Imran Ahmad
INTRODUCTION
Awake endotracheal intubation can be achieved using a variety of equipment, such as video laryngoscopes, optical stylets, and fiber-optic scopes. Appropriate topical anesthesia of the airway and sedation can enable any of these techniques to be used successfully. The commonest method used to perform awake endotracheal intubation is with a flexible fiberscope, and awake fiber-optic intubation is regarded as the gold standard for the endotracheal intubation of patients with an anticipated difficult airway. This procedure requires skills and knowledge that should be familiar to all anesthesiologists.
Recently, there have been many advances in regional anesthesia, allowing for more complicated and innovative procedures to be done under regional block techniques; however, not all of these cases can be done solely under regional anesthesia. Often, a combination of regional and general anesthesia is required; therefore, all anesthesiologists must be familiar with awake intubation techniques, especially if the patient has an anticipated difficult airway. Anesthetizing patients with an anticipated difficult airway is often a source of anxiety and trepidation, but appropriate airway topicalization and sedation techniques can create the appropriate conditions for a safe and stress-free procedure for both the patient and the anesthesiologist.
It is difficult to give precise figures on the incidence of difficult airways due to a variety of reasons, including population differences, operator skill variation, operator reporting, and an inconsistency in the definition of a difficult airway. In the general population, the approximate figures for the incidence of Cormack and Lehane laryngoscopy grades 3 and 4 is 10%, difficult intubation is 1%, and difficult bag mask ventilation is 0.08%–5%.
Endotracheal intubation is usually performed under general anesthesia, but if a difficult airway is anticipated, then this should ideally be done under regional anesthesia (with or without sedation) as this allows the patient to breathe spontaneously, maintain airway patency, and cooperate with the operator. If any untoward difficulties are experienced, then the procedure can be abandoned with minimum risk to the patient. There are obvious exceptions to performing awake intubation, such as patient refusal, young children, and uncooperative patients (due to confusion or learning disabilities).
To successfully perform awake endotracheal intubation, one should be familiar with the following:
- Sensory innervation of the upper airway
- Agents available for topicalization
- Application techniques available to topicalize the airway
- Regional anesthesia techniques, landmark or ultrasound-guided
- Safe sedation techniques
SENSORY INNERVATION OF THE AIRWAY
The upper airway is divided into the nasal and oral cavities, the pharynx, and the larynx. The sensory innervation to the upper airway is supplied by the trigeminal, glossopharyngeal, and vagus nerves (Figure 1).
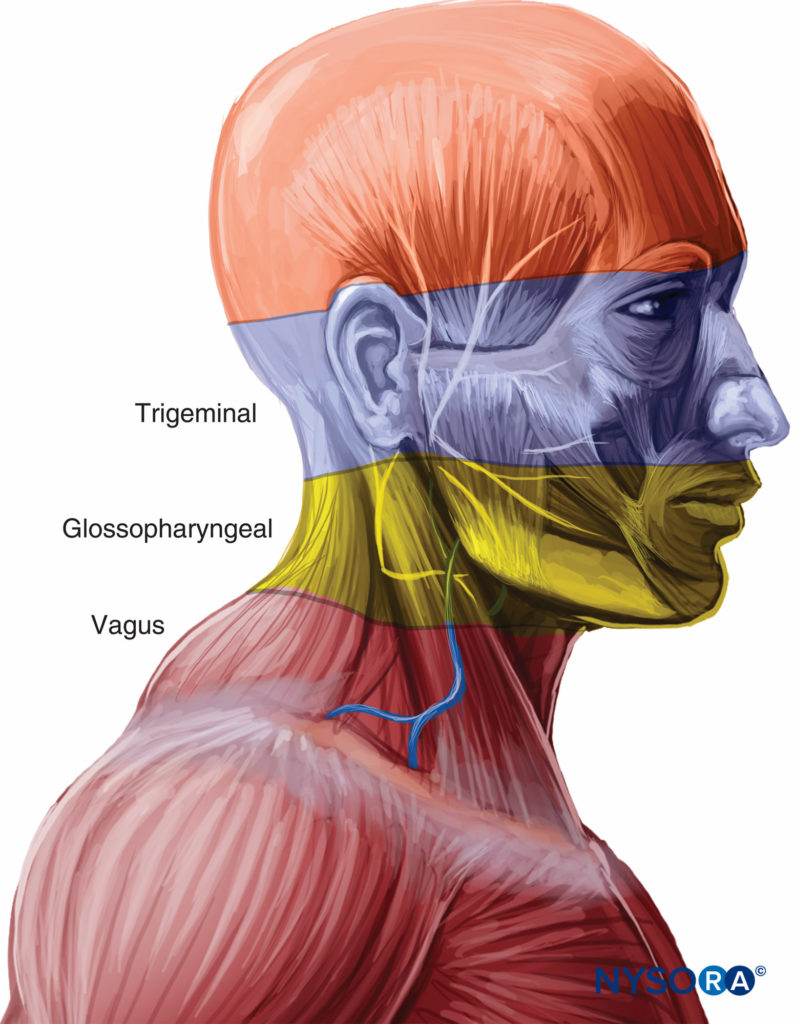
FIGURE 1. Innervation of the upper airway.
Nose
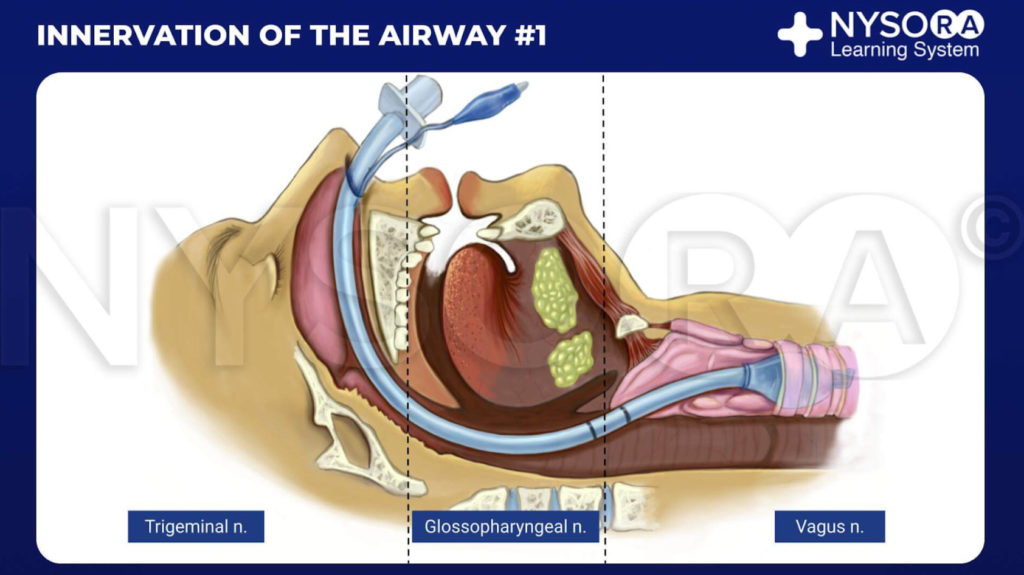
From the Compendium of Regional Anesthesia: Innervation of the airway #1 infographic.
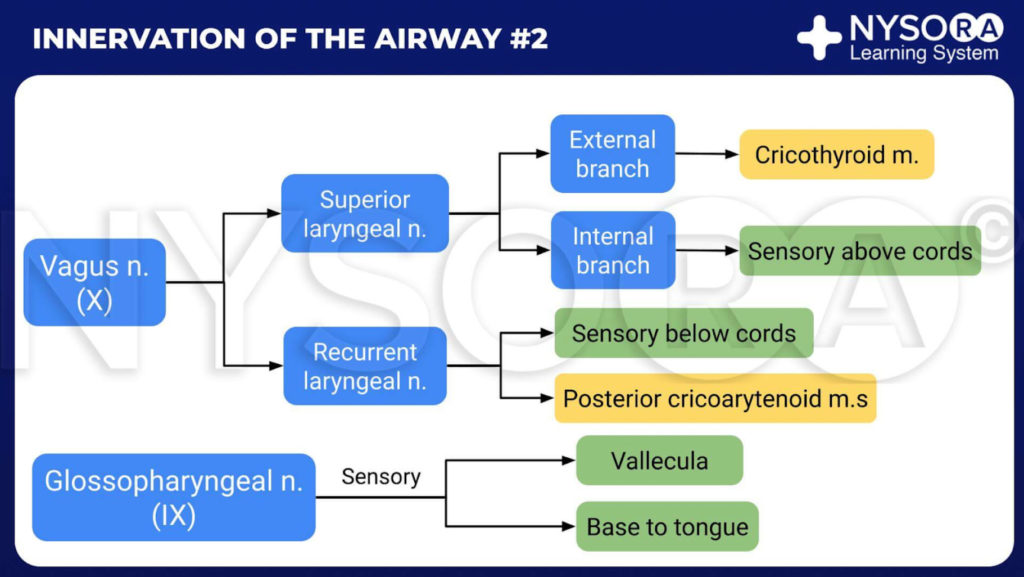
From the Compendium of Regional Anesthesia: Innervation of the airway #2 infographic.
The nose is entirely innervated by branches of the trigeminal nerve. Septum and anterior parts of the nasal cavity are affected by the anterior ethmoidal nerve (a branch of the ophthalmic nerve). The rest of the nasal cavity is innervated by the greater and lesser palatine nerves (branches of the maxillary nerve).
