhaphy. Orthopedic Clin North Am 1977;8:377–386.
- Sala-Blanch X, Reina MA, Ribalta T, Prats-Galino A. Sciatic nerve structure and nomenclature: Epineurium to paraneurium. Is this a new paradigm? Reg Anesth Pain Med 2013;38:463–465.
- 29. Sala-Blanch X, Vandepitte C, Laur J, et al: Practical review of perineural versus intraneural injections: a call for standard nomenclature. Int Anesth Clin 2011;49:1–12.
- Millesi H, Hausner T, Schmidhammer R, Trattnig S, Tschabitscher M: Anatomical structures to provide passive motility of peripheral nerve trunks and fascicles. Acta Neurochir Suppl 2007;100:133–135.
- Reina MA (ed): Atlas of Functional Anatomy for Regional Anesthesia and Pain Medicine: Human Structure, Ultrastructure and 3D Reconstruction Images. Springer, 2015.
- Tran de QH, Dugani S, Pham K, Al-Shaafi A, Finlayson RJ: A randomized comparison between subepineural and conventional ultrasound-guided popliteal sciatic nerve block. Reg Anesth Pain Med 2011;36:548–552.
- Orebaugh SL, McFadden K, Skorupan H, Bigeleisen PE: Subepineurial injection in ultrasound-guided interscalene needle tip placement. Reg Anesth Pain Med 2010;35:450–454.
- Spinner RJ, Wang H, Carmichael SW, Amrami KK, Scheithauer BW: Epineurial compartments and their role in intraneural ganglion cyst propagation: An experimental study. Clin Anat 2007;20:826–833.
- Ip V, Tsui B. Injection through the paraneural sheath rather than circumferential spread facilitates safe, effective sciatic nerve block. Reg Anesth Pain Med 2013;38:373.
- Ip VH, Tsui BC. Kill 2 birds with 1 stone: Injection at the bifurcation during popliteal sciatic nerve block. Reg Anesth Pain Med 2011;36: 633–634.
- Sala-Blanch X, López A, Prats-Galino A. Vloka sciatic nerve sheath: a tribute to a visionary. Reg Anesth Pain Med. 2015 Mar-Apr;40(2):174.
- Popitz-Bergez S, Lee-Soon S, Strichartz GR, Thalhammer JG: Relation between functional deficit and intraneural local anesthetic during peripheral nerve block. A study in the rat sciatic nerve. Anesthesiology 1995;83:583–592.