The palatine nerves are relayed through the pterygopalatine ganglion, found in the pterygopalatine fossa, which is situated close to the sphenopalatine fossa, located just posterior to the middle turbinate.
Pharynx
The pharynx is largely innervated by the glossopharyngeal nerve. Innervation of the whole pharynx, posterior third of tongue, the fauces, tonsils, and epiglottis is from the glossopharyngeal nerve.
Oropharynx
The oropharynx is innervated by branches of the vagus, trigeminal, and glossopharyngeal nerves. The posterior third of the tongue, vallecula, and anterior surface of the epiglottis are innervated by the tonsillar nerve (a branch of the glossopharyngeal nerve). The posterior and lateral wall of the pharynx are innervated by the pharyngeal nerve (a branch of the vagus nerve). The tonsillar nerve affects the tonsils. The anterior twothirds of the tongue are innervated by the lingual nerve (branch of the mandibular division of the trigeminal nerve).
Larynx
The larynx is innervated by the vagus nerve (Figure 2). Above the vocal cords (base of tongue, posterior epiglottis, aryepiglottic folds, and arytenoids), the internal branch of the superior laryngeal nerve (a branch of the vagus nerve) supplies innervation. For the vocal cords and below the vocal cords, the recurrent laryngeal nerve (a branch of the vagus nerve) is the supplier.
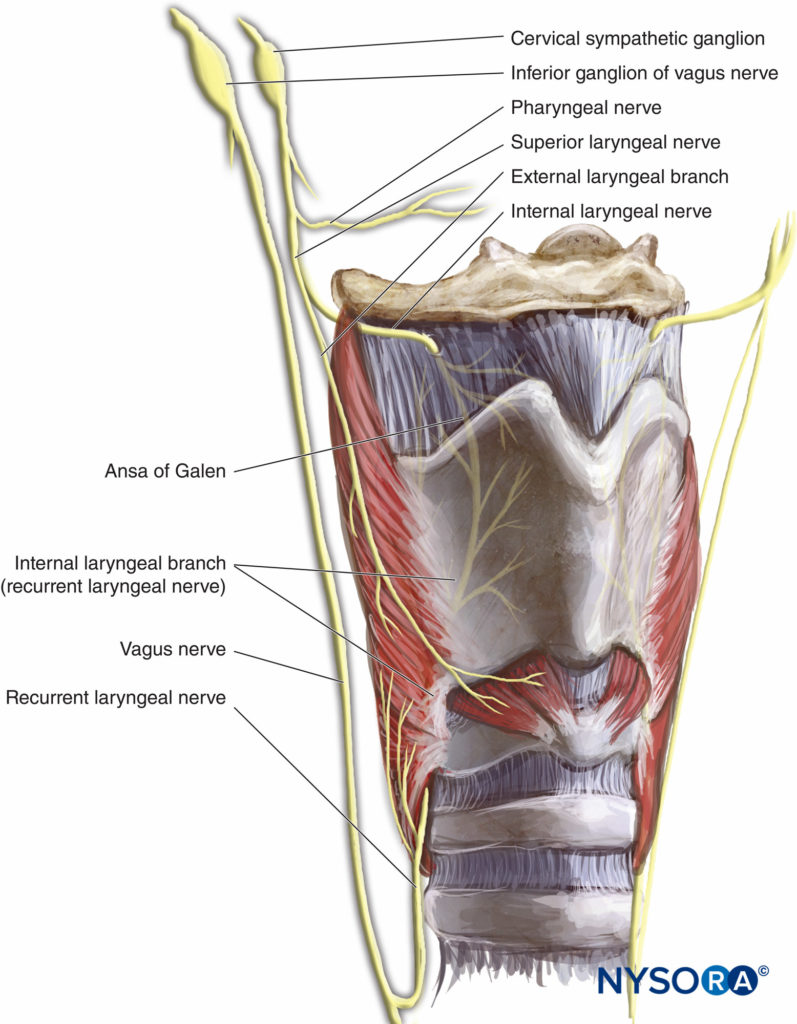
FIGURE 2.Innervation of the larynx.
NYSORA Tips
• The greater and lesser palatine nerves provide sensation to the nasal turbinates and posterior two-thirds of the nasal septum.
• The anterior ethmoid nerve innervates the remainder of the nasal passage.
• The glossopharyngeal nerve provides sensory innervation to the posterior third of the tongue, the vallecula, the anterior surface of the epiglottis (lingual branch), the walls of the pharynx (pharyngeal branch), and the tonsils (tonsillar branch).
• The superior laryngeal nerve innervates the base of the tongue, posterior surface of the epiglottis, aryepiglottic fold, and the arytenoids.
• The recurrent laryngeal nerve provides sensory innervation to the trachea and vocal folds.
TOPICAL ANESTHESIA
Cocaine
Cocaine is the only local anesthetic with vasoconstrictor properties; therefore, it is particularly useful for topical anesthesia of the nasopharynx, which is highly vascular. Cocaine is available as a 5% or 10% solution and in paste form; the maximum recommended dose is 1.5 mg/kg. It should be used with caution in patients with coronary artery disease, hypertension, and pseudocholinesterase deficiency.
The mixture of 2 mL of 10% cocaine, 1 mL 1:1000 adrenaline, 2 mL sodium bicarbonate, and 5 mL sodium chloride makes 10 mL of Moffett’s solution. This is commonly used in rhinological procedures to provide local anesthesia, vasoconstriction, and decongestion. It is also used to topicalize the nasal mucosa to provide the optimal conditions for nasal intubations.
Lidocaine
Lidocaine is the most commonly used local anesthetic for airway topicalization. The 4% solution and 10% spray are most often used (Figure 3). Systemic absorption from topical application to the upper airways is lower than expected, so in practice higher doses can be used than the recommended 2 mg/kg.
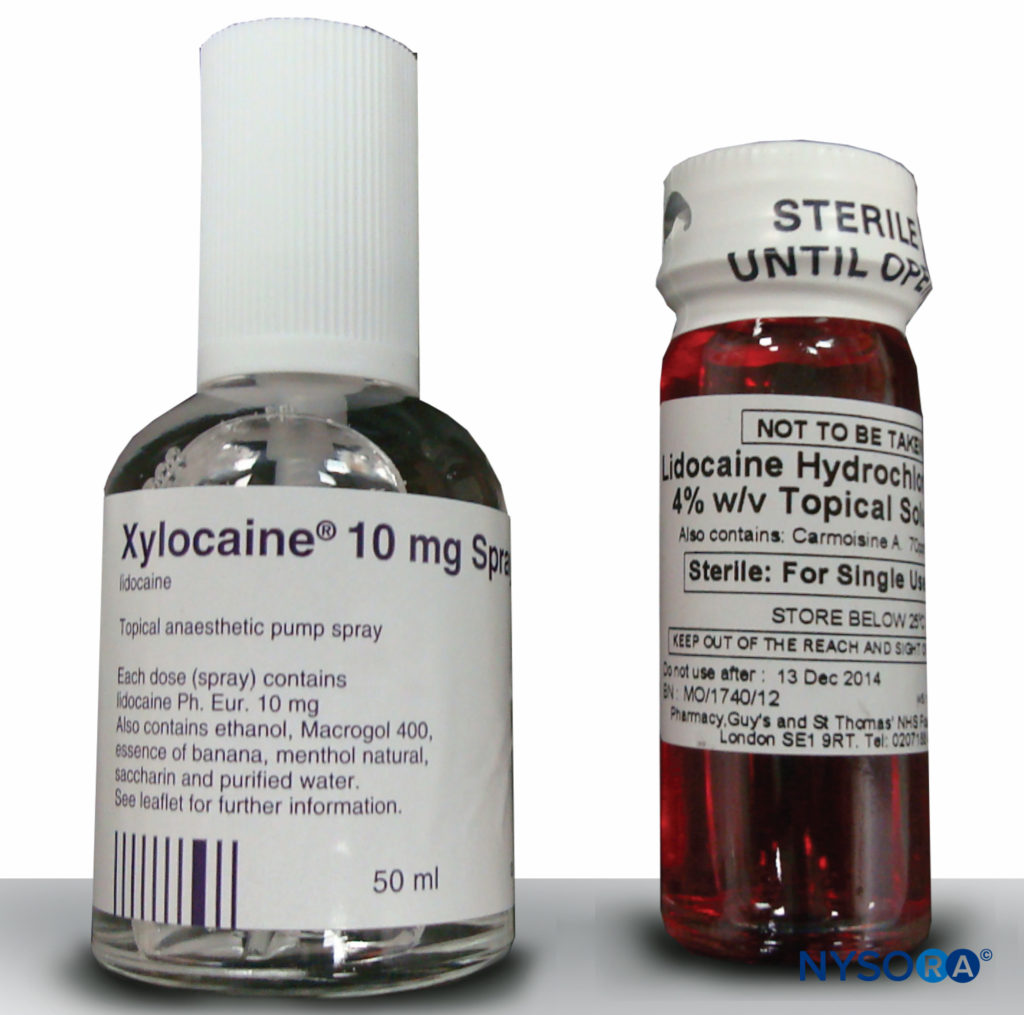
FIGURE 3.Lidocaine, 10%, and 4%.
Vasoconstrictors
Vasoconstrictors should be used when the nasal mucosa is being anesthetized; this is because the mucosa is highly vascular, and bleeding can readily occur on instrumentation, which can obscure the view seen on the fiberscope.
As mentioned, cocaine has inherent vasoconstrictor properties, so it is a suitable agent to use for the nasal mucosa. Vasoconstrictor agents such as xylometazoline and phenylephrine are prepared with lidocaine to produce local anesthesia and vasoconstriction.
These mixtures are also suitable agents for the preparation of the nasal mucosa.
NYSORA Tips
• The use of vasoconstrictors reduces the bleeding “shrinks” the nasal mucosa resulting in better surgical exposure.
• Shrinking of the nasal mucosa increases the size of the nasal airway passages, creating more space for the fiberscope and endotracheal tube.
• Appropriate time should be allowed for the vasoconstrictor to take effect before commencing fiberoscopy
APPLICATION TECHNIQUES
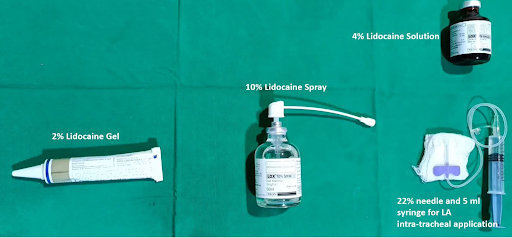
From the Compendium of Regional Anesthesia: Equipment and local anesthetic preparations for anesthesia of the airway.
There are various techniques available to topicalize the upper airway in preparation for awake intubation. The nasopharynx and oropharynx can be sprayed directly from the container of local anesthetic preparations, sprayed using the McKenzie technique, or sprayed via a mucosal atomization device (MAD).
The McKenzie technique uses a 20-gauge cannula attached to oxygen bubble tubing via a three-way tap.
The other end of bubble tubing is then attached to an oxygen source, which is turned on to deliver a flow of 2–4 L/min. As the local anesthetic is slowly administered via a 5-mL syringe attached to the top port of the cannula, a jetlike spray effect is seen, which greatly increases the surface area of the local anesthetic and allows directed topicalization of the nasal and oral mucosa (Figure 4).
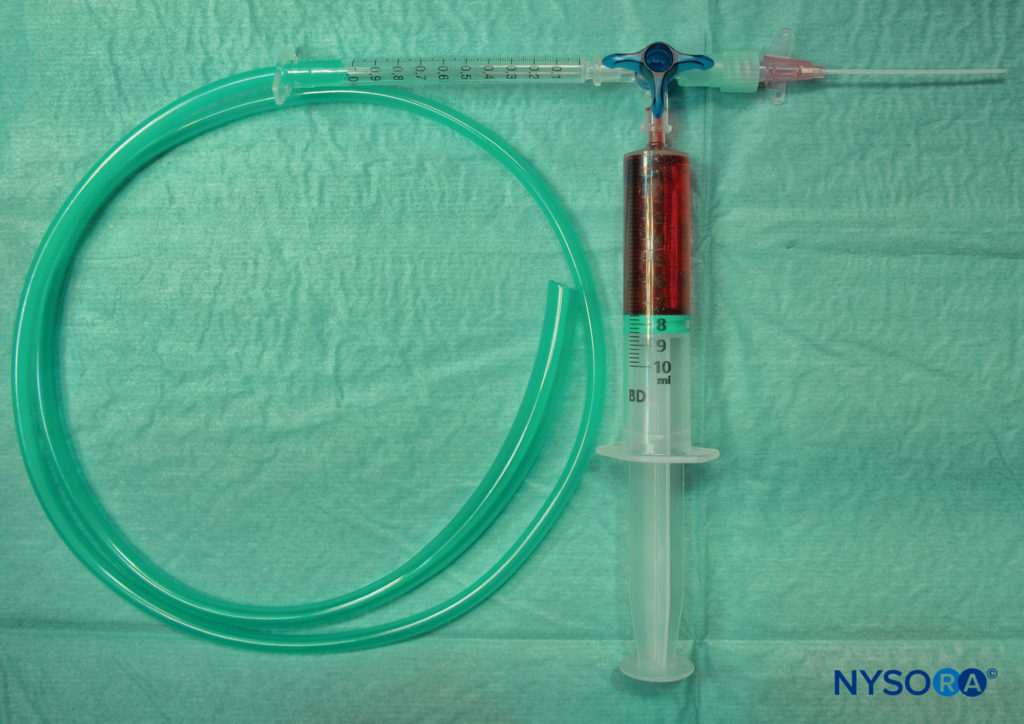
FIGURE 4. Setup for McKenzie technique.
NYSORA Tips
• Maintain a tight seal between the tubing and the cannula to prevent leakage of local anesthetic from these areas.
• Slow, continuous pressure on the 5-mL syringe containing local anesthesia will result in a “hissing” sound as a fine mist is sprayed out of the cannula.
Commercially available mucosal atomizers allow a similar mistlike effect as seen with the McKenzie technique by just attaching them to the end of a syringe (Figure 5). These devices are available for nasal and oral applications.

FIGURE 5. Mucosal atomization device (MAD).
Adding approximately 5 mL of 4% lidocaine to a nebulizer, then delivering it with oxygen for up to 30 minutes is a safe and noninvasive way to topicalize the airway all the way down to the trachea (Figure 6). It is well tolerated and is a useful technique to topicalize the whole airway. It also allows the topicalization of patients with limited mouth opening, where atomizers cannot be passed into the mouth to topicalize the oropharynx.
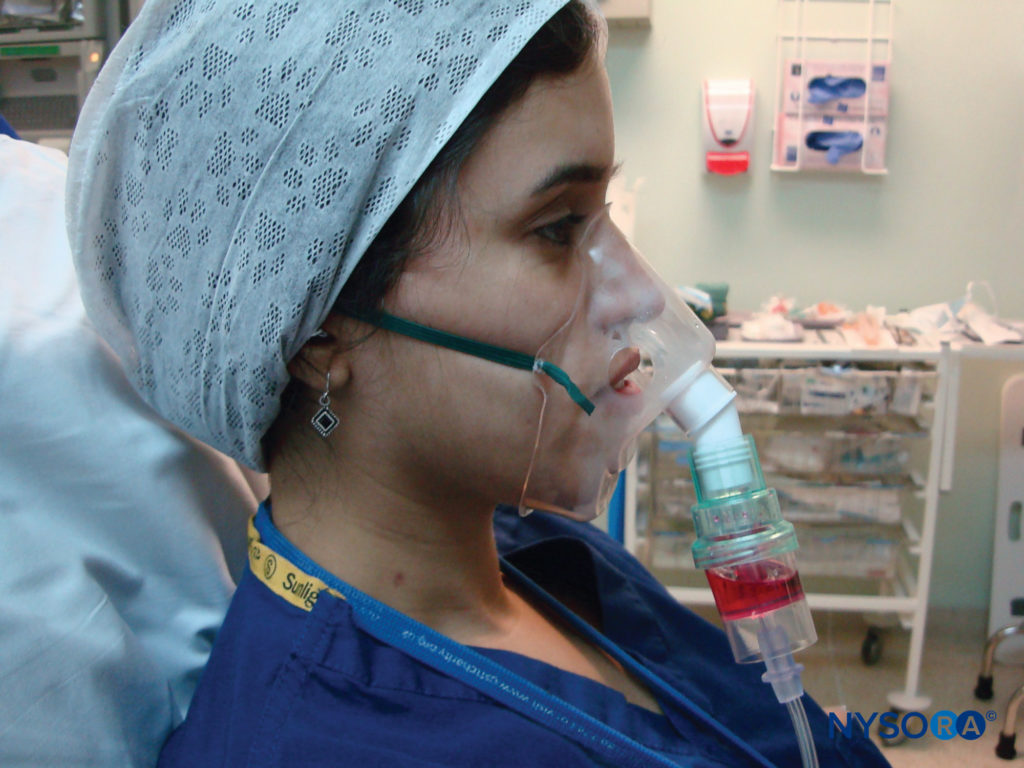
FIGURE 6. Administration of nebulized lidocaine.
The vocal cords can also be sprayed directly with local anesthetic using the spray-as-you-go (SAYGO) technique. Here, the distal end of a 16-gauge epidural catheter is cut 3 cm from the end and then fed through the working channel of a fiberscope.
The Luer lock connector is connected to the proximal end of the catheter and then attached to a 5-mL syringe prepared with 4% lidocaine. The distal end should protrude out of the fiberscope, so that the tip is just visible. The local anesthetic is then dripped onto the vocal cords prior to the fiberscope being introduced into the trachea. This reduces patient discomfort and coughing when the fiberscope and endotracheal tube are introduced into the trachea.
Usually, a combination of techniques (Table 1) is used to deliver local anesthetic to the airway mucosa in preparation for awake intubation. For example, to prepare the nasopharynx, preprepared local anesthetic solution can be sprayed into the nasal mucosa using the nozzle from the container. The oropharynx could be prepared using local anesthetic sprayed using the McKenzie technique, and the vocal cords could be sprayed using the SAYGO method. Alternatively, the MADs can be used to spray the nasal and oral mucosa. Whichever technique or combination of techniques is used, the aim should be to have an airway adequately anesthetized in preparation for instrumentation.
TABLE 1. Application techniques.
| Spray from container Local anesthetic soaked in ribbon gauze Cotton applicators McKenzie technique Mucosal atomization device Inhalation of nebulized lidocaine “Spray as you go” via epidural catheter |
NYSORA Tips
• Sitting the patient in an upright position will help with oxygenation and topicalization.
• Always administer supplementary oxygen.
• Start and establish the sedation before commencing the topicalization process, which can be uncomfortable.
• Asking the patient to “sniff ” while spraying the nasopharynx can aid in the distribution of local anesthetic.
REGIONAL ANESTHESIA TECHNIQUES
Nerve blocks can provide anesthesia for awake intubation but can be technically more challenging to perform than topical anesthesia of the airway. They do carry a higher risk of complications, such as intravascular injection and nerve damage, and more than one nerve needs to be blocked. These are the glossopharyngeal, superior laryngeal, and recurrent laryngeal nerves, as they supply the innervation to the oropharynx and larynx. Therefore, the nerve blocks required to anesthetize the airway are the glossopharyngeal, superior laryngeal, and translaryngeal blocks.
The nasal passages are supplied by the palatine nerves and anterior ethmoidal nerve. These nerves need to be blocked to allow for awake nasal fiber-optic intubation. These nerves are usually blocked by the topical application of local anesthetic to the nasal passages, usually by inhalation, spray topicalization, or the application of cotton applicators soaked in anesthetic.
Landmark Technique
Glossopharyngeal Nerve Block
The glossopharyngeal nerve provides sensation to the posterior third of the tongue and the vallecula and provides the sensory limb for the gag reflex; therefore, this block is particularly useful in abolishing this reflex. There are two approaches described for this block: intraoral and peristyloid.
For the intraoral approach, the patient requires sufficient mouth opening to allow adequate visualization and access to the base of the posterior tonsillar pillars (palatopharyngeal arch) (Figure 7). After adequate topical anesthesia (lidocaine spray) has been applied, the tongue is retracted medially with a
laryngoscope blade or a tongue depressor, allowing access to the posterior tonsillar pillar. Then, using a 22- or 25-gauge needle, 2–5 mL of 2% lidocaine are injected submucosally, after negative aspiration. The point of injection is at the caudal aspect of the posterior tonsillar pillar (approximately 0.5 cm lateral to the lateral edge of the tongue where it joins the floor of the mouth; Figure 8). This is then repeated on the other side.
Alternatively, a gauze soaked in local anesthetic can be firmly applied to this region for a few minutes. This method avoids the risk of intravascular injection but is not as successful as when the local anesthetic is injected.
The peristyloid approach aims to infiltrate local anesthetic just posterior to the styloid process where the glossopharyngeal nerve lies. In close proximity to this is the internal carotid artery, so care must be taken when using this approach.
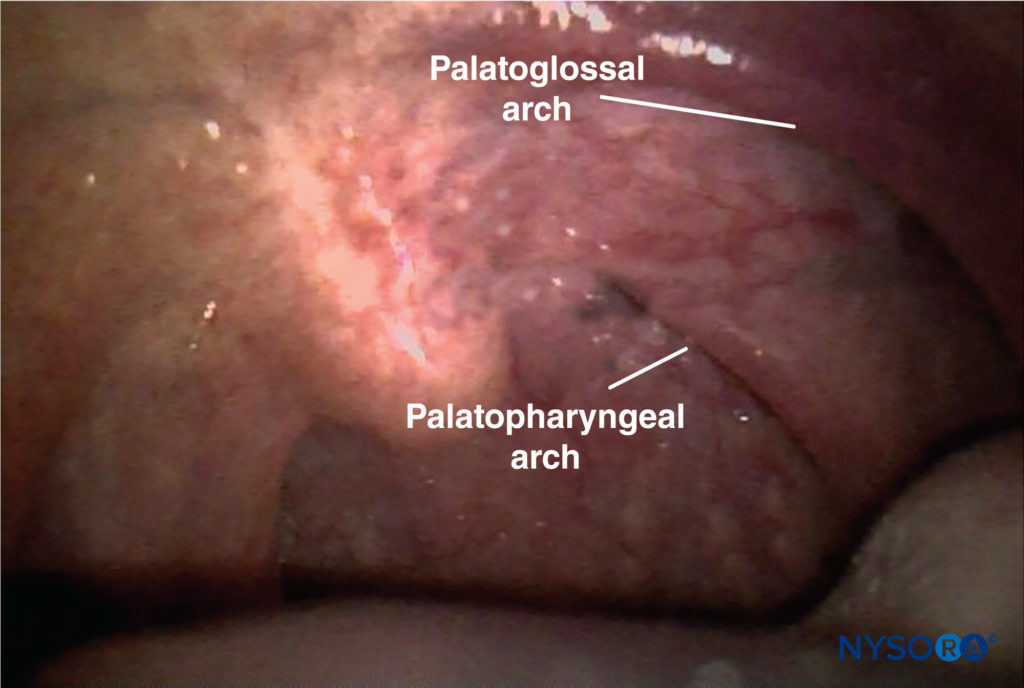
FIGURE 7. Palatopharyngeal arch.
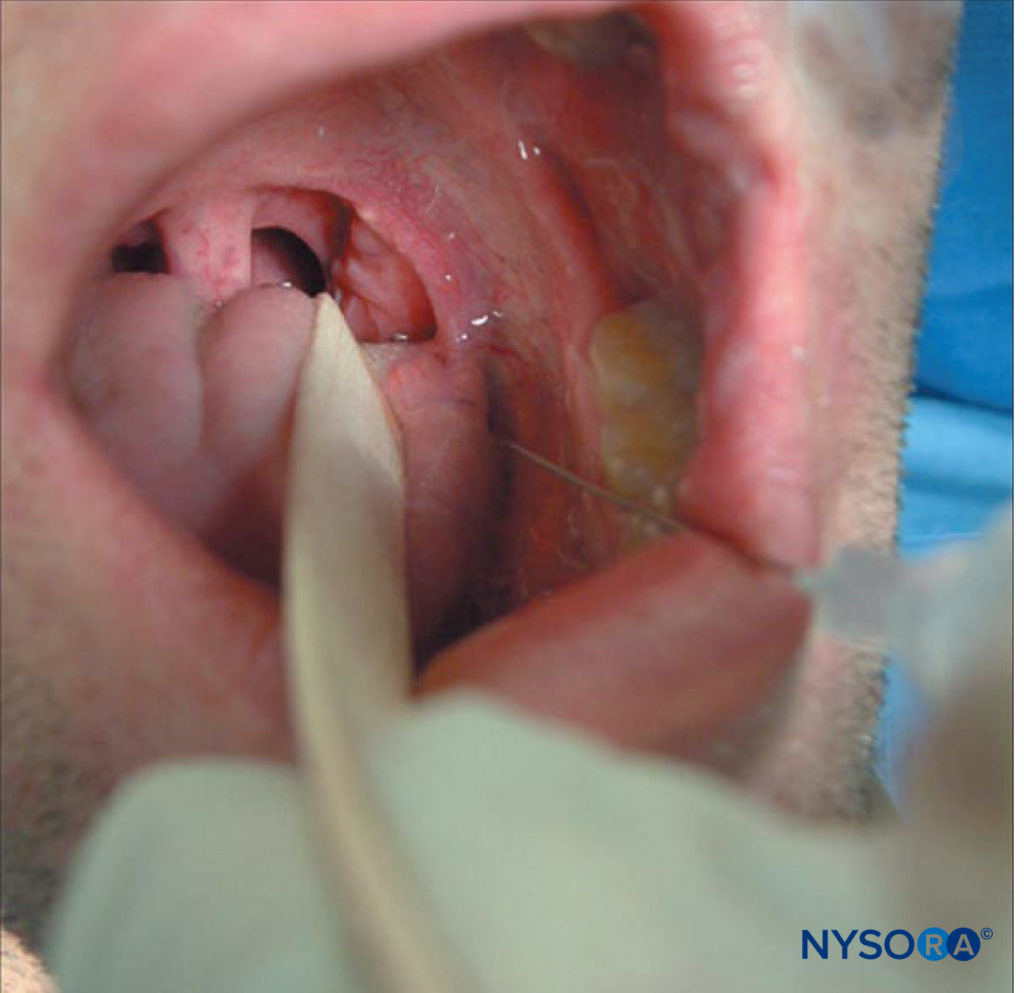
FIGURE 8. Glossopharyngeal nerve block.
The patient should be placed in a supine position with the head placed neutrally. The styloid process is located at the midpoint of a line drawn from the angle of the jaw to the tip of the mastoid process. It can be palpated using deep pressure, but this may be uncomfortable for the patient; a needle is inserted perpendicular to the skin, aiming to hit the styloid process. Once contact has been made (usually 1–2 cm deep), the needle should be reangled posteriorly and walked off the styloid process until contact is lost, then 5–7 mL of 2% lidocaine can be injected after negative aspiration. This is then repeated on the other side.
NYSORA Tips
• The glossopharyngeal nerve is most easily blocked where it crosses the palatoglossal arch.
• It can be blocked by spraying local anesthetic, by applying gauze or pledgets soaked in local anesthetic directly over the nerve, or by direct injection of local anesthetic around the nerve.
• This helps to abolish the gag reflex, but this block on its own will not provide adequate conditions for awake fiberoptic intubation.
Superior Laryngeal Nerve Block
The superior laryngeal nerve provides sensation to the laryngeal structures above the vocal cords and lies inferior to the greater cornu of the hyoid bone; here, it splits into the internal and external branches. The internal branch then penetrates the thyrohyoid membrane about 2–4 mm inferior to the greater cornu, continuing submucosally in the piriform recess (Figure 9 and Figure 10). The external branch does not penetrate the thyrohyoid membrane; it descends on the larynx deep to the sternothyroid muscle. The superior laryngeal nerve can be blocked using the external or internal approach.
To perform the block using the external approach, the patient is placed in the supine position and will need a degree of neck extension to facilitate identification of the hyoid bone.
Once identified, the hyoid bone is gently displaced to the side where the block is to be performed and a 25-gauge needle is inserted from the lateral side of the neck, aiming toward the greater cornu.
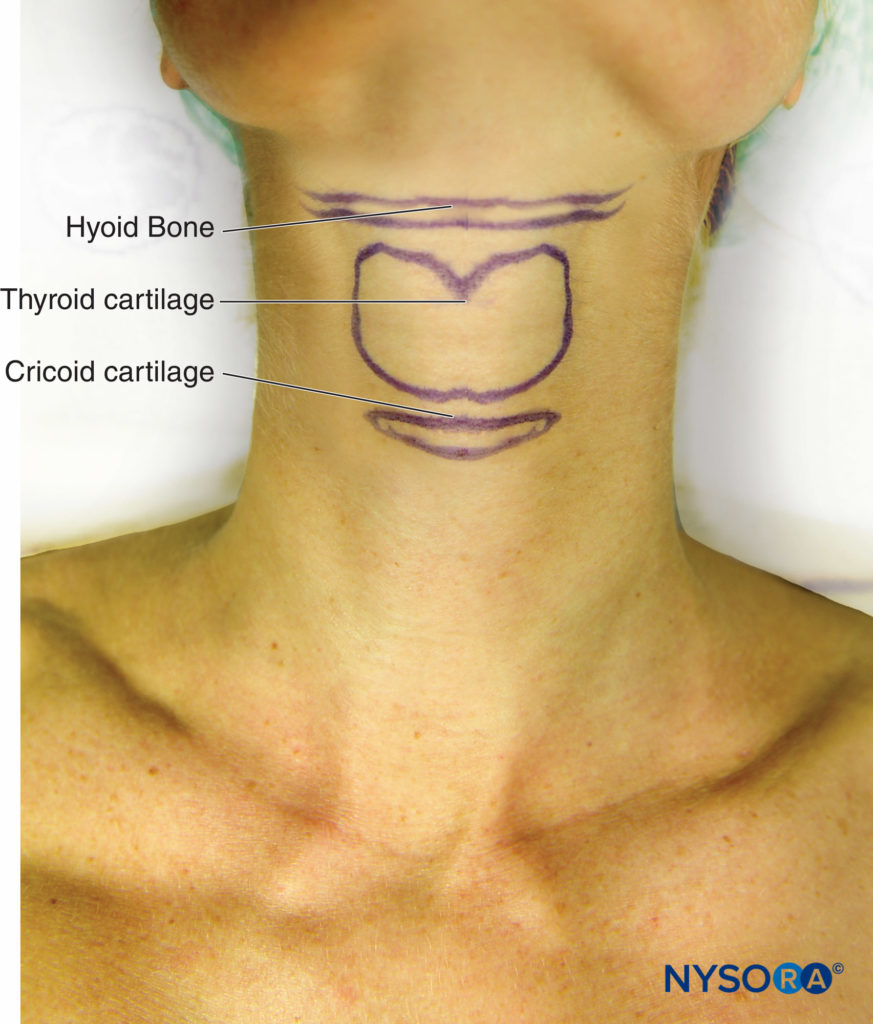
FIGURE 9. Surface anatomy of hyoid bone, thyroid, and cricoid cartilages.
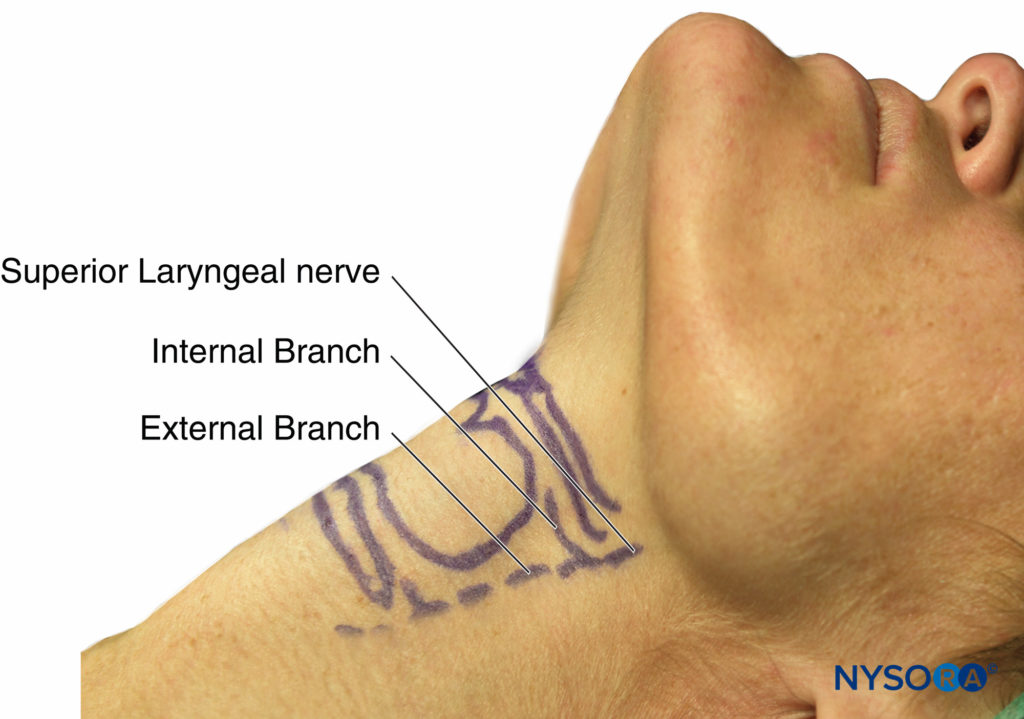
FIGURE 10. Surface anatomy of superior laryngeal nerve and branches.
Once contact has been made, the needle is walked off the bone inferiorly, and injecting 2 mL of 2% lidocaine here will block both the internal and the external branches of the superior laryngeal nerve (Figure 11). If the needle is advanced a few millimeters, it will pierce the thyrohyoid membrane, and a “give” is felt. Injecting local anesthetic here will result in only the internal branch of the superior laryngeal nerve being blocked. As with all blocks, careful aspiration must be performed prior to injection, especially as the carotid artery is in close proximity.
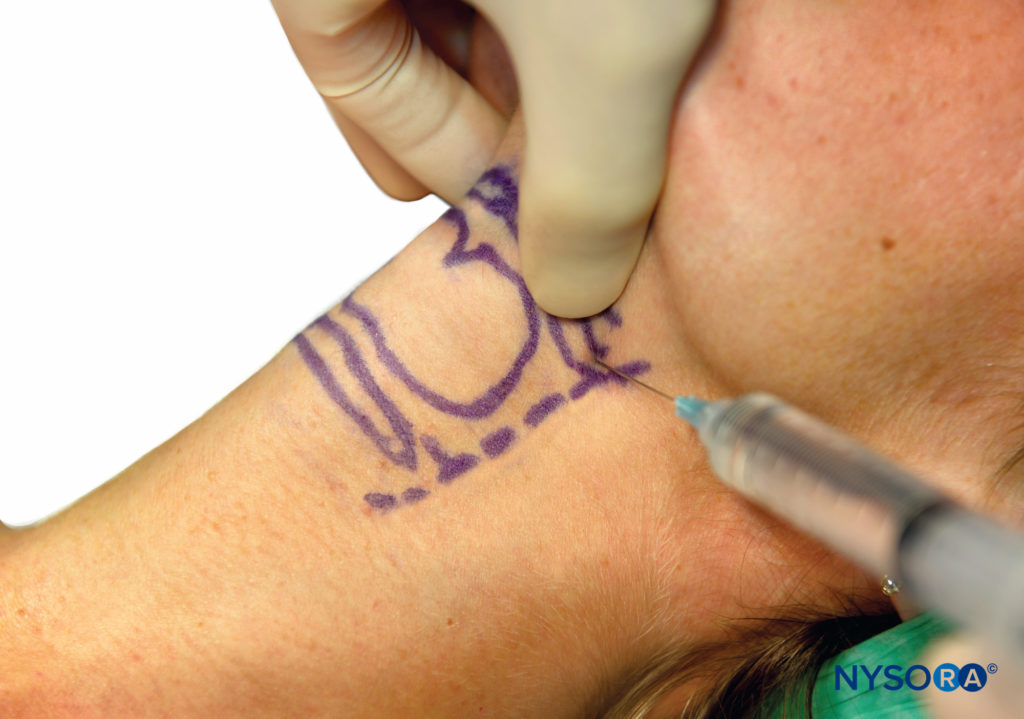
FIGURE 11. Superior laryngeal nerve block.
If it is difficult to identify the hyoid bone, the superior cornu of the thyroid cartilage can be identified instead. This is located by identifying the thyroid notch, tracing the upper edge posteriorly until the superior cornu can be palpated as a small round structure. This lies just inferior to the greater cornu of the hyoid bone. The needle can be inserted, aiming for the superior cornu of the thyroid cartilage, then walked cephalad, then local anesthetic is injected once the needle loses contact with the superior cornu. If the thyrohyoid membrane is pierced, then inject 2 mL of local anesthetic here and a further 2 mL as the needle is withdrawn; this will increase the chances of both the internal and external branches of the superior laryngeal nerve being blocked.
The internal approach uses gauze or pledgets soaked in local anesthetic and placed in the piriform fossae using Krause’s forceps. These need to be kept in place for 5-10 minutes to allow sufficient time for the local anesthetic to take effect.
Recurrent Laryngeal Nerve Block
The sensory innervation of the vocal cords and trachea is supplied by the recurrent laryngeal nerves. These ascend along the tracheoesophageal groove and also provide the motor supply to all the intrinsic muscles of the larynx except the cricothyroid muscle. Direct recurrent laryngeal nerve blocks are not performed as they can result in bilateral vocal cord paralysis and airway obstruction, as both the motor and the sensory fibers run together. Therefore, this nerve is blocked using the translaryngeal block.
To perform this, the patient should be supine, with the neck extended be identified in the midline, then the palpating finger should be moved in a caudad direction until the cricoid cartilage is palpated. The cricothyroid membrane lies between these two structures, immediately above the cricoid cartilage. The thumb and third digit of one hand should stabilize the trachea at the level of the thyroid cartilage, then a 22 or 20 gauge needle should be inserted perpendicular to the skin with the aim to penetrate the cricothyroid membrane (above the cricoid cartilage) (Figure 12). This should be done with continuous aspiration of the syringe, as the appearance of bubbles will indicate that the needle tip is now in the trachea. At this point, immediately stop advancing the needle; otherwise, the posterior laryngeal wall can be punctured. Rapid injection (and then removal of the needle) of 5 mL of 4% lidocaine will result in coughing, which will help to disperse the local anesthetic and block of the recurrent laryngeal nerve.
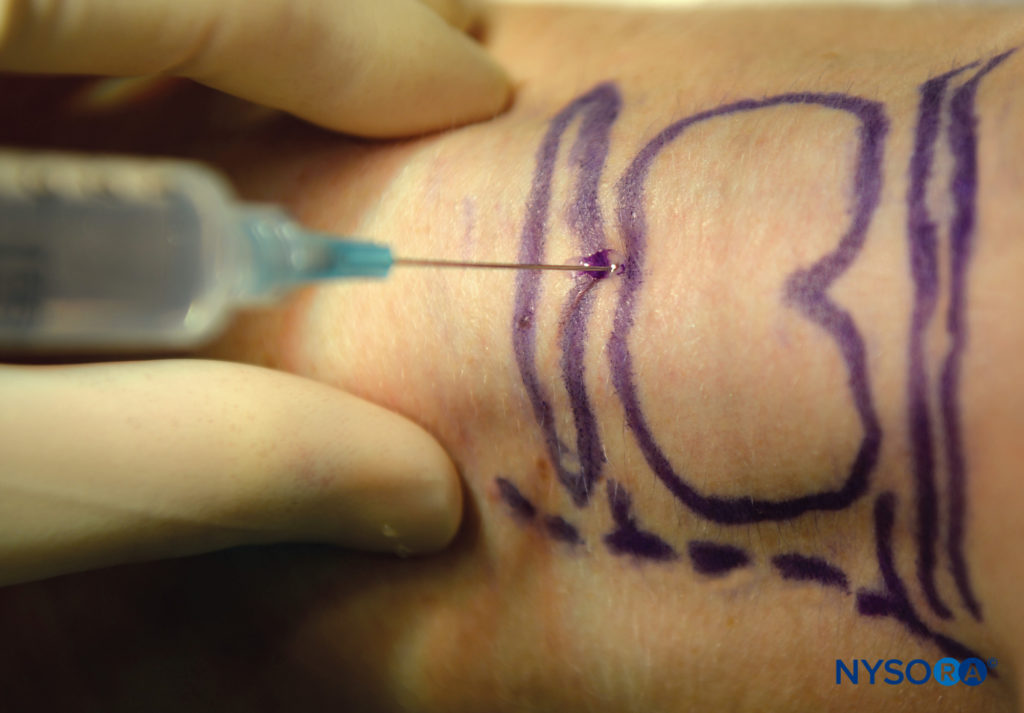
FIGURE 12. Translaryngeal block.
NYSORA Tips
• Appropriate position of the patient will aid in the correct identification of the cricoid and thyroid cartilages and the cricothyroid membrane.
• The neck should be extended, which makes these structures more prominent.
• Placing a liter bag of infusion fluids between the shoulder blades can help achieve this position.
Ultrasound-Guided Techniques
Ultrasound can be used to help increase the success rate of performing some of the blocks described (Table 2). Ultrasound can increase the accuracy of the deposition of local anesthetic around the greater cornu of the hyoid bone for the superior laryngeal nerve block and can be used to identify the cricothyroid membrane for translaryngeal blocks.
TABLE 2. Structures that can be identified on ultrasound.
| Hyoid bone Thyroid cartilage Thyrohyoid membrane Superior laryngeal artery Superior laryngeal nerve |
Superior Laryngeal Nerve Block
Sometimes, it can be difficult to identify the landmarks (eg, in obese patients) when trying to perform this block. Ultrasound can therefore be used to facilitate the deposition of local anesthetic to the correct place. The hyoid bone can be visualized on ultrasound (Figure 13), and an in-plane technique can be used to deposit local anesthetic around the surface of the greater cornu of the hyoid bone to achieve the block.
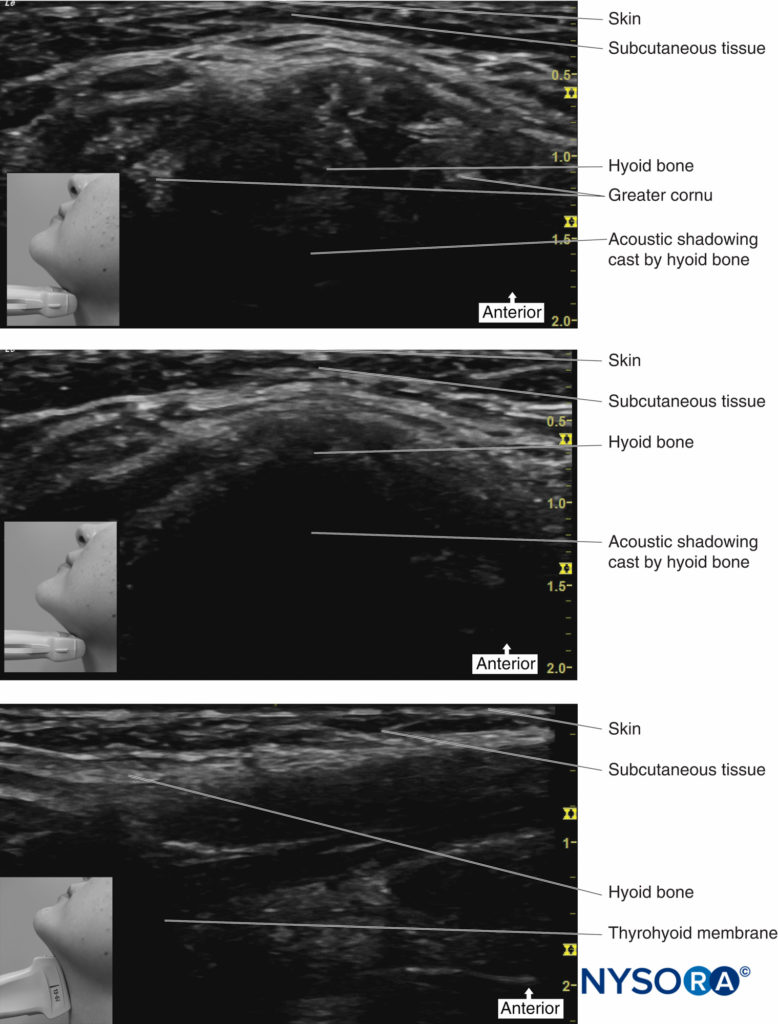
FIGURE 13. Ultrasound images of hyoid bone.
Place the transducer probe in the sagittal plane to identify the greater cornu of the hyoid bone; the transducer is then rotated transversely to identify the superior lateral aspect of the thyrohyoid membrane. The superior laryngeal nerve can be seen superficial to the thyrohyoid membrane when the medial aspect of the probe is rotated cephalad. The internal branch of the superior laryngeal nerve runs along with the superior laryngeal artery, just below the greater cornu of the hyoid bone.
An alternative approach is to identify the hyoid bone, which appears as a hyperechoic curved bright structure on ultrasound in the midline. If the probe is moved laterally, the greater cornu of the hyoid bone can be seen as a bright structure medial to the superior laryngeal artery. The internal branch of the superior laryngeal nerve runs with the superior laryngeal artery just below the level of the greater cornu of the hyoid bone. Using an in-plane technique, a needle is passed perpendicular to the skin, aiming just below the greater cornu of the hyoid bone.
Then, 1–2 mL of local anesthetic can be injected here after negative aspiration (Figure 14).
This technique has been shown to have a success rate of over 90%. Failure is thought to be due to variations in the anatomical position of the superior laryngeal nerve in relation to the hyoid bone.
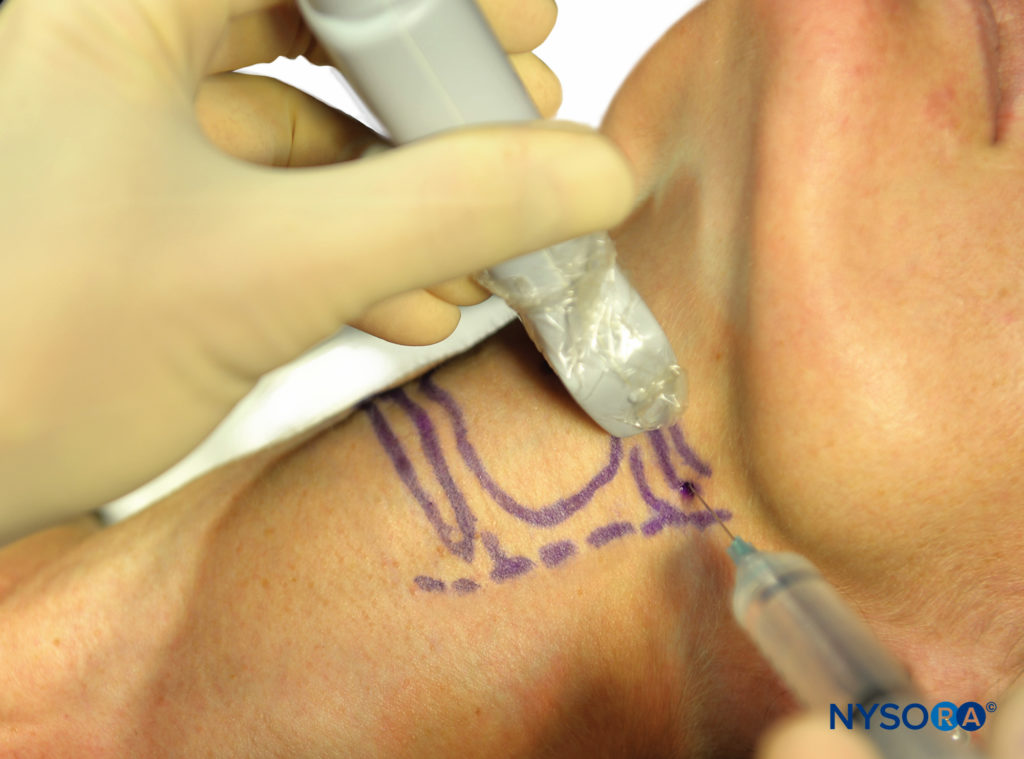
FIGURE 14. Ultrasound-guided superior laryngeal nerve block.
Translaryngeal Block
Sometimes, the correct location of the cricothyroid membrane is difficult to identify by palpation only. Ultrasound can be used to identify the thyroid and cricoid cartilages and the cricothyroid membrane (Table 3), ensuring that the local anesthetic is deposited correctly and a successful translaryngeal block is achieved19 (Figure 15).
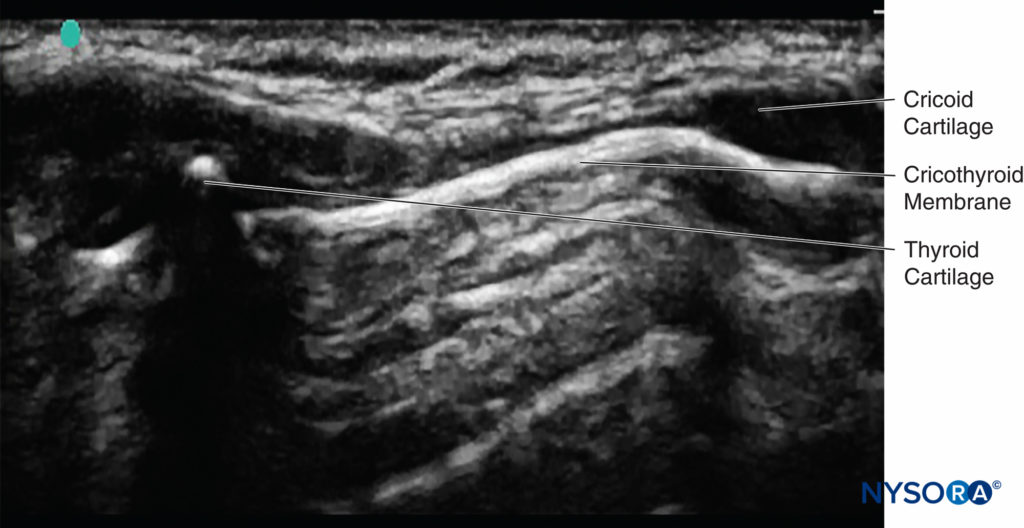
FIGURE 15. Ultrasound image of cricoid cartilage, thyroid cartilage, saggital plane, and cricothyroid membrane.
TABLE 3. Readily identifiable structures.
| Tracheal rings Cricoid cartilage Thyroid cartilage Cricothyroid membrane |
If the probe is placed longitudinally in the midline of the neck, the tracheal rings can be seen. If the probe is then advanced cranially, the cricoid cartilage can be seen next; this is a slightly elongated structure that is larger and more superficial than the tracheal rings. If the probe is further advanced cranially, the thyroid cartilage can be seen. The cricothyroid membrane lies between the caudal border of the thyroid cartilage and the cephalad border of the cricoid cartilage. Keep the probe in the midline with the cricothyroid membrane in the middle of the image seen on the monitor; then, the exact location on the patient’s neck can be marked using a marker pen. Now that the position of the cricothyroid membrane has been located, the translaryngeal block can be performed.
The block can also be performed under real-time sonography by simply tilting the probe from the midline to a parasagittal position, keeping the cricoid cartilage in view. The needle entry point should be just cranial to the cricoid cartilage and can be seen on the ultrasound monitor (Figure 16). Once air is aspirated, this confirms that the needle is through the membrane and in the trachea.
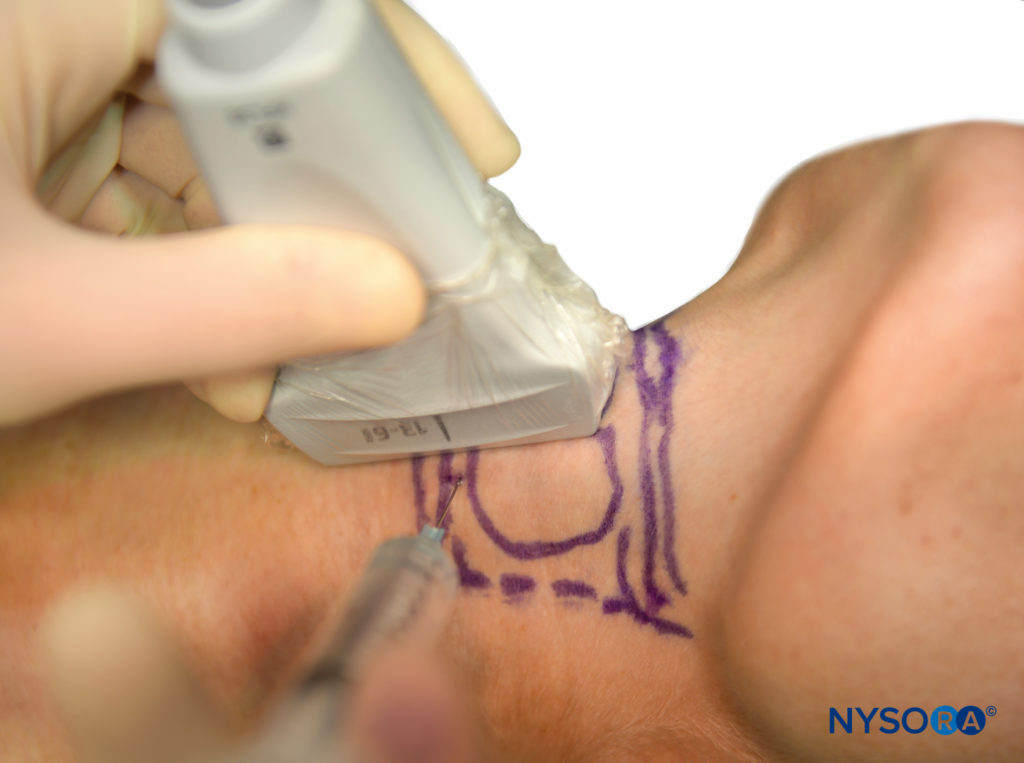
FIGURE 16. Ultrasound-guided translaryngeal block.
SEDATION TECHNIQUES
Awake endotracheal intubation can be an unpleasant experience for the patient, even if thorough topicalization of the airway has been done. The aim of conscious sedation is not only to allow the patient to tolerate the procedure but also to provide optimal intubating conditions.
There are various techniques available to achieve the desired level of sedation; whichever is used, the priority is to avoid oversedation of the patient. Oversedation could lead to an unresponsive patient with loss of airway, which could result in serious consequences.
The ideal sedation conditions would involve a comfortable patient responsive to commands with a maintained airway, spontaneous breathing, and a degree of amnesia (Table 4).
Two drugs are becoming increasingly popular and have growing evidence to support their use for conscious sedation: remifentanil and dexmedetomidine.20 Remifentanil is an ultrashort-acting opioid, and dexmedetomidine is a highly selective α2 agonist (Table 5).
Remifentanil has been found to provide good intubating conditions, is well tolerated, and has high patient satisfaction scores, although there is a high incidence of recall when used as a solo agent. Best results are seen when a target controlled infusion (TCI) technique is used.
TABLE 4. Ideal sedation conditions.
| Anxiolysis Amnesia Analgesia Suppression of gag and cough reflexes Easily titratable Minimal respiratory side effects Rapidly reversible |
TABLE 5. Examples of sedation techniques.
| Boluses of benzodiazepines (eg, diazepam, midazolam) Boluses of opioids (eg, fentanyl, alfentanyl, morphine) Boluses of α2 agonists (eg, clonidine, dexmedetomidine) Boluses of anesthetic agents (propofol, ketamine) Combination of agents (eg, benzodiazepines and opioids) Intravenous infusion (propofol, remifentanil, dexmedetomidine) Combination of intravenous infusions (propofol and remifentanil) |
The advantage of dexmedetomidine is that a state of cooperative sedation is achieved; it also has antisialagogue effects. There is level 1 evidence to support its use for good intubating conditions, patient tolerance, and patient satisfaction. It is usually administered as a slow bolus over 120 minutes followed by an infusion. Benzodiazepines are usually administered in combination with an opioid as intermittent boluses and have been used as a sedative for awake fiberoptic intubation. The disadvantage of using boluses of benzodiazepines is that intermittent boluses are associated with overshooting; therefore, there is a risk of oversedation and apnea.
Propofol can be administered as intermittent boluses or as an infusion. Both techniques have been shown to be safe and well tolerated by patients. There is now increasing popularity of administering propofol as a TCI, either as a sole agent or in combination with remifentanil. Whichever technique is used, it is important to maintain a balance of an appropriate level of sedation and avoidance of underdosing or overdosing.
The combination of propofol and remifentanil TCI has proven to be a safe technique for fiber-optic intubation with consistent pharmacodynamic effects and allows for a more predictable level of sedation.
NYSORA Tips
• Safe sedation can be achieved by slowly administering the sedative drugs and continually communicating with the patient.
• Bispectral Index (BIS) monitoring can also be used to aid and guide sedation level.
AUTHOR’S PREFERRED TECHNIQUE FOR PERFORMING AN AWAKE FIBER-OPTIC INTUBATION
There are numerous techniques available for performing awake fiberoptic intubation. Next is described as a well-accepted and successful technique I use on a regular basis:
- Sit patient as upright as tolerable.
- Administer supplemental oxygen (via Hudson mask or nasal cannulae).
- Attach full monitoring.
- Start remifentanil (1–3 ng/mL) and propofol (0.5–1 μg/mL) TCI infusion. Do not give a bolus dose. Titrate the dose according to the patient’s level of sedation.
- Start to topicalize the nasopharynx with Moffett’s solution sprayed via MAD.
- Topicalize the oropharynx with 4% lidocaine using a MAD.
- After topicalization, suction any secretions using a soft suction catheter; this also tests the effectiveness of the local anesthetic.
- If patient does not tolerate the suction catheter, spray oropharynx with 2–4 sprays of 10% lidocaine.
- Preload the fiberscope with a nasal endotracheal tube (ETT) (size 6/6.5 outer diameter [OD]).
- Start fiberoscopy via the nasopharynx and visualize the vocal cords.
- Pass the fiberscope into the trachea.
- “Railroad” the lubricated ETT over the scope gently into the trachea, trying not to touch the carina with the fiberscope.
- Confirm correct placement of the ETT by visualizing the carina and ETT.
- Connect the ETT to the anesthetic circuit and capnography.
- Gently inflate the cuff of the ETT.
- Keep hold of the ETT until it has been safely secured.
- Patient is now safe to anesthetize.
SUMMARY
To successfully perform awake intubation in a patient with an anticipated difficult airway, it is important that you have an understanding of and are competent in all of the following:
- Innervation of the upper airway
- Knowledge of appropriate local anesthetic techniques and vasoconstrictor drugs
- Techniques available to topicalize/anesthetize the upper airway
- Prudent sedation techniques
- Oxygenation techniques during the procedure
- Techniques used for the correct placement of the endotracheal tube
This will enable a safe, stress-free, and successful awake intubation with high levels of patient satisfaction.
REFERENCES
- Cheney FW, Posner KL, Caplan RA: Adverse respiratory events infrequently leading to malpractice suits. A closed claims analysis. Anaesthesiology 1991;75:932–939.
- Cheney FW, Posner KL, Lee LA, Caplan RA, Domino KB: Trends in anaesthesia-related death and brain damage: A closed claims analysis. Anaesthesiology 2006;105:1081–1086.
- Popat M (ed). Difficult Airway Management. Oxford University Press, 2009.
- Rose DK, Cohen MM: The airway: Problems and predictions in 18,500 patients. Can J Anaesth 1994;41:372–383.
- Benjamin E, Wong DK, Choa D: “Moffett’s” solution: A review of the evidence and scientific basis for the topical preparation of the nose. Clin Otolaryngol Allied Sci 2004;29(6):582–587.
- Simmons ST, Schleich AR: Airway regional anaesthesia for awake fiberoptic intubation. Reg Anesth Pain Med 2002;27:180–192.
- Curran J, Hamilton C, Taylor T: Topical analgesia before tracheal intubation. Anaesthesia 1975;30:765–768.
- Morris IR: Fibreoptic intubation. Can J Anaesth 1994;41:996–1008.
- Vloka JD, Hadzic A, Kitain E: A simple adaptation to the Olympus LF1 and LF2 fiberoptic bronchoscopes for instillation of local anesthetic.
Anesthesiology 1995;82:792. - Furlan JC: Anatomical study applied to anesthetic block technique of the superior laryngeal nerve. Acta Anaesthesiol Scand 2002;46:199–202.
- Wheatley JR, Brancatisano A, Engel LA: Respiratory-related activity of cricothyroid muscle in awake normal humans. J Appl Physiol 1991;70:2226–2232.
- Curran J, Hamilton C, Taylor T: Topical analgesia before tracheal intubation. Anaesthesia 1975;30:765–768.
- Tsui BC, Dillane D: Finucane. Neural block for surgery to the neck and head: Clinical applications. In: Cousins MJ, Bridenbaugh PO, Carr D,
et al (eds). Cousin and Bridenbaugh’s Neural block in Clinical Anesthesia and Management of Pain, 4th ed. Lippincott Williams and Wilkins; 2008:479-491. - Green JS, Tsui BCH: Applications of ultrasonography in ENT: Airway assessment and nerve block. Anaesthesiology Clin 2010;28:541–553.
- Singh M, Chin KJ, Chan VWS, Wong DT, Prasad GA, Yu E: Use of sonography for airway assessment. An observational study. J Ultrasound Med 2010;29:79–85.
- Kristensen MS: Ultrasonography in the management of the airway. Acta Anaesthesiol Scand 2011;55:1155–1173.
- Manikandan S, Neema PK, Rathod RC: Ultrasound-guided bilateral superior laryngeal nerve block to aid awake endotracheal intubation in a patient with cervical spine disease for emergency surgery. Anaesth Intensive Care 2010;38:946–948.
- Kaur B, Tang R, Sawka A, Krebs C, Vaghadia H: A method for ultrasonographic visualization and injection of the superior laryngeal nerve: Volunteer study and cadaver simulation. Anaesth Analg 2012;115(5):1242–1245.
- De Oliveira GS Jr, Fitzgerald P, Kendall M: Ultrasound-assisted translaryngeal block for awake fibreoptic intubation. Can J Anesth/J Can Anesth 2011;58:664–665.
- Johnson KD, Rai MR; Conscious sedation for awake fibreoptic intubation: A review of the literature. Can J Anaesth 2013;60(6):584–599.
- Rai MR, Parry TM, Dombrovskis A, Warner OJ: Remifentanil target controlled infusion vs propofol target-controlled infusion for conscious sedation for awake fibreoptic intubation: A double blond randomized controlled trial. Br J Anaesth 2008;100:125–130.
- Puchner W, Egger P, Punringer F, Lockinger A, Obwegeser J, Gombotz H: Evaluation of remifentanil as a single drug for awake fibreoptic intubation. Acta Anaesthesiol Scand 2002;46:350–354.
- Mingo OH, Ashpole KJ, Irving CJ, Rucklidge MW: Remifentanil sedation for awake fibreoptic intubation with limited application of local anaesthetic in patients for elective head and neck surgery. Anaesthesia 2008;63:1065–1069.
- Maroof M, Khan RM, Jain D, Ashraf M: Dexmedetomidine is a useful adjunct for awake intubation. Can J Anesth 2005;52:776–777.
- Unger RJ, Gallagher CJ: Dexmedetomidine sedation for awake fibreoptic intubation. Semin Anesth Perioper Med Pain 2006;25:65–70.
- Bergese SD, Khabiri B, Roberts WD, Howie MB, McSweeney TD, Gerhardt MA: Dexmedetomidine for conscious sedation in difficult awake fibreoptic intubation cases. J Clin Anesth 2007;19:141–144.
- Sidhu VS, Whitehead EM, Ainsworth QP, Smith M, Calder I: A technique for awake fibreoptic intubation. Experience in patients with cervical spine disease. Anaesthesia 1993;48:910–913.
- JooHS, Kapoor S, Rose DK, Naik VN: The intubating laryngeal mask airway after induction of anaesthesia versus awake fibreoptic intubation in patients with difficult airways. Anesth Analg 2001;92:1342–1346.
- Lallo A, Billard V, Bourgain JL: A comparison of propofol and remifentanil target-controlled infusions to fascilitate fibreoptic nasotracheal intubation. Anesth Analg 2009;108:852–857.